Published online Aug 14, 2012. doi: 10.3748/wjg.v18.i30.3977
Revised: November 23, 2011
Accepted: June 8, 2012
Published online: August 14, 2012
AIM: To investigate the mechanisms of Lactobacillus plantarum (L. plantarum) action on gut barrier in preoperative and postoperative experimental obstructive jaundice in rats.
METHODS: Forty rats were randomly divided into groups of sham-operation, bile duct ligation (BDL), BDL + L. plantarum, BDL + internal biliary drainage (IBD), and BDL + IBD + L. plantarum. Ten days after L. plantarum administration, blood and ileal samples were collected from the rats for morphological examination, and intestinal barrier function, liver function, intestinal oxidative stress and protein kinase C (PKC) activity measurement. The distribution and expression of the PKC and tight junction (TJ) proteins, such as occludin, zonula occludens-1, claudin-1, claudin-4, junction adhesion molecule-A and F-actin, were examined by confocal laser scanning microscopy, immunohistochemistry, Western blotting, real-time fluorescent quantitative polymerase chain reaction assay.
RESULTS: L. plantarum administration substantially restored gut barrier, decreased enterocyte apoptosis, improved intestinal oxidative stress, promoted the activity and expression of protein kinase (BDL vs BDL + L. plantarum, 0.295 ± 0.007 vs 0.349 ± 0.003, P < 0.05; BDL + IBD vs BDL + IBD + L. plantarum, 0.407 ± 0.046 vs 0.465 ± 0.135, P < 0.05), and particularly enhanced the expression and phosphorylation of TJ proteins in the experimental obstructive jaundice (BDL vs BDL + L. plantarum, 0.266 ± 0.118 vs 0.326 ± 0.009, P < 0.05). The protective effect of L. plantarum was more prominent after internal biliary drainage ( BDL + IBD vs BDL + IBD + L. plantarum, 0.415 ± 0.105 vs 0.494 ± 0.145, P < 0.05).
CONCLUSION: L. plantarum can decrease intestinal epithelial cell apoptosis, reduce oxidative stress, and prevent TJ disruption in biliary obstruction by activating the PKC pathway.
-
Citation: Zhou YK, Qin HL, Zhang M, Shen TY, Chen HQ, Ma YL, Chu ZX, Zhang P, Liu ZH. Effects of
Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. World J Gastroenterol 2012; 18(30): 3977-3991 - URL: https://www.wjgnet.com/1007-9327/full/v18/i30/3977.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i30.3977
Biliary tract surgery in patients with obstructive jaundice is associated with high a morbidity and mortality rate[1]. Evidence accumulated over the past several years indicates that the absence of bile in the gastrointestinal tract promotes bacterial overgrowth and increases intestinal permeability, leading to significant translocation of bacteria and endotoxin following bile duct obstruction[2,3]. The mechanism underlying the increased intestinal permeability in obstructive jaundice has been obscure. However, recent experimental studies have shown that the regional decrease in tight junction (TJ)-associated protein levels in the intestinal epithelium[4], increased apoptosis of enterocytes in intestinal crypts[5], and intestinal oxidative stress[6] are the key factors in the pathogenesis of hepatic and intestinal injury in obstructive jaundice[7].
Probiotic bacteria have been shown to have beneficial effects in the intestinal barrier function. For example, live Bifidobacterium lactis has been shown to inhibit toxic effects in epithelial cell culture[8]. Lactobacillus plantarum (L. plantarum) has been found to inhibit epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-α[9] and prevent cytokine-induced apoptosis in intestinal epithelial cells[10]. L. plantarum stabilizes the cellular TJ, thereby preventing enteropathogenic Escherichia coli-induced redistribution of integral TJ proteins[11]. Based on the excretion of orally administered 14C, White et al[12] demonstrated that enteral administration of the probiotic bacterium L. plantarum 299 reduced intestinal hyperpermeability associated with experimental biliary obstruction. However, these authors did not clarify the mechanism for the protective effect of the probiotics on the intestinal barrier in obstructive jaundice. A recent clinical study reported that preoperative oral administration of synbiotics could enhance immune responses, attenuate systemic post-operative inflammatory responses, and improve the intestinal microbial environment after hepatobiliary surgery for obstructive jaundice[13].
