Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4257
Revised: August 14, 2012
Accepted: August 18, 2012
Published online: August 28, 2012
AIM: To analyze gene expression profiles in an experimental pancreatitis and provide functional reversal of hypersensitivity with candidate gene endothelin-1 antagonists.
METHODS: Dibutyltin dichloride (DBTC) is a chemical used as a polyvinyl carbonate stabilizer/catalyzer, biocide in agriculture, antifouling agent in paint and fabric. DBTC induces an acute pancreatitis flare through generation of reactive oxygen species. Lewis-inbred rats received a single i.v. injection with either DBTC or vehicle. Spinal cord and dorsal root ganglia (DRG) were taken at the peak of inflammation and processed for transcriptional profiling with a cDNA microarray biased for rat brain-specific genes. In a second study, groups of animals with DBTC-induced pancreatitis were treated with endothelin (ET) receptor antagonists [ET-A (BQ123) and ET-B BQ788)]. Spontaneous pain related mechanical and thermal hypersensitivity were measured. Immunohistochemical analysis was performed using anti-ET-A and ET-B antibodies on sections from pancreatic tissues and DRG of the T10-12 spinal segments.
RESULTS: Animals developed acute pancreatic inflammation persisting 7-10 d as confirmed by pathological studies (edema in parenchyma, loss of pancreatic architecture and islets, infiltration of inflammatory cells, neutrophil and mononuclear cells, degeneration, vacuolization and necrosis of acinar cells) and the pain-related behaviors (cutaneous secondary mechanical and thermal hypersensitivity). Gene expression profile was different in the spinal cord from animals with pancreatitis compared to the vehicle control group. Over 260 up-regulated and 60 down-regulated unique genes could be classified into 8 functional gene families: circulatory/acute phase/immunomodulatory; extracellular matrix; structural; channel/receptor/transporter; signaling transduction; transcription/translation-related; antioxidants/chaperones/heat shock; pancreatic and other enzymes. ET-1 was among the 52 candidate genes up-regulated greater than 2-fold in animals with pancreatic inflammation and visceral pain-related behavior. Treatments with the ET-A (BQ123) and ET-B (BQ-788) antagonists revealed significant protection against inflammatory pain related mechanical and thermal hypersensitivity behaviors in animals with pancreatitis (P < 0.05). Open field spontaneous behavioral activity (at baseline, day 6 and 30 min after drug treatments (BQ123, BQ788) showed overall stable activity levels indicating that the drugs produced no undesirable effects on normal exploratory behaviors, except for a trend toward reduction of the active time and increase in resting time at the highest dose (300 μmol/L). Immunocytochemical localization revealed that expression of ET-A and ET-B receptors increased in DRG from animals with pancreatitis. Endothelin receptor localization was combined in dual staining with neuronal marker NeuN, and glia marker, glial fibrillary acidic protein. ET-A was expressed in the cell bodies and occasional nuclei of DRG neurons in naïve animals. However, phenotypic expression of ET-A receptor was greatly increased in neurons of all sizes in animals with pancreatitis. Similarly, ET-B receptor was localized in neurons and in the satellite glia, as well as in the Schwann cell glial myelin sheaths surrounding the axons passing through the DRG.
CONCLUSION: Endothelin-receptor antagonists protect against inflammatory pain responses without interfering with normal exploratory behaviors. Candidate genes can serve as future biomarkers for diagnosis and/or targeted gene therapy.
- Citation: Oz HS, Lu Y, Vera-Portocarrero LP, Ge P, Silos-Santiago A, Westlund KN. Gene expression profiling and endothelin in acute experimental pancreatitis. World J Gastroenterol 2012; 18(32): 4257-4269
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4257
Abdominal pain ranging from mild to severe pain is the chief symptom of patients with pancreatic disorders. Neural innervation of the pancreas is important in the initiation and maintenance of inflammation. Activation of pancreatic sensory neurons causes release of neurotransmitters in the spinal cord and neurogenic activation signals in the pancreas itself producing plasma extravasation and neutrophil infiltration. Abundant evidence has suggested that endothelins (ETs) may play a role in the transmission of nociceptive information in animals[1-5] and in humans[1,6,7]. ET1 has been shown to induce abdominal constrictions in mice, incapacitation in dogs, and intradermal injection into humans caused wheal and flare and itching responses[1]. Additionally, ductal fibrosis induced constriction is enhanced through endothelin autocrine loops caused by stellate cell activation. Therefore, endothelin cascade is implicated as a major contributing factor in pancreatic pain in both pancreatitis and pancreatic cancer[8-11].
Multifunctional ETs[1-3] comprise a family of peptides of 21 amino acids which interact with their specific receptor subtypes that are involved in regulation of blood flow, cell proliferation, muscle contraction or relaxation, secretion and ion transport[12]. The ETs are expressed by a variety of cell types including endothelial cells, macrophages, astrocytes and neurons. Previous data also indicate that ETs can have direct effects on the peripheral sensory nervous system (including neurons and glial cells and may directly be involved in signaling nociceptive events in peripheral tissues)[3]. In inflammatory states the levels of ETs are increased[1]. In mammals, ETs produce their biological effects via activation of two receptors subtypes, the endothelin-A (ET-A) receptor and the endothelin-B (ET-B) receptor[3]. ET-A and ET-B receptors are expressed in different cell types in peripheral nerve and sensory ganglia and are involved in pain transmission[3]. ET receptor blockade in severe acute pancreatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival in rats[13,14]. ET-A receptors are mainly localized in dorsal root ganglia (DRG), enteric motor neurons[15], and brain blood vessels. In peripheral nerves, small unmyelinated fibers, are reported to express both ET-A and ET-B receptors. ET-B receptors are expressed primarily in the glia, epithelia, ependymal, in addition to neuronal cells. ET-A receptor action produces vasoconstriction and they are involved in hypoxia mediated neuropathic pain, while action of ET-B receptors results in vasodilatation that has been implicated in inflammatory pain and nociception[16]. ET-1 is a potent vasoconstrictor peptide increased in inflammatory states and known to induce pain in animals through its actions on endothelin receptors[3]. In addition, ET-1 increases capillary permeability changes and plays a role in aggravating the development of acute hemorrhagic pancreatitis through its action on the pancreatic microcirculation[17]. ET-1 is recognized as the key player in the immune-mediated hypernociception and inflammatory diseases as depicted in autoimmune pancreatitis[18]. Indeed, ET-1 is considered as the main cause of pancreas microcirculation disturbance during acute pancreatitis. ET-1 increases capillary permeability changes. Nitric oxide (NO) is the mediator of the cascade of inflammatory responses[19]. Normally, ET-1 and NO together are in a dynamic balance regulating the elasticity of blood vessels, and maintaining the peripheral resistance of vessels and local vasomotor function. Once this balance is disrupted, it leads to vasomotor dysfunction and microcirculation disturbances[20].
Objectives of the present study were to analyze the gene expression profile in the thoracic spinal cord and DRG to elucidate whether pancreatitis can induce gene regulation in these tissues. One of the genes upregulated by dibutyltin dichloride (DBTC)-induced pancreatitis was endothelin-1. As a test of phenotypic expression and functional significance, the present study examined localization of endothelin-A and B receptors in the pancreas and the DRG, as well as determined the effects of pharmacological agents known to be ET-A or ET-B receptor antagonists. Thus, we used the chemically-induced pancreatitis model by a single iv injection of the polyvinyl carbonate stabilizer/catalyzer, biocide in agriculture, antifouling agent in paint and fabric of DBTC that results in acute pancreatitis through reactive oxygen species. Pancreatitis induced by DBTC persists through seven days allowing a more clinically relevant study of the ongoing processes evoked by activation of viscero-nociceptive pathways that are likely maintaining the central sensitization state characteristic of experimental and clinical pancreatitis[21,22]. We hypothesized that the pancreatitis-induced hypersensitivity is mediated by the endothelin imbalance and its action on related receptors. Furthermore was tested the notion that pain related responses generated by endothelins could be pharmacologically reduced using specific endothelin receptor antagonists. To reduce the activation in viscero-nociceptive pathways, we assessed the ability of ET-A (BQ123) and ET-B (BQ788) receptor antagonists to reverse the behavioral syndrome induced by the experimental pancreatitis as described previously[21,22]. This behavioral syndrome is a measureable end point indicative of the central sensitization state ongoing in the spinal cord and peripheral nerves.
