Published online Aug 14, 2012. doi: 10.3748/wjg.v18.i30.4037
Revised: April 11, 2012
Accepted: April 18, 2012
Published online: August 14, 2012
AIM: To investigate M2 isoform of pyruvate kinase (PKM2) expression in gastric cancers and evaluate its potential as a prognostic biomarker and an anticancer target.
METHODS: All tissue samples were derived from gastric cancer patients underwent curative gastrectomy as a primary treatment. Clinical and pathological information were obtained from the medical records. Gene expression microarray data from 60 cancer and 19 non-cancer gastric tissues were analyzed to evaluate the expression level of PKM2 mRNA. Tissue microarrays were constructed from 368 gastric cancer patients. Immunohistochemistry was used to measure PKM2 expression and PKM2 positivity of cancer was determined by proportion of PKM2-positive tumor cells and staining intensity. Association between PKM2 expression and the clinicopathological factors was evaluated and the correlation between PKM2 and cancer prognosis was evaluated.
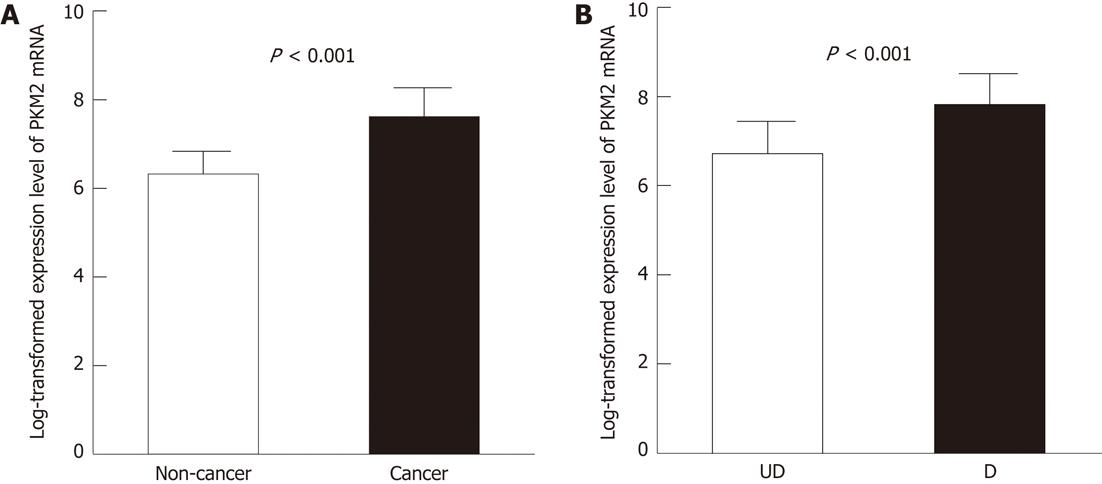
RESULTS: PKM2 mRNA levels were increased more than 2-fold in primary gastric cancers compared to adjacent normal tissues from the same patients (log transformed expression level: 7.6 ± 0.65 vs 6.3 ± 0.51, P < 0.001). Moreover, differentiated type cancers had significantly higher PKM2 mRNA compared to undifferentiated type cancers (log transformed expression level: 7.8 ± 0.70 vs 6.7 ± 0.71, P < 0.001). PKM2 protein was mainly localized in the cytoplasm of primary cancer cells and detected in 144 of 368 (39.1%) human gastric cancer cases. PKM2 expression was not related with stage (P = 0.811), but strongly correlated with gastric cancer differentiation (P < 0.001). Differentiated type cancers expressed more PKM2 protein than did the undifferentiated ones. Well differentiated adenocarcinoma showed 63.6% PKM2-positive cells; in contrast, signet-ring cell cancers showed only 17.7% PKM2-positive cells. Importantly, PKM2 expression was correlated with shorter overall survival (P < 0.05) independent of stage only in signet-ring cell cancers.