TJs, which represent the uppermost basolateral connection between neighboring enterocytes, are important components of the epithelial barrier[14]. TJ assembly and paracellular permeability are regulated by a network of signaling pathways that involves different protein kinase C (PKC) isoforms[15]. A substantial body of experimental data indicates that PKC regulates paracellular permeability of the epithelial barrier[16,17]. PKC regulates the assembly of TJ proteins through phosphorylation of zonula occludens-1 (ZO-1)[15]. Seth et al[18] suggested that PKCβI activation may be one of the initial events in the probiotic-mediated protection of TJs. PKCε may play a role in the downstream events of the signaling pathway involved in this process. These data suggest that PKC plays a crucial role in the mediation of intestinal epithelial TJ proteins.
This study aims to investigate the effects of L. plantarum on the intestinal mucosal barrier, oxidative stress, epithelial TJ-protein structure and phosphorylation, especially its impact on the expression and activity of PKC.
Rabbit polyclonal anti-occludin, rabbit polyclonal anti-junction adhesion molecule (JAM)-A, rabbit polyclonal anti-claudin-1, mouse monoclonal anti-claudin-4, and rabbit polyclonal anti-phosphoserine antibodies were supplied by Zymed (Invitrogen, Carlsbad, CA, United States). Rabbit polyclonal anti-ZO-1 and rabbit polyclonal anti-PKC were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Fluorescein isothiocyanate (FITC)-phalloidin was obtained from Sigma (St. Louis, United States). FITC-conjugated secondary antibodies were supplied by Zymed (Invitrogen). Biotin-labeled goat anti-rabbit immunoglobulin G (IgG) and horseradish peroxidase (HRP)-labeled streptavidin were supplied by DAKO (Glostrup, Denmark). All other reagents of analytical grade were purchased from Sigma (St. Louis, United States).
The L. plantarum (strain CGMCC No. 1258) used in this study was a gift from Dr. Xiao-Ming Hang (Onlly Institute of Biomedicine, Shanghai Jiao Tong University, Shanghai, China). L. plantarum cultures were prepared exactly as described previously[11].
Forty male albino Wistar rats weighing 250-320 g were purchased from Fudan University Medical Animal Center (Shanghai, China). They were housed in stainless-steel cages, three rats per cage, at controlled temperature (23 °C) and humidity and with a 12 h/12 h dark/light cycle. They were maintained on a standard laboratory diet with tap water ad libitum, except for an overnight fast before surgery. The study was conducted according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and it was approved by the Ethics and Research Committee of Shanghai Sixth People’s Hospital, Shanghai, China.
Animals were randomly divided into five groups of eight rats each as described below.
Group I, sham-operation: A 2.0-cm upper midline abdominal incision was made, and the common bile duct (CBD) was freed from the surrounding tissues without ligation or transection.
Group II, bile duct ligation: The CBD was double ligated in its middle third with a 4-0 silk suture and transected between the two ligatures.
Group III, bile duct ligation + L. plantarum: After bile duct ligation (BDL), a volume of 10 mL live L. plantarum (activity, 2 × 108 CFU/mL) divided into two equal doses was administrated daily to the rats by gavage for 10 d. After 10 d, the animals were sacrificed under ketamine anesthesia.
Group IV, BDL + IBD: The CBDs of animals were ligated and isolated. A polyethylene tube PE-10 (American Health and Medical Supply International Corp. Co., Ltd., Scarsdale, New York, United States) was inserted into the proximal CBD in a cephalad direction and fixed. The drainage end was tied and positioned in the right hepatorenal recess. After 5 d of obstructive jaundice, the abdomen was reopened through the previous incision. After releasing the biliary obstruction by transecting the tube, a distal 3-cm segment of the catheter was inserted into the duodenum for internal biliary drainage. The animals were sacrificed after another 5 d.
Group V, BDL + IBD + L. plantarum: Ten days after BDL, live L. plantarum was infused as described for Group III.
All non-L. plantarum control groups, including sham-operation (SHAM), were gavaged with the same volume of the same vehicle (Dulbecco’s phosphate buffered saline) used for the L. plantarum groups. The animals were sacrificed after 10 d.
All surgical procedures were performed under strict sterile conditions and ketamine anesthesia. At the end of the experiment on day 10, 4-5 mL blood sample was collected from each animal by puncturing the portal vein.