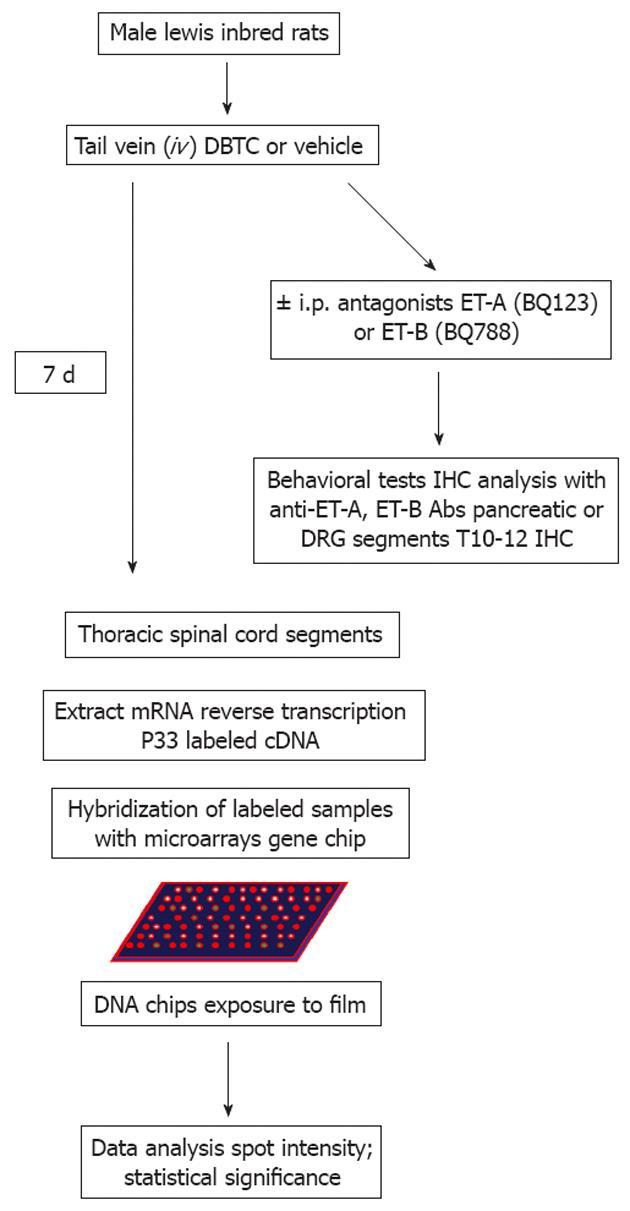
All animal procedures were approved by the Institutional Animal Care and Use Committee. Male Lewis-inbred rats (125-150 g) were purchased from Harlan Laboratories (Indianapolis, IN). Rats were housed 2 per cages with a 12-h/12-h light/dark cycle and allowed access to food and water ad libitum except during behavioral testing. Rats were monitored daily for continued weight gain and general health. Health Status and Procedures were documented daily on the post-operative evaluation form. After one week acclimatization/quarantine, they were assigned to experimental groups. Baseline behavioral measures were assessed prior to induction of pancreatitis with DBTC and one week later, before and after treatment with ET-A antagonist BQ123 or ET-B antagonist BQ-788. A flowchart for the experimental design is provided in (Figure 1).
Animals were divided into groups: Naïve control, Vehicle/phosphate buffer (PBS) and DBTC/PBS. The study was repeated for each of the endothelin antagonists (Table 1).
| No. | Groups (n = 5 per group) | iv injection | Drug administration (ip) |
| 1 | Naïve | Non | Non |
| 2 | Vehicle/PBS | Vehicle | 0.1 mol/L PBS |
| 3 | Vehicle/BQ-123 or BQ-788 | Vehicle | 300 μmol/L |
| 4 | DBTC/PBS | DBTC | 0.1 mol/L PBS |
| 5 | DBTC/BQ-123 or BQ-788 | DBTC | 33 μmol/L |
| 6 | DBTC/BQ-123 or BQ-788 | DBTC | 100 μmol/L |
| 7 | DBTC/BQ-123 or BQ-788 | DBTC | 300 μmol/L |
Induction of persistent acute pancreatitis: Acute persistent pancreatitis was induced in Lewis rats by a single tail vein injection with DBTC (Sigma-Aldrich, St Louis, MO). DBTC was dissolved in 95% ethanol (two parts) and then mixed with glycerol (three parts). In rats anesthetized with isoflurane inhalation, a maximum volume of 200 μL of DBTC (8 mg/kg body weight) was injected into the tail vein at a rate of 25 μL/min over 10 min using a syringe pump (Harvard Apparatus 22). Sham control rats received the vehicle (95% ethanol + glycerol, 2:3) also via tail vein. All animals (DBTC and sham controls) were fed Teklad 8626 chow and given 10% alcohol + 5% apple juice in their drinking water as a maintenance diet to support persistence pancreatitis in the model.
Tissue acquisition, RNA extraction, and gene chip analysis: Rat DRGs and segments of T10-T12 spinal cord were taken 7 d after injection at the peak of pancreatic inflammation and processed for transcriptional profiling using the rat brain-biased cDNA nylon array generated by Millennium Pharmaceuticals Inc. (Cambridge, MA) for gene discovery (both normal and subtractive). The cDNA libraries, array construction, hybridization, sequencing, process technology, informatics, TRACE for library construction, expressed sequence tag sequencing, data acquisition, and software have been detailed previously[23]. Therefore, rats were sacrificed and tissues were rapidly dissected from three animals per each group, frozen in dry ice, and stored at -80 °C before RNA isolation. Frozen tissues were homogenized in 1 mL of the TRIZOL reagent (Invitrogen, Carlsbad, CA) per 50-100 mg tissue and total RNA was extracted following the manufacturers protocol. Briefly, total RNA from each sample was column purified, and oligonucleotide primers flanking the cloning site was used to amplify the cDNA insert by polymerase chain reaction. After purification over CHROMA SPIN TE+30 columns, the labeled cDNA was annealed at 65 °C for 1 h with 10 μg poly (dA) > 200 (Amersham Pharmacia). At 2 × 106 cpm/mL, the annealed cDNA mixture was added to array filters in preannealing solution containing 100 mg/mL sheared salmon sperm DNA in 7% sodium dodecyl sulphate, 0.25 mol/L sodium phosphate, 1 mmol/L ethylene diamine tetraacetic acid, and 10% formamide. Following overnight hybridization the radioactive signals captured by a Fuji BAS 2500 phosphoimager (Fuji Medical Systems, Stamford, CT) and were quantified by using ARRAY VISION software (Imaging Research, St. Catherine’s, ON, Canada). Array hybridizations were performed in triplicate. Expression profiling data analysis and data clustering algorithm were done according to the published methodology[23]. Regulated genes were selected based on the array spot intensity that was normalized to the average of housekeeping genes and normal controls across the array. Total 3 animals/each group was used for the microarray analysis and the study repeated at least ×2. The average greater than or equal 2 fold changes in gene expressions were included in the study. Animal study design is shown in the Figure 1.
Pain-related behavioral assessments: Experimental procedures: Day 0: Baseline testing of abdominal nociceptive responses to mechanical and heat stimuli was applied to the upper left abdominal quadrant skin of rats as previously described[21,22,24]. Pain-related behavior was assessed throughout the study by an observer blinded to group assignment. (1) Assessment of Secondary Mechanical Hyperalgesia/Allodynia by Testing Abdominal Withdrawal Threshold. Mechanical hypersensitivity in the abdominal area was quantified by measuring the number of withdrawal events (either abdominal withdraw from the von Frey filament or consequent licking of the abdominal area, or whole body withdrawal) in response to normally innocuous or sub-threshold mechanical stimuli. The stimuli, applied to the upper left abdominal skin area, were von Frey filaments with bending forces of 3.52 mN, 10.78 mN and 47.24 mN (considered sub-threshold stimuli), and with bending forces of 215.6 mN (used as a supra-threshold stimulus). Reflex testing for “referred” secondary mechanical hyperalgesia/allodynia with von Frey fibers was developed by Max von Frey, who in 1896 identified “pain spots” on human skin, and has remained the standard in the field of pain assessment in humans and in animals. Mechanical nociceptive assay scores were expressed as an average percent response to ten applications of the von Frey filaments or the total number of withdrawal events to ten applications of three individual filaments as described previously[21,22,24]. Before testing, all animals were shaved on the rostral abdominal area and placed into clear plastic enclosures (7 cm × 4 cm × 4 cm) on the metal meshed (3 cm × 3 cm) platform (36 cm × 29 cm × 21.5 cm) and acclimated for 30 min. The von Frey filaments were applied from underneath through the mesh floor to the surface of the rostral abdominal area at different points. A single trial consisted of 10 applications of every von Frey filament. Each application was held for 1-2 s with a 5 s rest interval between applications to allow the animal to cease any response and return to a relatively inactive position. Totally, there were 3 trials through the graded series with a 10 min interval between trials. The withdrawal events for the three trials were averaged to get a single value per rat per time point. A percentage response was calculated (average of withdraw events/10 × 100) for the study. A positive response was defined as an abrupt withdrawal (flick response) of the abdomen during stimulation or immediately after the removal of stimulus. The abdominal withdrawal tests were performed at baseline; before DBTC insult (day 6); and on day 7 after endothelin receptor antagonist’ injection and von Frey filaments mechanic stimulation at 0 min, 20 min, 40 min, 75 min after BQ123 or BQ788 i.p. injection. Comparisons were made to baseline response; (2) Assessment of Secondary Thermal Hyperalgesia. In this assay, rats were placed on a regulated hotplate (52 °C) in a clear plastic enclosure (14 inches × 14 inches). The latency to responses (foot flick, jumping, licking, etc.) was determined on day 7 after DBTC insult and before (0) and 20 min, 40 min and 75 min after injection of endothelin receptor antagonists; and (3) Open Field Box Spontaneous Exploratory Behavioral Measures. On day 6, spontaneous exploratory behavioral activities were collected using open field 16 × 16 Photobeam Activity System (PAS) with FLEXFIELD software coupled to a PC (San Diego Instrument, Inc. CA). The PAS allows acquisition of movements in an x, y and z axis oriented grid system within an activity chamber (40 cm × 40 cm × 40 cm) by recording the number of times photobeams are obstructed. Data was collected in 5 min intervals for 30 min. Six different behavioral measures of spontaneous activity were examined: rearing events, rearing time (s), active time (s), rest time (s), distance traveled (inch), and total counts (number of beams broken). Resting time was defined as a period when the animal remained in place for 1 s or longer. Active and rest time are important to determine effect of the treatment on the total amount of time spent for both exploratory and stationary movements. Changes in each parameter were evaluated individually.