CONCLUSION: PKM2 expression might be an adverse prognostic factor for signet-ring cell carcinomas. Its function and potential as a prognostic marker should be further verified in gastric cancer.
- Citation: Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, Cho JY. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol 2012; 18(30): 4037-4043
- URL: https://www.wjgnet.com/1007-9327/full/v18/i30/4037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i30.4037
Gastric cancer is the second leading cause of cancer-related deaths worldwide[1]. Although surgery is the standard curative treatment for gastric cancer, relapses occur in many patients even after adjuvant therapy. Gastric cancer patients with the same stage of the disease present different clinical courses and have different prognosis[2]. This heterogeneity of gastric cancer is present at the molecular level and has a genetic predisposition to it[3-6]. Therefore, it is important to identify new molecular markers to predict patients’ outcomes and personalize treatments according to the individual biology of each cancer.
Cancer cells take up glucose at higher rates than do normal cells but produce energy mainly by glycolysis, rather than by mitochondrial oxidation of pyruvate[7]. This process, called aerobic glycolysis or the Warburg effect, is very important for tumor growth[8]. Glycolysis increases lactate production resulting in acidification of the extracellular environment, which is believed to facilitate cell invasion and metastasis[9]. The M2 isoform of pyruvate kinase (PKM2) was identified as a driver of aerobic glycolysis, and has been shown to be the isoform preferentially overexpressed in tumor cells[10]. Other isoenzymes of pyruvate kinase (pyruvate kinase type M1, pyruvate kinase type L, pyruvate kinase type R) are expressed depending upon the metabolic responsibilities of the various non-cancerous cells and tissues[10].
Several studies have shown that PKM2 is selectively stained in cancer cells in immunohistochemical assay[11,12]. It has been suggested that plasma PKM2 could be a valuable tumor marker for diagnosis or monitoring of lung, pancreas, kidney, breast, tongue, and gastrointestinal cancers[11-17]. However, little is known about the biological function of PKM2 in cancer and its potential as an anti-cancer target. Previous studies reported that PKM2 protein level was increased in both the tumors and plasma of gastric cancer patients[17], and that it positively correlated with cisplatin sensitivity in gastric cancer cell lines[18]. However, clinical and prognostic implications of PKM2 as a marker for gastric cancer are still unclear. Therefore, we decided to analyze whether PKM2 expression is correlated with cancer progression and prognosis in human gastric cancer patients.
The previously generated gene expression data from 60 gastric cancer patients is available in the NCBI’s GEO public database (microarray data accession number, GSE13861)[2]. All patients underwent curative gastrectomy as a primary treatment in 2005 at Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea. Clinical and pathologic data were obtained by review of the Severance Hospital medical records. The gene expression data of 60 cancer and 19 non-cancer gastric tissues from 60 gastric cancer patients were analyzed. Class comparison using two sample t test (significance P < 0.001, 10 000 random permutation) identified gastric cancer specific genes.
We selected primary gastric adenocarcinoma patients who had undergone curative gastrectomy as the primary treatment between 1999 and 2007 at Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea. Patients were followed up more than 36 mo after surgery or presented recurrence or death within 36 mo after surgery. We obtained paraffin-embedded tissues and clinical data from patients. The demographic details of the cases analyzed are described in Table 1. Clinical and pathological information were obtained from the medical records. Tumors were staged according to the 7th edition of the American Joint Committee Guidelines on cancer staging issued in 2010. Tumor histology was classified as differentiated (well and moderately differentiated adenocarcinoma) and undifferentiated (poorly differentiated adenocarcinoma and signet ring cell carcinoma) type. The median follow-up duration was 70.6 mo (range: 3.6-144.6 mo). A total of 125 (34%) patients did not receive any adjuvant chemotherapy, and most of their cancers were classified as stage I. No radiation was given to any of the patients. The study was approved by the Investigational Review Board of Gangnam Severance Hospital.