Endothelin receptor antagonist administration: ET-A receptor antagonist (BQ-123) and ET-B receptor antagonist (BQ-788) from American Peptide, CA, were tested in this model to assess their effect on pain related behavioral modification. The drugs were dissolved in PBS with final concentration in 1 mL and administrated (i.p.). Rats were treated with ET-A and ET-B receptor antagonists (0 min, 20 min, 40 min and 75 min) day 7 after induction of pancreatitis. The concentrations used are based on the respective Ki for the drugs. The Ki for BQ-123 has been reported to be 10 nmol/L[25]. The Ki for BQ-788 has been reported to be 100 nmol/L[26]. The 33 μmol/L, 100 μmol/L and 300 μmol/L treatments were used for generating the BQ-123 dose response curve by examining the effects of post-treatment on behavioral testing in animals with pancreatitis (n = 5 per each dose). Then, the maximal effective dose (300 μmol/L) was selected for the rest of study including for the drug, ET-B receptor antagonist (BQ-788, American Peptide, CA). Control rats received PBS injections (i.p.).
Therefore, seven different experimental groups were designed: BQ-123 or BQ-788 treated rats with or without pancreatitis; PBS treated rats with or without pancreatitis as well as naïve rats. Five rats in each group were included in this study (Table 1).
Morphine administration: Morphine was injected in cumulative doses of 1, 5 and 10 mg/kg body weight or sham saline given i.p. to rats (n = 6/group). Systemic administration of morphine is used as a gold standard analgesic in different pain related behavioral modification studies.
Perfusion and tissue collection: At the end of the experiment, rats were given an overdose of pentobarbital (75 mg/kg). Fresh pancreatic tissues were collected. Dorsal root ganglia (DRG) and spinal cord from 10th-12th thoracic segments were dissected as they receive the sensory information from the neuronal fibers that innervate the pancreas. Samples were flash frozen in liquid nitrogen and kept at -80 °C and processed for transcriptional profiling microarray analysis biased for rat brain-specific genes as mentioned above and for immunohistochemistry. The study design is shown in the Figure 1.
Spinal cord, DRG and pancreatic tissues collected for immunohistochemical analysis: At the end of the experiment, rats were given an overdose of pentobarbital and transcardially perfused with 4% buffered parafomaldehyde. Spinal cord segments T7-T12 and DRGs were taken 7 d after injection at the end of the experiment. Tissue were removed and stored overnight at 4 °C followed by immersing in 30% sucrose. The frozen sections were cut as 30 μm thickness. The fresh pancreas was directly fixed in 4% paraformaldehyde for 24 h followed by storing in 70% alcohol. The tissue was processed for paraffin sections (4 μm) which were mounted onto the chrome-gelatin pre-coated glass slides. After deparaffinization in xylene for 5 min (× 2), the sections were hydrated gradually with 100%, 95%, 80% and 70% alcohol followed by washes with distilled water. Polyclonal rabbit anti- ET-A and ET-B receptor (Alomone Labs, Jerusalem, Israel) were used for staining frozen sections of the DRG. In addition, paraffin sections of pancreas were prepared from rats with different treatments. Then, immunohistochemical staining procedures for both frozen and paraffin section were performed. After rinsing in 0.1 mol/L PBS, the tissues were blocked with 5% normal goat serum in 0.1 mol/L PBS plus 0.05% Triton X-100 and 0.3% bovine serum (NGSTB) for 40 min. Then, the tissues were double stained with specific antibodies produced in two different species in the following combinations: (1) Endothelin receptor A and neuronal marker (NeuN); and (2) Endothelin receptor B and glial fibrillary acidic protein marker for all glia (GFAP) (satellite and Schwann cells). This was accomplished by incubating the cut tissue sections with rabbit anti-ET-AR (1:400) and mouse anti-NeuN (1:5000) or ET-BR (1:200) and mouse anti-GFAP (1:500) diluted in 1% NGSTB overnight at room temperature. After rinsing with 0.1 mol/L PBS, the tissues were incubated for 1 h in a fluorescent tagged secondary antibody, red Alexa fluor 568 goat anti-rabbit IgG (1:1000) and green Alexa fluor 488 goat anti-mouse IgG (1:1000) diluted in 1% NGSTB. Finally, the tissues were rinsed with 0.1 mol/L PBS, cover-slipped with mounting media hard set with hard set mounting media with blue 4’,6-diamidino-2-phenylindole counterstain (Vector Labs, Burlingame, CA). Pictures were taken using the Act-1 program with a Nikon E1000 microscope.
All data are presented as mean ± SD unless otherwise stated. Statistical analysis was performed by the ANOVA-test (two way analysis of variance). If significant differences were found Bonferroni post hoc and when appropriate student t test statistical analysis were performed to assess changes over time for each group separately to compare (1) baseline and 7 d after induction of pancreatitis; (2) before and after drug treatment; and (3) drug treated group compared to controls without drug treatment. Statistical significance was set at P≤ 0.05.
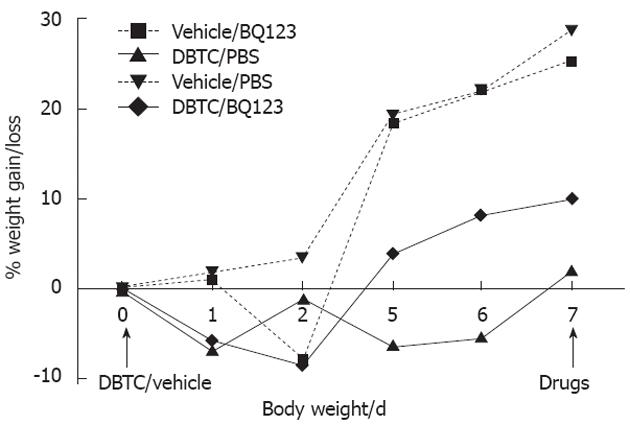
Animals that received sham vehicle significantly gained weight (27%) on the diet (vehicle: D1 vs D7, P < 0.01) compared the DBTC induced pancreatitis rats which differed (vehicle vs DBTC) by an average of 21% (Figure 2).