| Characteristics | M2 isoform of pyruvate kinase expression | |||
| Total (n = 368) | Negative (n = 224) | Positive (n = 144) | P | |
| Median follow-up (70.6 mo) | ||||
| Relapse | 143 (39.0) | |||
| Death | 138 (37.7) | |||
| Adjuvantchemotherapy | 243 (66.0) | |||
| Age (yr) | 0.027 | |||
| ≤ 60 | 230 | 150 (65.2) | 80 (34.8) | |
| > 60 | 138 | 74 (53.6) | 64 (46.4) | |
| Gender | 0.263 | |||
| Male | 222 | 130 (58.6) | 92 (41.4) | |
| Female | 146 | 94 (64.4) | 52 (35.6) | |
| AJCC 7th stage | 0.811 | |||
| I | 105 | 67 (63.8) | 38 (36.2) | |
| II | 89 | 51 (57.3) | 38 (42.7) | |
| III | 172 | 105 (61.0) | 67 (39.0) | |
| IV | 2 | 1 (50.0) | 1 (50.0) | |
| T stage | 0.009 | |||
| T1 | 94 | 62 (65.9) | 32 (34.1) | |
| T2 | 42 | 30 (71.4) | 12 (28.6) | |
| T3 | 87 | 40 (45.9) | 47 (54.1) | |
| T4 | 145 | 92 (63.4) | 53 (36.6) | |
| N stage | 0.086 | |||
| N0 | 131 | 80 (61.1) | 51 (38.9) | |
| N1 | 62 | 33 (53.2) | 29 (46.8) | |
| N2 | 69 | 37 (53.6) | 32 (46.4) | |
| N3 | 106 | 74 (69.8) | 32 (30.2) | |
| Histology | < 0.001 | |||
| Welldifferentiatedadenocarcinoma | 22 | 8 (36.4) | 14 (63.6) | |
| Moderatelydifferentiatedadenocarcinoma | 96 | 39 (40.6) | 57 (59.4) | |
| Poorlydifferentiatedadenocarcinoma | 143 | 91 (63.6) | 52 (36.4) | |
| Signetringcellcarcinoma | 79 | 65 (82.3) | 14 (17.7) | |
The paraffin-embedded tissue microarray blocks of gastric cancer tissue specimens obtained from 368 patients were used. Each block had a 3-mm core of gastric cancer tissue. Immunohistochemistry was performed on 4 μm-thick tissue microarray tissue sections an Enzyme-conjugated polymer backbone: Dextran (EnVision Detection kit, DAKO Cytomation, Glostrup, Denmark) according to the manufacturer’s instructions after microwave-based antigen retrieval. Antibody to PMK2 1:500, Cell Signaling, Cambridge, MA, United States) was applied to the sections, which were incubated for 2 h at room temperature. The sections were incubated with secondary antibody (HRP-Rabbit/Mouse) for 15 min at room temperature, and developed using a NovaRED substrate kit (VECTOR Laboratory, Burlingame, CA, United States) and counterstained with Harris hematoxylin. The slides were photographed using a Zeiss microscope. The degree of immunostaining was scored independently by 2 observers based on the proportion of positively stained tumor cells and the intensity of staining. Tumor cell proportion was classified as follows: 0%, 10%-25%, 25%-50%, and > 50% PKM2-positive tumor cells. Staining intensity was classified as none, weak and strong staining.
We measured PKM2 expression in non-cancer gastric epithelial cells and malignant lesions. Tumors with more than 25% PKM2-positive cells were considered tumors with positive PKM2 expression, and those with less than 25% PKM2-positive cells were considered negative for PKM2 expression.
The correlation between the immunohistochemical expression scores and patient survival after surgery was estimated using the Kaplan-Meier method, followed by univariate comparison between the groups using the log-rank test. To adjust for potential confounding variables and to single out independent predictors of survival, a multivariate analysis of survival was done using the Cox’s proportional hazard model using a forward stepwise mode. Statistical analyses were done with GraphPad Prism 5 (GraphPad Software, San Diego, CA) and PASW Statistics 18.0 (SPSS Inc., Chicago, IL). Association between PKM2 expression and the clinicopathological factors was tested using the χ2 test. Two-tailed P values of 0.05 or less were considered statistically significant.