Rat DRG segments of T10-12 spinal cord were taken at the peak of inflammation and processed for gene expression profiling with a microarray biased for rat brain-specific genes. An average of 3 animals/each group was used for the microarray analysis and the study repeated at least twice. The genes were normalized with sham controls and average greater than or equal 2 fold changes in gene expressions were demonstrated in the Table 2. Modified genes were classified into groups according to their functionality (Table 3). The identified genes included: circulatory/acute phase, extracellular matrix, signaling transduction, transcription/translation-related, antioxidants/chaperones/heat shock and pancreatic and other enzymes. Candidate genes are among those modified in comparison to normals and are involved in different signaling pathways in DBTC induced pancreatic inflammation as shown in the Table 2.
| Group | Regulated genes | Abbreviation | Fold |
| Up-regulated genes1 | |||
| 1 | B-lymphocyte surface antigen | 7.3 | |
| 1 | Endothelin-1 | ET-1 | 3.2 |
| 1 | Eosinophil chemotactic protein | ECP | 11 |
| 1 | T-cell surface glycoprotein CD5 | 2.6 | |
| 1 | Tumor necrosis factor | TNF-α | 4 |
| 2 | Laminin α 5 | LAMA5 | 2.5 |
| 2 | Matrix gla protein | MGP | 7.4 |
| 2 | Integrin α | 27 | |
| 3 | Epithelial-cadherin | E-CADHERIN | 3.1 |
| 3 | Transmembrane ER-resident protein, type I | IRE1b | 23.5 |
| 4 | ATP binding cassette | ABC-transporter | 23 |
| 4 | C-C chemokine receptor type 8 | C-C CKR-8, GPR-CY6 | 3.4 |
| 4 | GABAA receptor | GABAA R | 14.3 |
| 4 | Glutamate receptor ionotropic, AMPA 1 | GluR-1 | 9.1 |
| 4 | Putative G protein-coupled receptor | GPR68 (OGR-1) | 3.1 |
| 5 | Calcium binding protein | CHP | 2.6 |
| 5 | Caveolin-1 | CAV1 | 3.8 |
| 5 | Iron response regulator | IRR | 2.8 |
| 5 | LDL receptor-related protein 6 | LDL-R6 | 3.6 |
| 5 | Insulin-like growth factor binding complex acid label chain precursor | ALS | 2.7 |
| 5 | Proto-oncogene tyrosine-protein kinase FES/FPS | C-FES | 8 |
| 5 | RAS GTPase-activating-like protein | RAN | 3.9 |
| 5 | Retinoic acid-inducible gene-1 | RAIG1 | 6.4 |
| 5 | Transforming growth factor-b | TGFb | 2.8 |
| 5 | Guanine nucleotide binding protein | G (Y) α-11 | 4.7 |
| 6 | Nuclear factor of activated T cells | NF-ATc | 3.5 |
| 6 | Signal transducer and activator of transcription 5B | STAT5b | 3 |
| 6 | Transcription factor | TF | 11.8 |
| 7 | Glutathione peroxidase gastrointestinal | GSHPX_GI | 2.5 |
| 7 | Extracellular superoxide dismutase | EC-SOD | 2.9 |
| 7 | Heat shock related 70 kD proteins2 | 2.3 | |
| 7 | NADH dehydrogenase (ubiquinone) | 2.3 | |
| 7 | Serum amine oxidase | SAO | 2.2 |
| 8 | ACE-related carboxypeptidase | ACE2 | 3.6 |
| 8 | Aminopeptidase N | APN | 2.3 |
| 8 | ATP-dependent RNA helicase P54 | DDX6 | 2 |
| 8 | a enolase | 2 | |
| 8 | a-Tryptase | TRYPTASE 1 | 2.6 |
| 8 | cAMP cAMP-inhibited cGMP 3’5’-cyclic phosphodiesterase | 9.4 | |
| 8 | Cholecystokinin type A receptor | CCK-AR | 2.8 |
| 8 | Dopamine β-hydroxylase | DBH | 5.4 |
| 8 | GAMMA-glutamyltransferase 1 | Gamma-GT | 5.6 |
| 8 | (GDP-D-MANNOSE dehydratase | GMD | 3.5 |
| 8 | Glycolipid transfer protein | GLTP | 2.6 |
| 8 | Lipase | 3.8 | |
| 8 | Lysophosphatidic acid acyltransferase-b | LPAAT- b | 3.4 |
| 8 | Malonyl-CoA decarboxylase | MLYCD | 4.8 |
| 8 | NTPDase 3; CD39L 3 | ENTPD3 | 7.2 |
| 8 | Pancreatic isozyme | Glucokinase | 3.4 |
| 8 | Phosphate regulating neutral endopeptidase | NEP | 11 |
| 8 | Putative NAD (P)-dependent cholesterol dehydrogenase | MDH | 3 |
| 8 | Red cell phosphatase1, isozyme F | ACP1 | 2.3 |
| Down-regulated genes1 | |||
| 1 | Insulin precursor | 2.8 | |
| 1 | IG GAMMA-3 chain C region | HDC | 2 |
| 1 | Interlaken 1 receptor 1 precursor | IL-1R, IL-1Rα | 3.8 |
| 1 | Growth regulated protein (neutrophil-activating protein 3) | NAP-3 | 2.3 |
| 1 | Natural killer cells antigen CD94 | KP43 | 4.2 |
| 1 | Monocyte chemotactic protein 1 | 4.3 | |
| 1 | T-cell surface glycoprotein CD3 e chain precursor | 5.2 | |
| 1 | Heparin-binding growth factor 1 | 7.3 | |
| 2 | EGF-containing fibulin-like extracellular matrix protein 1 | FIBL-3 | 2.1 |
| 2 | Homocontig12 | 2.3 | |
| 2 | MT4-MMP | 2 | |
| 3 | Glocosyl structure | 2 | |
| 3 | Microtubule-associated protein 2 | MAP2C | 2.1 |
| 3 | Mucosal addressin cell adhesion molecule-1 | 2.1 | |
| 3 | Peripheral myelin protein 22 | PMP-22 | 3.8 |
| 4 | Growth factor regulated channel 5 | GRC5 | 2.1 |
| 4 | Growth hormone secretagogue receptor type 1 | 2 | |
| 4 | a platelet-derived growth factor receptor (CD104A antigen) | PDGFRa | 2.1 |
| 4 | Glucose transporter type 5, small intestine (fructose transporter) | 2.2 | |
| 4 | Dopamine receptor | 2.3 | |
| 4 | C-C chemokine receptor type 9 | CCR-9 | 2.4 |
| 4 | Monocarboxylate-transporter 3 | MCT 3 | 2.9 |
| 4 | Orphan receptor | GRF | 3.6 |
| 4 | G protein-coupled receptor | GRP1 | 3.6 |
| 4 | Neuropeptide Y Receptor Type 4 | NPY4-R | 10.6 |
| 4 | b 3 Adrenergic Receptor | 2.5 | |
| 5 | G25K GTP-binding protein, placental isoform (GP) | 2 | |
| 5 | MAD 2 | 2 | |
| 5 | Ribosomal S6 protein kinase | 2 | |
| 5 | Metalloproteinase Inhibitor 3 | TIMP-3 | 2.2 |
| 5 | GTPase RhoD | RhoD | 2.4 |
| 5 | Ankyrins | 2.7 | |
| 5 | Putative RHO/RAC effector protein | 4.2 | |
| 5 | Protein farnesyltransferase β subunit (RAS Proteins prenyltransferase) | FTASE-β | 2.4 |
| 6 | GTP-binding protein | RHEB | 2.2 |
| 6 | GTP-binding protein 1 | GP-1 | 2.3 |
| 6 | hLHX 6 1α | 2 | |
| 6 | AMP-binding protein | 2.7 | |
| 7 | Thyroid peroxidase | TPO | 2.1 |
| 8 | Ubiquitin-conjugating enzyme E2 (ubiquitin-protein ligase) | UBE2 | 2 |
| 8 | Steryl-sulfatase (steroid sulfatase) (steryl-sulfate sulfhydrolase) | ASC | 2 |
| 8 | Histone deacetylase 2 | HD2 | 2.1 |
| 8 | Corticosteroid 11-β-dehydrogenase, isozyme 2 | 4.3 | |
| 8 | Hydroxymethylglutaryl-CoA synthase | 4.4 | |
| 8 | ATP synthase oligomycin sensitivity conferral protein | OSCP | 4.9 |
| 8 | Indoleamine 2,3-dioxygenase | IDO | 2.1 |
| 8 | Lysozyme | 2.2 | |
| 8 | ubiquitin C-terminal hydrolase | UCH | 2.3 |
| 8 | Tyrosine aminotransferase,L-tyrosine: 2-oxoglutarate Aminotransferase | TAT | 2.7 |
| 8 | Corticosteroid 11-β-dehydrogenase, isozyme 2 | 4.3 | |
| 8 | Hydroxymethylglutaryl-CoA synthase | 4.4 | |
| 8 | ATP synthase oligomycin sensitivity conferral protein | OSCP | 4.9 |
| 8 | Glycogen phosphorylase | 8.5 |
| Groups | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | % | Total |
| Up | 17 | 17 | 8 | 30 | 46 | 23 | 9 | 67 | 44 | 81.3 | 261 |
| Down | 7 | 3 | 5 | 11 | 12 | 4 | 1 | 14 | 3 | 18.7 | 60 |
| Sum | 24 | 20 | 13 | 41 | 58 | 27 | 10 | 81 | 47 | 321 | |
| Total% | 7.5 | 6.2 | 4.1 | 12.8 | 18.1 | 8.4 | 3.1 | 25.2 | 14.6 | 100 | |
| Sum | 8.7 | 4.62 | 5.86 | 13.98 | 16.56 | 6.24 | 1.86 | 20.52 | 7.26 | 118.7 | 334 |
Subtraction of overlapping normal genes revealed 321 unique genes that were classified into 9 different functional groups (Table 3). The candidate genes included 261 that were up-regulated and 60 downregulated genes in this model. Amongst these, fifty-two distinct genes that were upregulated greater than 2-fold were chosen as candidates for the pancreatitis, visceral pain and pancreatic cancer pathway specific and listed in Table 2. Additionally, 53 selected genes which were down regulated in this pancreatitis model are shown in Table 2.