From sixty gastric cancer patients, 60 gastric cancer tissues and 19 non-cancer adjacent gastric tissues were used for gene expression microarray analysis. PKM2 was identified as one of 3360 gastric cancer-specific genes by class comparison using the 2-sample t test (Data not shown). PKM2 mRNA levels were increased > 2-fold in human primary gastric cancers compared to normal adjacent tissues from the same patients (log transformed expression level: 7.6 ± 0.65 vs 6.3 ± 0.51, P < 0.001, Figure 1A). Among cancer types, differentiated type cancers displayed > 2-fold increase in PKM2 levels compared to undifferentiated type cancers (log transformed expression level: 7.8 ± 0.70 vs 6.7 ± 0.71, P < 0.001, Figure 1B).
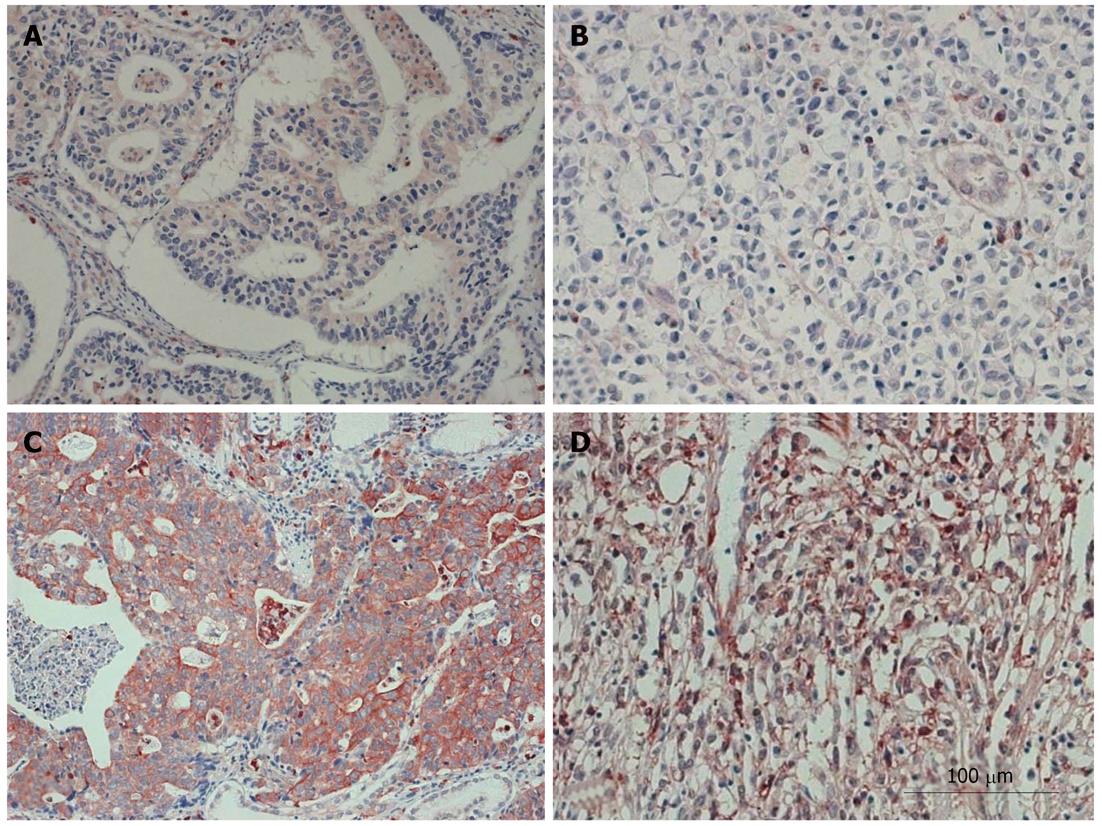
To examine whether PKM2 protein upregulation was linked to the clinical characteristics of gastric cancers, the following samples were subjected to immunohistochemistry with a human PKM2 antibody: 368 paraffin-embedded, archived gastric cancer tissue samples, including 194 cases of stages I/II and 174 cases of stage III/IV tumors. The results are summarized in Table 1. PKM2 protein was detected in 144 of 368 (39.1%) human gastric cancer cases. Strong cytoplasmic staining of PKM2 was detected in 42 (11.4%) tumors and weak staining was detected in 102 (27.7%) tumors. As shown in Figure 2, PKM2 was mainly localized in the cytoplasm of primary cancer cells. Diffuse and/or intense cytoplasmic statining was noted in only cancer cells. In contrast, PKM2 was either undetectable or only marginally detectable in the normal epithelial body gland of noncancerous tissues in the adjacent section regions (Figure 2).