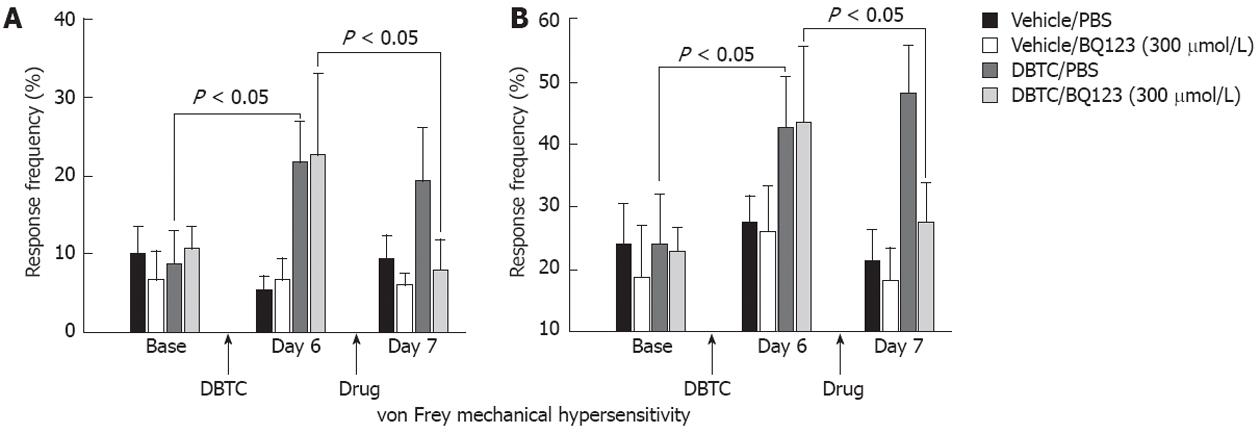
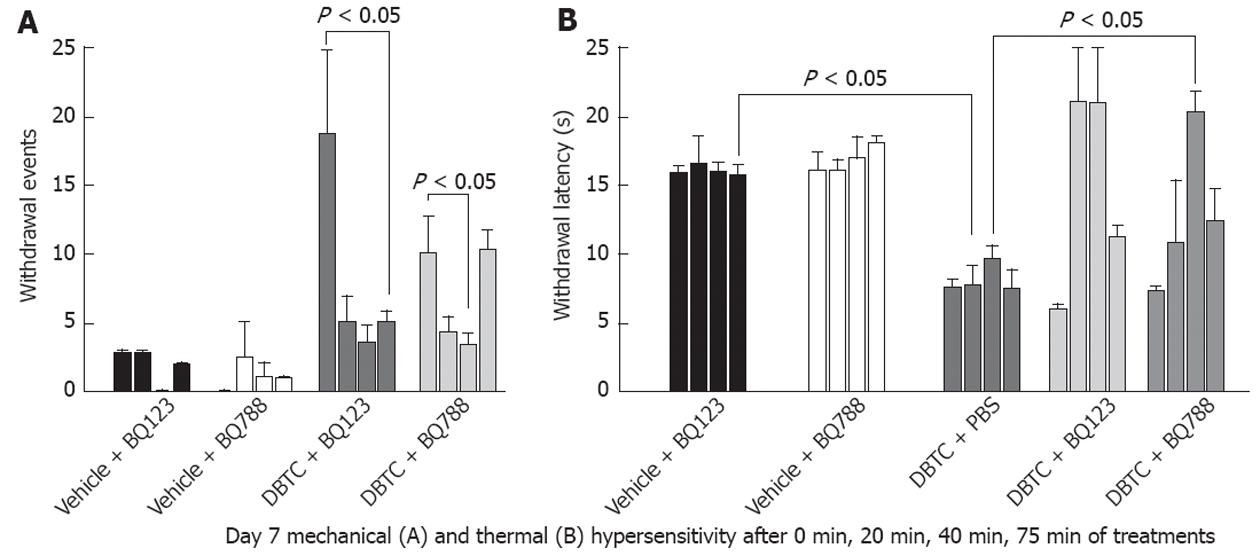
Secondary mechanical hyperalgesia: Animals developed persistent pancreatic inflammation during the study as detected in pathological samples and secondary pain-related cutaneous mechanical hypersensitivity. Abdominal mechanical testing with von Frey microfilament demonstrated statistically significant increases in withdrawal events on day 6 post DBTC injection in pancreatitis animals and are presented as % number of events (n = 5) (Figure 3). In contrast, no changes were detected in the sham control rats. Data obtained were consistent for 2 different microfilaments except more intense responses were detected with the larger microfilament (3.52 mN vs 10.78 mN) as expected, demonstrating consistency for abdominal response with the von Frey mechanical test (Figure 3A and B).
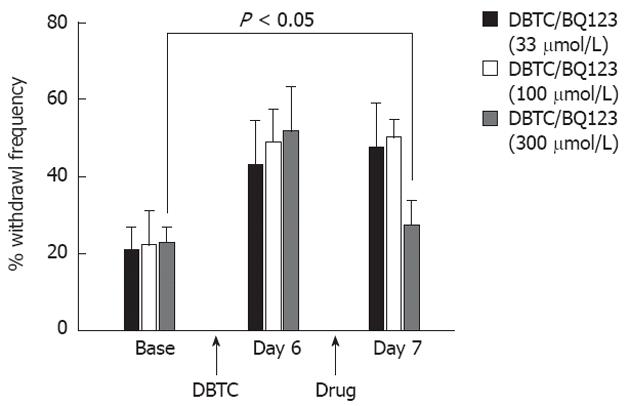
On day 7 after induction of pancreatitis, groups of animals were simultaneously treated (i.p. injection) with 3 different doses of ET-A antagonist (BQ123, 33, 100 and 300 μmol/L). Animals with pancreatitis had dose dependent responses in contrast to control animals that were not affected by the antagonists. While 33 μmol/L BQ123 did not provide a statistically significant effect (Figure 4), 300 μmol/L provided significant protection against inflammatory pain related behaviors in pancreatitic animals tested at 75 min after drug administration. Therefore, studies continued only with the 300 μmol/L dose (Figure 4).
Next we compared efficacy of BQ123 (ET-A receptor antagonist) to the BQ788, an ET-B antagonist (Figure 5). Both drugs were effective in normalizing mechanical hypersensitivity response. However, ET-A receptor antagonist had a longer lasting effect compared to ET-B receptor antagonist, with a more robust response. The ET-A receptor antagonist (BQ123) and the ET-B receptor antagonist (BQ788) abolished abdominal mechanical hypersensitivity as early as 20 min (Figure 5A). The reduced events using BQ123 demonstrated more vigorous response persisting through 75 min post treatment (n = 5), while the number of withdrawal events increased again at 75 min for the ET-B receptor antagonist demonstrating less efficacy and persistence of effect for this agent than for the ET-A receptor antagonist in management of pain related behavioral modifications in this model.
Hotplate whole-body thermal responses: Rats with pancreatitis reacted to the hotplate with reduced response times compared to naïve rats indicating increased thermal hypersensitivity (Figure 5B). Both ET-A receptor antagonist (BQ123) and ET-B receptor antagonist (BQ788) significantly increased response latencies, reinstating response times back to the levels of the vehicle control animals.
Additionally as expected abdominal hypersensitivity to von Frey mechanical stimulation and hyperalgesia (data not shown) were attenuated by the i.p. administration of the morphine in a cumulative, dose dependent manner in rats on day 7 (n = 6). As systemic administration of morphine is currently a gold standard therapy in different experimental pain models, data for the endothelin receptor antagonists used in this study correlated well to those obtained with morphine administration (correlation not shown).
Open field exploration: Spontaneous behavioral activity was conducted before induction of pancreatitis (baseline), 6 d after induction of pancreatitis, as well as 30 min after drug treatments (BQ123, BQ788) for comparison to sham control animals. The overall stable activity levels indicate that the drugs produced no undesirable effects on normal exploratory behaviors (data not shown). No changes were noted in the six behavioral measures except a trend toward reduction of the active time and an increase in resting time measured at the highest dose (300 μmol/L). These data indicate that the agents do not interfere with normal exploratory behaviors.