As shown in Table 1, there was no correlation between stage and PKM2 expression (P = 0.811). PKM2 expression was strongly correlated with gastric cancer differentiation (P < 0.001). Differentiated type cancers expressed more PKM2 protein than did the undifferentiated ones. Well differentiated adenocarcinoma showed 63.6% PKM2-positive cells; in contrast, signet-ring cell cancers showed only 17.7% PKM2-positive cells.
We evaluated whether PKM2 expression could be a prognostic factor for gastric cancer. Two out of 368 patients died of non-cancer and were excluded from analysis. Table 2 shows that recurrence-free survival (RFS) and overall survival (OS) are significantly different between patients with different clinical stage (P < 0.001), T classification (P < 0.001), and N classification (P < 0.001). There was no significant difference in prognosis according to PKM2 expression.
| Characteristics | RFS | OS | ||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (yr) | ||||
| ≤ 60 | ||||
| > 60 | 1.12 (0.80-1.57) | 0.488 | 1.16 (0.83-1.64) | 0.373 |
| Gender | ||||
| M | ||||
| F | 1.18 (0.85-1.65) | 0.315 | 1.09 (0.78-1.53) | 0.600 |
| PKM2 | ||||
| Negative | ||||
| Positive | 0.93 (0.66-1.32) | 0.713 | 0.92 (0.65-1.30) | 0.637 |
| T stage | ||||
| T1/2/3 | ||||
| T4 | 6.12 (4.25-8.81) | < 0.001 | 5.04 (3.51-7.22) | < 0.001 |
| N stage | ||||
| N0/1/2 | ||||
| N3 | 6.02 (4.29-8.46) | < 0.001 | 5.64 (4.01-7.95) | < 0.001 |
| Stage | ||||
| I/II | ||||
| III | 8.42 (5.48-12.94) | < 0.001 | 6.70 (4.41-10.16) | < 0.001 |
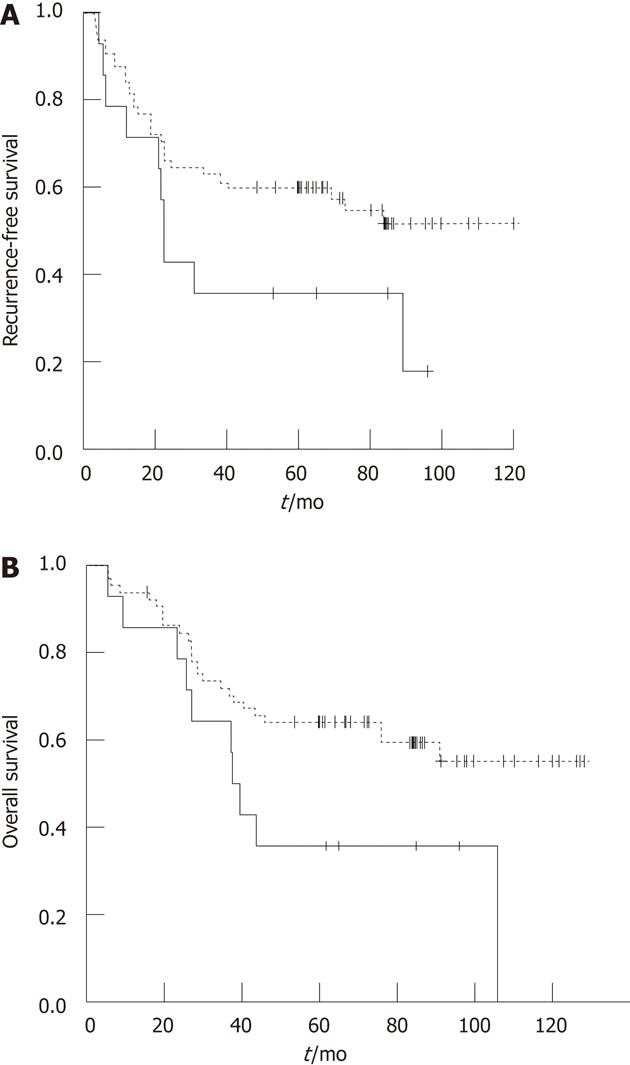
We performed subgroup analysis at each tumor stage. In stages II or III patients, PKM2 expression showed no significant correlation with RFS or OS. However, in stage I early gastric cancer patients (n = 99), PKM2 expression was significantly correlated with poor RFS (P = 0.006) and OS (P = 0.015). Based on the observation that PKM2 expression rate was remarkably different according to cancer histology (Table 1), the prognostic value of PKM2 