Pancreatic sections stained with HE from naïve animals receiving sham treatment showed normal histological architecture and islets. In contrast DBTC treated rats develop persistent pancreatitis demonstrated as edema in parenchyma, loss of pancreatic architecture, infiltration of inflammatory cells including neutrophil and mononuclear cells, degeneration vacuolization and necrosis of acinar cells (not shown).
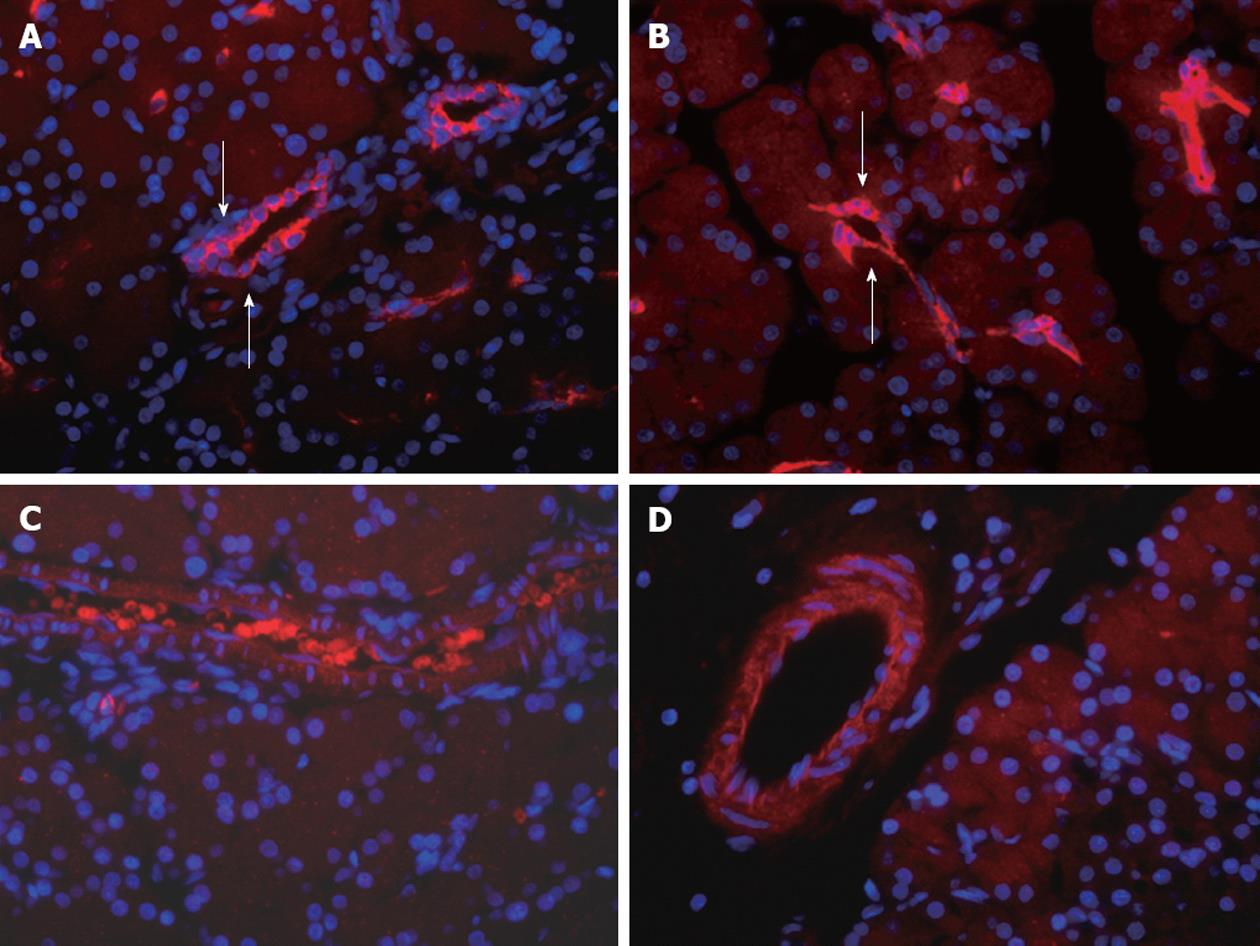
Endothelin-A and B receptor expression in naïve or pancreatitis DBTC rats was observed in the vascular endothelia (Figure 6). ET-A receptor appeared to be more condensed around constricted vessels in pancreatitic compared to naïve control animals. However, no quantitation was done since overall, minor detectable differences were evident in ET-A and ET-B receptors expression in the pancreas in comparisons between naïve and pancreatitis animals.
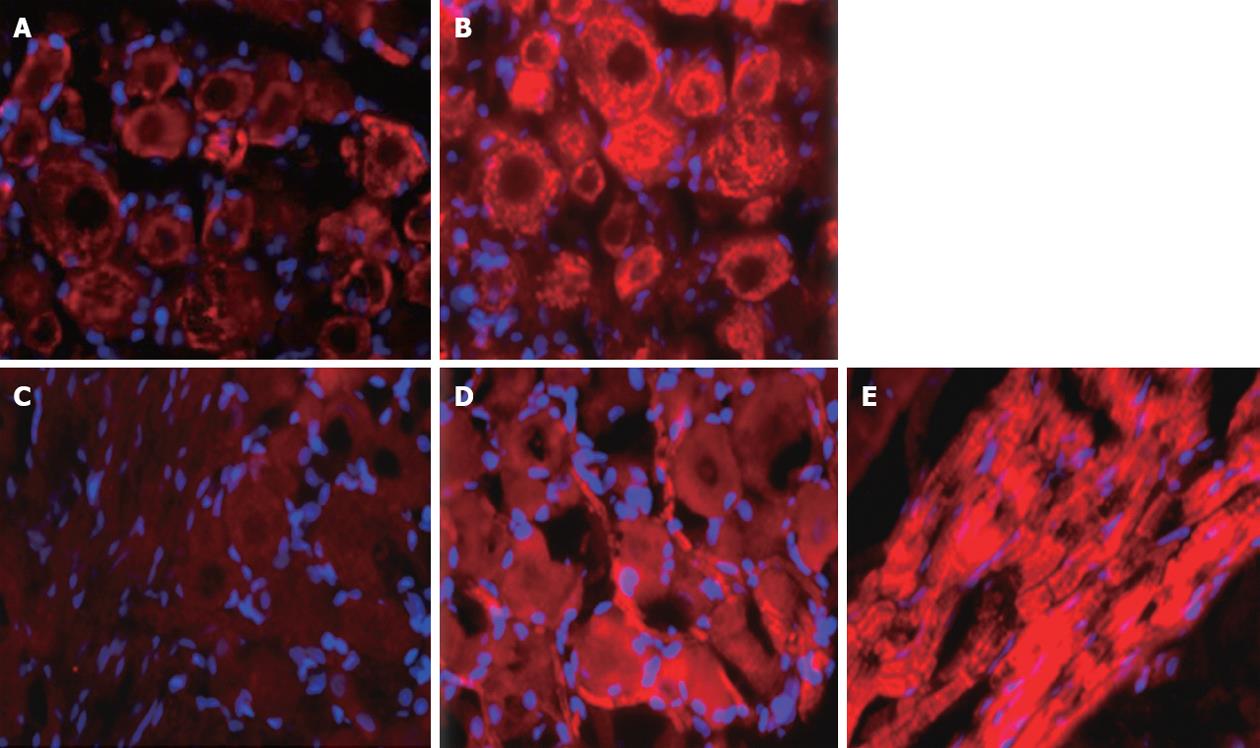
Based on immunocytochemical localization, ET-A and ET-B receptors were increased in DRG (T10-12) from pancreatitis compared to naïve rats (Figure 7). ET-A receptor expression was observed in all sizes of primary sensory neurons of the DRG. In contrast, ET-B receptors were primarily localized on the Schwann cells (myelin sheaths) surrounding the axons that were passing through the DRG from naïve or pancreatitic rats.