expression in patient subgroups was evaluated according to the histology. We found that in signet-ring cell carcinomas PKM2 expression correlated with poor prognosis (P = 0.042 for OS, Table 3 and Figure 3). Moreover, univariate and multivariate analyses showed that PKM2 expression, as well as clinical stage, were independent prognostic factors for survival (Table 3).
| Characteristics | Groups | HR (95% CI) | P value |
| Univariate analysis | |||
| Age (yr) | > 60 vs ≤ 60 | 1.11 (0.52-2.37) | 0.785 |
| Gender | F vs M | 1.08 (0.56-2.07) | 0.817 |
| PKM2 | Positive vs negative | 2.13 (1.02-4.44) | 0.042 |
| T stage | T4 vs T1/2/3 | 6.25 (3.03-12.85) | < 0.001 |
| N stage | N3 vs N0/1/2 | 5.70 (2.90-11.22) | < 0.001 |
| Stage | III vsI/II | 6.84 (2.83-16.53) | < 0.001 |
| Multivariate analysis | |||
| PKM2 | Positive vs negative | 2.12 (1.02-4.42) | 0.044 |
| Stage | III vsI/II | 6.84 (2.83-16.55) | < 0.001 |
In this study, we report the characterization of PKM2 expression in human gastric cancers, and present its correlation with clinicopathological parameters and patients’ prognosis. First, our study revealed that PKM2 is overexpressed in gastric cancers both at the mRNA and protein levels compared to normal gastric tissues. Well and moderately differentiated adenocarcinoma showed significantly higher expression of PKM2 (60% PKM2-positive cells); in contrast, signet-ring cell cancers showed only 17.7% PKM2-positive cells (Table 1). Because PKM2 is mainly localized in the cytoplasm of primary cancer cells and signet-ring cells contain a large amount of mucin and scanty cytoplasm, we hypothesize that this might explain the lower levels of PKM2 expression in these cells. This finding might be explained by the different glucose utilization rates of the various gastric cancer subtypes. Fluorine-18 fluoro deoxy-D-glucose positron emission tomography detected glucose uptake of tumor cells, and differentiated gastric cancers showed higher fluoro deoxy-D-glucose uptake rates than did undifferentiated ones[19].
Second, PKM2 protein expression was found to negatively correlate with survival in signet-ring cell gastric cancer patients, as higher expression of PKM2 is associated with patients’ shorter survival time (P = 0.042) after curative resection (Figure 3). Signet-ring cell carcinomas have a distinct biology and generally have worse prognosis than do other types of gastric cancer[20]. A recent study reported that higher glucose uptake was indicative of a more aggressive disease especially in advanced signet-ring cell cancers[21], although no biological mechanism was proposed to explain it. This finding is in agreement with our results. Thus, our study suggests that higher PKM2 expression, which indicates a higher rate of glycolysis in the tumor, might represent a novel prognostic marker for the clinical outcome of these types of gastric cancers.
PKM2 expression was related with poor prognosis only in stage I gastric cancer patients who did not receive chemotherapy. Only 4 of 99 patients showed relapse after curative gastrectomy, and in all cases, cancer cells were positive for PKM2 expression compared to the 36% patients overall who expressed PKM2. Furthermore, 3 patients had early relapses, within 1 year from the surgery, and all expressed high levels of PKM2 in the resected tissues. As cancer relapse in stage I patients are rare and four recurrent cases in our result are small number, it seems too early to conclude that PKM2 expression correlated with poor prognosis of stage I gastric cancer. However, it is clinical value to expand investigation in large cases. For stages II and III patients, there were no significant differences in survival. In a previous study, PKM2 was shown to positively correlate with the response to cisplatin in human gastric cancer cell lines[18]. Cisplatin is the main chemotherapeutic agent for gastric cancers as either adjuvant or palliative aim. As cisplatin was administered as adjuvant therapy to 62.8% (147/234) of stages II or III gastric cancer patients after curative gastrectomy, the negative prognostic effect of PKM2 might be cancelled by cisplatin-based chemotherapy.
The possibility of using PKM2 as a target for the development of anti-cancer therapies has been evaluated in the preclinical setting[22,23]. PKM2 knockdown by short hairpin RNA reduced the ability of human cancer cell lines to form tumors in nude mouse xenografts[10,24]. If anti-cancer strategies based on targeting PKM2 treatment are feasible, stage I or signet-ring cell cancer patients with PKM2 expression would be suitable candidates for such treatments.
The molecular function of PKM2 and its role in cancer are not completely understood yet. It was recently shown that PKM2 allows cancer cells to mount an antioxidant response and thereby support cell survival under acute oxidative stress[25] and also induces epidermal growth factor receptor (EGFR)-dependent β-catenin transactivation, which leads to cell proliferation and tumorigenesis[26]. These data are in agreement with our microarray study in which we also identified EGFR and β-catenin signaling, and hypoxic stress are linked to gastric cancer. Altogether, these studies suggest that the function of PKM2 in gastric cancer is very complex and needs to be further elucidated. In addition, the mechanisms of the regulation of PKM2 expression specifically in gastric tumors should be studied.
In conclusion, this study showed that PKM2 was overexpressed in gastric cancers. Moreover, PKM2 expression is an independent prognostic factor for signet ring cell carcinomas. The biological role of PKM2 in the development of these tumors needs to be further elucidated.
Gastric cancer is the major cause of cancer-related deaths worldwide. It is important to identify molecular markers to predict patients’ outcomes and personalize treatments according to the individual biology. Clinical and prognostic implications of M2 isoform of pyruvate kinase (PKM2) as a marker for gastric cancer were unclear. The authors evaluated whether PKM2 expression is correlated with cancer progression and prognosis in human gastric cancer patients.
PKM2 was identified as a driver of aerobic glycolysis, and has been shown to be overexpressed in tumor cells. PKM2 was selectively expressed in cancer cells and suggested valuable tumor marker for diagnosis or monitoring of various cancers. The biological function of PKM2 in cancer has been elucidated and PKM2 might be a candidate for anti-cancer target.
This study revealed that PKM2 was overexpressed in gastric cancers both at the mRNA and protein levels compared to normal gastric tissues and was found to negatively correlate with survival in signet-ring cell gastric cancer patients. PKM2 expression might be an adverse prognostic factor for signet-ring cell carcinomas. Its function and potential as a prognostic marker should be further verified in gastric cancer.