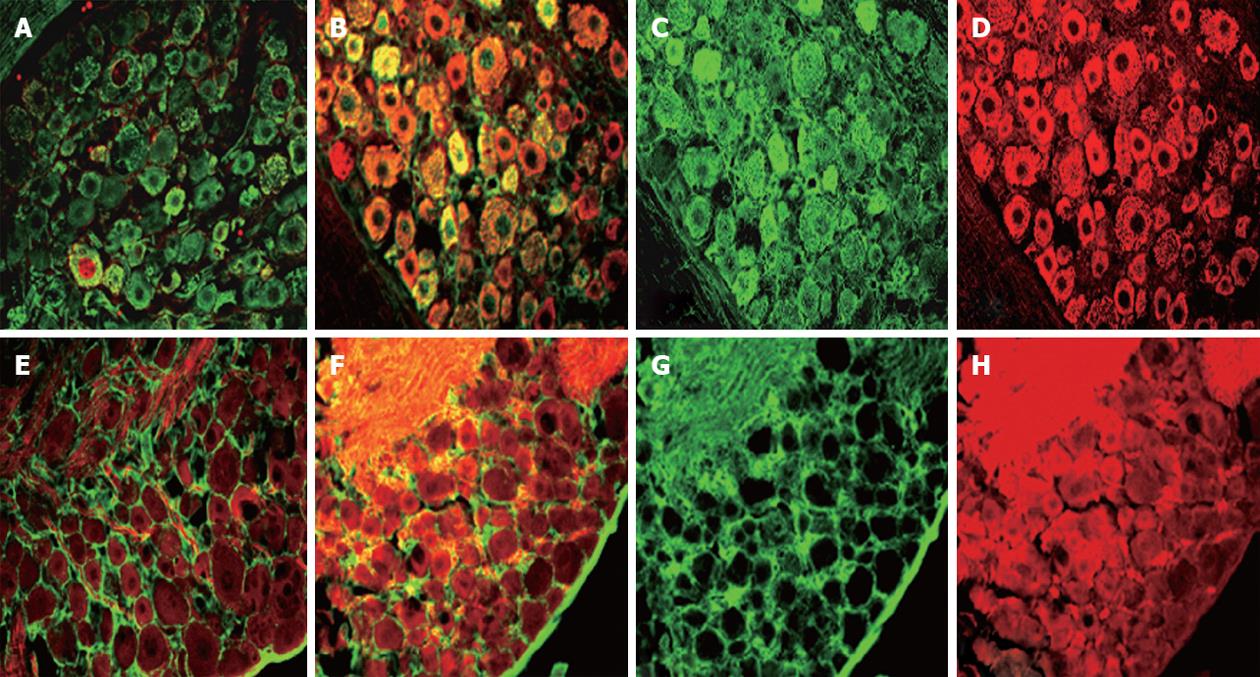
Endothelin receptor localization was combined in dual NeuN and GFAP (Figure 8). ET-A was expressed in the cell bodies and occasional nuclei of neurons of different sizes in the DRG in naïve animals. However, phenotypic expression of ET-A receptor was greatly increased in neurons of all sizes in rats with DBTC induced pancreatitis. Similarly, ET-B receptor was also localized in neurons of all sizes, in the satellite glia, as well as in the Schwann cell glial myelin sheaths surrounding the axons passing through the DRG and satellite glia.
In this investigation, DBTC-treated animals developed persistent pancreatic inflammation detected in pathological analysis and secondary pain-related hypersensitivity. The DRG and spinal cord from 10th-12th thoracic segments were selected for gene microarray analysis, as they portray the major source of sensory neuronal fibers in the pancreas. Amongst the 52 candidate genes upregulated in three animals in each experimental group (run in duplicate) was ET-1. ET-1 was upregulated greater than 2-fold in the animals with pancreatic inflammation and visceral pain-related behavior. This finding illustrates the benefits of gene array analysis in identifying relevant genes with possible direct roles in mediating pain in visceral inflammatory states. Visceral mechanical testing with von Frey microfilaments demonstrated functional significance showing increases in abdominal withdrawals on day 6-7 post DBTC insult in pancreatitis animals. In contrast, no changes were detected in normal rats receiving sham vehicle alone. Similarly, spontaneous pain related behaviors were unchanged in naïve rats treated with endothelin antagonists indicating that the agent had no effect on normal exploratory behaviors. Single highest dose treatment with ET-A antagonist (BQ123) provided significant protection against inflammatory pain related behaviors in animals with pancreatitis, while the ET-B antagonist (BQ788) had a short lasting effect.
In the current study, ET-A and ET-B receptors were both detected in DRG. ET-A receptors trigger vascular constriction and ET-B receptors act as vasodilators, but their role in neurons is not defined.
Although, ET-A and ET-B receptors are both present in the thoracic spinal segment, and DRG, ET-B receptors are primarily expressed in DRG satellite cells and ensheathing Schwann cells[27] where they can stimulate the synthesis and release of prostaglandin E2, an active compound in inflammatory pain[16]. In addition, ET-B receptors present on keratinocytes mediate the release of β-endorphin from these cells with a local analgesic effect[18]. ET-1 has a mitogenic effect, promoting cancer cell growth in colon and pancreas in which there is also upregulation of ET-A receptors and moderate downregulation of ET-B receptors[28].
In our hands, ET-A receptors appeared to be more localized around constricted vasculature in pancreatic tissues of animals with DBTC-induced pancreatitis. While, modest detectable differences were evident in the ET-B expression of the pancreatic tissues in comparisons of animals with pancreatitis to naïve controls.
Animals with pancreatitis demonstrated trend toward a reduction in active time and an increase in resting time. Immunohistological analysis revealed increased ET-A in DRG neurons of all sizes and ET-B in myelin sheath and satellite glia. The changes in spontaneous behaviors induced by pancreatitis were normalized by application of the ET-A antagonist (BQ123), suggesting that this receptor has a role in pancreatitis-induced behavioral changes.
Injection of ET-1 is reported to cause severe pain via activation of ET-A receptors, in the sciatic nerve chronic constriction injury model[29]. In the present study, both the endothelin-A (BQ-123) and B (BQ-788) receptor antagonists significantly reversed the thermal hypersensitivity assessed using the hot plate method at 20 min, 40 min after injection. However, the ET-B antagonist was ineffective at the 75 min time point while the efficacy for the endothelin-A receptor antagonist persisted. Neither agents affected vehicle injected animals. Additionally, we demonstrated that the thermal and mechanical hyperalgesia induced in pancreatitis animals were equally well normalized by the administration of morphine (10 mg/kg) with a dose dependent response, whereas a lower dose of BQ-123 and BQ-788 were required to reverse the nocifensive responses in the pancreatitic rats. Similarly, administration of the ET-A receptor antagonist reversed the attenuation of spontaneous exploratory behaviors observed in pancreatitic animals.
These data reveal that nocifensive responses invoked by the persistent pancreatitis in animals can be ameliorated by systemic post-treatment with endothelin-A receptor and to a lesser degree endothelin-B antagonist similar to conventional morphine administration. As has been suggested for neuropathic pain[29], ET-A receptor antagonists deserve future study as potential novel therapy including against inflammatory pain.
Previous studies indicate that treatment with non-selective ET-A and ET-B (LU 302872) and selective ET-A (LU 302146) antagonists had no effect on the pancreatic pathological (edema and inflammatory infiltration) nor on trypsinogen activation 4h after caerulein-induced acute pancreatitis model. A slight increase in the pathological necrosis and vacuolization suggested the possible undesired effects of these compounds in the model[30]. In contrast ET-1 at the high dose was found to be beneficial on morphological changes and trypsinogen activation in that model[31]. In our hands we did not detect any undesirable pathological effects or any histological improvement in treated animals after single dose i.p. administration of endothelin-1 receptor antagonists (BQ123, BQ788).
In severe acute pancreatitis, microcirculatory disorders and increased capillary permeability contribute to multiple organ dysfunction syndrome, while, ET-A receptors stabilize capillary leakage and improved organ function[32]. Of interest, upregulated genes reported here substantiate this finding. For example, multifunction pro-inflammatory cytokine IL-6 levels were significantly upregulated in DRG of rats with pancreatitis on day 6 after DBTC injection[20]. Relative quantification of target cDNA levels in primary stellate cultures using real-time polymerase chain reaction revealed a dose-dependent reduction of endothelin-1 after treatment with inhibitors of histone deacetylases[33]. This is relevant to anti-cancer activities and, therefore, of growing clinical interest[34-36]. It is well established that diabetes can occur in acute pancreatitis as well as chronic pancreatitis. Insufficient pancreatic enzyme activity and dosing is treated in pancreatic steatorrhea with administration of lipase with meals in patients[37]. In this model we detected modulation of genes for the inflammatory markers of pancreatitis, including upregulated lipase, α enolase and α tryptase, while insulin precursor, glucose transporter type 5, and glycogen phosphorylase were down regulated. In accordance with these findings the increased levels of the serum amylase and lipase were reported on day 3 and peaked on day 7 in this DBTC induced pancreatitis model, consistent with pancreatitis in patients[22]. Finally, amongst those genes found to be downregulated were peripheral myelin protein 22, α platelet-derived growth factor receptor, dopamine receptor, and neuropeptide Y receptor. Further studies are warranted to investigate the role of these candidate genes as novel targeted therapeutic modalities in patients. ET-A receptor antagonists deserve future study as a potential novel therapy against inflammatory and neuropathic pain.