It is plausible to use PKM2 as an adverse prognostic marker of signet-ring cell cancer patients. If anti-cancer strategies based on targeting PKM2 treatment are feasible, signet-ring cell cancer patients with PKM2 expression would be suitable candidates for such treatments.
Aerobic glycolysis: Many tumor cells have elevated rates of glucose uptake but reduced rates of oxidative phosphorylation. This persistence of high lactate production by tumors in the presence of oxygen was known as aerobic glycolysis. This metabolic switch may be required to support cell growth. High aerobic glycolysis by malignant tumors is utilized clinically to diagnose and monitor treatment responses of cancers and also to treat cancer using antagonist.
This study investigated PKM2 expression in 368 gastric cancers and evaluated its potential as a prognostic biomarker based on relapse and survival data of patients. The results indicate that PKM2 positive expression could be used as an adverse prognostic marker in signet-ring cell gastric cancer.
Peer reviewer: Dr. Ismail Matalka, MD, FRCPath, Professor, Department of Pathology and Laboratory Medicine, King Abdullah University Hospital and School of Medicine, Jordan University Of Science and Technology, Irbid 22110, Jordan
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 2. | Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 3. | Tay ST, Leong SH, Yu K, Aggarwal A, Tan SY, Lee CH, Wong K, Visvanathan J, Lim D, Wong WK. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003;63:3309-3316. [PubMed] |
| 4. | Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, Choi K, Lee SG, Lee K, Lee Y. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248-8255. [PubMed] |
| 5. | Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, Chan AS, Law S, Troyanskaya OG, Wong J. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003;14:3208-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476-485, 485.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9935] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 8. | Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 9. | Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3472] [Cited by in RCA: 3610] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 10. | Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2170] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 11. | Schneider J, Neu K, Grimm H, Velcovsky HG, Weisse G, Eigenbrodt E. Tumor M2-pyruvate kinase in lung cancer patients: immunohistochemical detection and disease monitoring. Anticancer Res. 2002;22:311-318. [PubMed] |
| 12. | Wechsel HW, Petri E, Bichler KH, Feil G. Marker for renal cell carcinoma (RCC): the dimeric form of pyruvate kinase type M2 (Tu M2-PK). Anticancer Res. 1999;19:2583-2590. [PubMed] |
| 13. | Cerwenka H, Aigner R, Bacher H, Werkgartner G, el-Shabrawi A, Quehenberger F, Mischinger HJ. TUM2-PK (pyruvate kinase type tumor M2), CA19-9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. Anticancer Res. 1999;19:849-851. [PubMed] |
| 14. | Lüftner D, Mesterharm J, Akrivakis C, Geppert R, Petrides PE, Wernecke KD, Possinger K. Tumor type M2 pyruvate kinase expression in advanced breast cancer. Anticancer Res. 2000;20:5077-5082. [PubMed] |
| 15. | Schulze G. The tumor marker tumor M2-PK: an application in the diagnosis of gastrointestinal cancer. Anticancer Res. 2000;20:4961-4964. [PubMed] |
| 16. | Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer. 2008;123:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Chen JY, Chen DD, Wang GB, Shen P. Tumor type M2 pyruvate kinase expression in gastric cancer, colorectal cancer and controls. World J Gastroenterol. 2004;10:1643-1646. [PubMed] |
| 18. | Yoo BC, Ku JL, Hong SH, Shin YK, Park SY, Kim HK, Park JG. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int J Cancer. 2004;108:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, Schwaiger M, Fink U. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol. 2011;104:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 23. | Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Peng XC, Gong FM, Zhao YW, Zhou LX, Xie YW, Liao HL, Lin HJ, Li ZY, Tang MH, Tong AP. Comparative proteomic approach identifies PKM2 and cofilin-1 as potential diagnostic, prognostic and therapeutic targets for pulmonary adenocarcinoma. PLoS One. 2011;6:e27309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 924] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 26. | Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 859] [Article Influence: 66.1] [Reference Citation Analysis (0)] |