In conclusion, we report 8 different groups of genes modified in this model of pancreatitis. These candidate genes may be useful as future biomarkers for diagnostic and/or targeted gene therapy. As an example, endothelin-1 gene was upregulated and subsequently, ET-A and ET-B receptor antagonists were found to reverse inflammatory pain responses. These results demonstrate the potential utility of the gene microarray analysis to identify candidate genes for analgesic development.
Abdominal pain ranging from mild to severe pain is the chief symptom of patients with pancreatic disorders. Neural innervation of the pancreas is important in the initiation and maintenance of inflammation. Activation of pancreatic sensory neurons causes release of neurotransmitters in the spinal cord and neurogenic activation signals in the pancreas itself producing plasma extravasation and neutrophil infiltration. Endothelins (ET) cascade is implicated as a major contributing factor in pancreatic pain in both pancreatitis and pancreatic cancer.
Multifunctional ETs comprise a family of peptides which interact with their receptors that are involved in regulation of blood flow, cell proliferation, muscle contraction or relaxation, secretion and ion transport. The ETs are expressed by endothelial cells, macrophages, astrocytes and neurons. ETs activate the peripheral sensory nervous system and may directly be involved in signaling nociceptive events in peripheral tissues. ETs are increased in inflammatory conditions. ETs produce their biological effects via activation of the ET-A receptor and the ET-B receptor.
The ET-1 was amongst the 52 candidate genes upregulated (average of 3 animals/group and study repeated X2) greater than 2-fold in an experimental pancreatitis model. ET receptor antagonists were safe and effective in ameliorating mechanical and thermal hypersensitivity in doses lower than the gold standard morphine with exception of no detectable side effects.
The candidate genes reported in this investigation may be useful as future diagnostic tool and targeted gene therapies. ET-A and to a lesser extent ET-B receptor antagonists can reverse pancreatic inflammatory pain responses. The results demonstrate the possible utility of the gene array analysis to identify candidate genes for analgesic development.
The pain systems are comprised of peripheral neurons and their receptors, the nociceptors, central neuronal transmitting pathways and neurons with excitatory or inhibitory effects on nociceptive information. Nociceptors are specialized receptors on nerve endings that respond to noxious to mechanical and thermal stimuli interpreted as pain. Dorsal root ganglia are the cell bodies of peripheral nerves and are located adjacent to the spinal cord in the vertebral column. ET interacts with its receptors and regulates blood flow, cell proliferation, muscle contraction or relaxation, secretion and ion transport.
This paper reports a potentially interesting approach to an important problem, pain in pancreatitis. The paper is an original report focusing on the gene profiling to provide rationale for potential casual mechanisms and pharmacological block of functional pain related behaviors with specific antagonists. The agent dose response data present important information for potential future human translational studies.
Peer reviewers: Julian Swierczynski, MD, PhD, Professor, Department of Biochemistry, Medical University of Gdansk, 80-211 Gdansk, Poland; Espen Melum, MD, Medical Department, Rikshospitalet University Hospital, Sognsvannsvn. 20, 0027 Oslo, Norway
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13 Suppl 5:S220-S222. [PubMed] |
| 2. | Piovezan AP, D'Orléans-Juste P, Frighetto M, Souza GE, Henriques MG, Rae GA. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol. 2004;141:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999-1006. [PubMed] |
| 4. | De-Melo JD, Tonussi CR, D'Orléans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77:261-269. [PubMed] |
| 5. | Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279-2283. [PubMed] |
| 6. | Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 1997;156:280-285. [PubMed] |
| 8. | Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Klonowski-Stumpe H, Reinehr R, Fischer R, Warskulat U, Lüthen R, Häussinger D. Production and effects of endothelin-1 in rat pancreatic stellate cells. Pancreas. 2003;27:67-74. [PubMed] |
| 10. | Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11:6144-6151. [PubMed] |
| 11. | DiMagno MJ, Dimagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2006;22:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Rubanyi GM. The discovery of endothelin: the power of bioassay and the role of serendipity in the discovery of endothelium-derived vasocative substances. Pharmacol Res. 2011;63:448-454. [PubMed] |
| 13. | Eibl G, Forgacs B, Hotz HG, Buhr HJ, Foitzik T. Endothelin A but not endothelin B receptor blockade reduces capillary permeability in severe experimental pancreatitis. Pancreas. 2002;25:e15-e20. [PubMed] |
| 14. | Foitzik T, Faulhaber J, Hotz HG, Kirchengast M, Buhr HJ. Endothelin mediates local and systemic disease sequelae in severe experimental pancreatitis. Pancreas. 2001;22:248-254. [PubMed] |
| 15. | Eaker E, Sallustio J, Kohler J, Visner G. Endothelin-1 expression in myenteric neurons cultured from rat small intestine. Regul Pept. 1995;55:167-177. [PubMed] |
| 16. | Koyama Y, Mizobata T, Yamamoto N, Hashimoto H, Matsuda T, Baba A. Endothelins stimulate expression of cyclooxygenase 2 in rat cultured astrocytes. J Neurochem. 1999;73:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Liu X, Nakano I, Ito T, Kimura T, Nawata H. Is endothelin-1 an aggravating factor in the development of acute pancreatitis? Chin Med J (Engl). 1999;112:603-607. [PubMed] |
| 18. | Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Oz HS, Zhong J, de Villiers WJ. Pegylated arginine deiminase downregulates colitis in murine models. Mediators Inflamm. 2012;2012:813892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Nolan RP, Spanos NP. Hypnotic analgesia and stress inoculation: a critical reexamination of Miller and Bowers. Psychol Rep. 1987;61:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Vera-Portocarrero LP, Lu Y, Westlund KN. Nociception in persistent pancreatitis in rats: effects of morphine and neuropeptide alterations. Anesthesiology. 2003;98:474-484. [PubMed] |
| 22. | Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155-2164. [PubMed] |
| 23. | Chiang LW, Grenier JM, Ettwiller L, Jenkins LP, Ficenec D, Martin J, Jin F, DiStefano PS, Wood A. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc Natl Acad Sci USA. 2001;98:2814-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Westlund KN, Zhang L, Ma F, Oz HS. Chronic inflammation and pain in a tumor necrosis factor receptor (TNFR) (p55/p75-/-) dual deficient murine model. Transl Res. 2012;160:84-94. [PubMed] |
| 25. | Marsault R, Feolde E, Frelin C. Receptor externalization determines sustained contractile responses to endothelin-1 in the rat aorta. Am J Physiol. 1993;264:C687-C693. [PubMed] |
| 26. | Webber KM, Pennefather JN, Head GA, van den Buuse M. Endothelin induces dopamine release from rat striatum via endothelin-B receptors. Neuroscience. 1998;86:1173-1180. [PubMed] |
| 27. | Raffa RB, Schupsky JJ, Lee DK, Jacoby HI. Characterization of endothelin-induced nociception in mice: evidence for a mechanistically distinct analgesic model. J Pharmacol Exp Ther. 1996;278:1-7. [PubMed] |
| 28. | Hans G, Deseure K, Adriaensen H. Endothelin-1-induced pain and hyperalgesia: a review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides. 2008;42:119-132. [PubMed] |
| 29. | Długosz JW, Andrzejewska A, Nowak K, Wróblewski E, Dabrowski A. The cumulative effect of nuclear factor-kappaB (NF-kappaB) inhibition and endothelins in early cerulein-induced acute pancreatitis in rats. Rocz Akad Med Bialymst. 2005;50:230-236. [PubMed] |
| 30. | Andrzejewska A, Dlugosz JW. Differential effects of endothelins on histological and ultrastructural changes and trypsinogen activation in the secretagogue-induced acute pancreatitis in rats. Exp Toxicol Pathol. 2011;63:371-378. [PubMed] |
| 31. | Klass M, Hord A, Wilcox M, Denson D, Csete M. A role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerve. Anesth Analg. 2005;101:1757-1762. [PubMed] |
| 32. | Werner MF, Trevisani M, Campi B, André E, Geppetti P, Rae GA. Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain. 2010;14:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Bülow R, Fitzner B, Sparmann G, Emmrich J, Liebe S, Jaster R. Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem Pharmacol. 2007;74:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Bai J, Demirjian A, Sui J, Marasco W, Callery MP. Histone deacetylase inhibitor trichostatin A and proteasome inhibitor PS-341 synergistically induce apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun. 2006;348:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Huang L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol. 2006;209:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Chen JM, Férec C. Genetics and pathogenesis of chronic pancreatitis: The 2012 update. Clin Res Hepatol Gastroenterol. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Dimagno MJ, Dimagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2012;28:523-531. [PubMed] |