The serum total bilirubin and alanine aminotransferase (ALT) levels were determined using a kit (Jiancheng Biological Co., Ltd., Nanjing, China) and a Hitachi Model 7600 series automatic analyzer (Hitachi Co., Tokyo, Japan).
Endotoxin concentrations were determined using a quantitative chromogenic Limulus Amebocyte Lysate test kit (Shanghai Med. and Chem. Institute, Shanghai, China). Samples were processed according to the manufacturer’s instructions[19].
Plasma D-lactate levels were measured by an enzymatic spectrophotometric assay[20] using a serum D-lactate quantitative colorimetric detection kit according to the manufacturer’s instructions (GMS19038.6, Genmed, Boston, MA, United States). Results were expressed as mol/mL. Plasma diamine oxidase (DAO) activities were determined with an enzymatic spectrophotometric assay[21] using a Serum DAO detection kit according to the manufacturer’s instructions (Jiancheng Biological Co., Ltd., Nanjing, China). Results were expressed as U/L.
Plasma glutathione (GSH) and glutathione (GSSG) were determined by an enzymatic spectrophotometric assay[22] using the GSH and GSSG detection kits according to the manufacturer’s instructions (Jiancheng Biological Co., Ltd.). Results were expressed as mol/mL.
Superoxide dismutase (SOD) activity was detected using Sun et al’s[23] nitroblue tetrazolium method. Malondialdehyde (MDA) levels were measured using the thiobarbituric acid test according to Ohkawa et al[24]. Intestinal tissue samples were thawed, weighed, and homogenized 1:9 (w/v) in 0.9% saline. The homogenates were centrifuged at 3000 ×g for 10 min at 4 °C, and the supernatant was removed for the measurement of MDA content, SOD activity, and total protein. Total intestinal protein concentration was determined by a Coomassie blue method, with bovine serum albumin (BSA) as a standard. SOD activity and MDA levels were detected with kits according to the manufacturer’s instructions (Jiancheng Bioengineering Ltd., Nanjing, China). Results were expressed as U/mg protein and nmol/mg protein.
Samples 1 cm in length were collected from the terminal ileum. To avoid mucosal damage, the intestinal lumen was carefully cannulated and gently washed with normal saline before sampling. Specimens were fixed by immersion in 10% buffered formaldehyde solution and embedded in paraffin. Sections (5 µm thick) were cut and stained for routine light microscopy using HE.
Samples 3-4 mm in length were collected from the terminal ileum. These samples were longitudinally cut and immersed in 2.5% phosphate-buffered glutaraldehyde solution for 24 h at 4 °C. Specimens were then washed with phosphate-buffered solution (PBS), fixed in 1% osmium tetroxide for 2 h at 4 °C, dehydrated in ethanol and propylene oxide, and embedded in Epon 812 for 48 h. Sections (1 µm thick) were cut and stained with methylene blue. Ultrathin sections were then cut with a diamond knife, stained with uranyl acetate and lead citrate, and observed under transmission electron microscopy (TEM).
Four-μm thick sections were collected on poly-L-lysine-coated glass slides. The nuclear DNA fragmentation of apoptotic cells was labeled in situ by the terminal deoxyuridine nick-end labeling immunohistochemical method[25] using an ApopTag® Plus Peroxidase In Situ Apoptosis Detection Kit (CHEMICON, Billerica, MA, United States) according to the manufacturer’s instructions.
Terminal ileum tissues were fixed in 3% paraformaldehyde for 3 h, washed with PBS, and embedded in paraffin. Sections (5 μm thick) were cut and attached to glass slides. After deparaffinization and rehydration, sections were permeabilized with 0.2% Triton X-100 in PBS for 20 min. Slides were washed with PBS extensively and blocked with 5% normal goat serum PBS containing 0.05% Tween-20 and 0.1% BSA for 20 min at room temperature. Primary antibodies were added to the slides and incubated overnight at 4 °C in a humidity chamber. After washing, sections were incubated with FITC-conjugated specific secondary antibody (Sigma) at room temperature for 2 h in the dark. The slides were again washed extensively and then mounted with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, United States). Sections were observed under a confocal laser microscope (LSM 510, Zeiss, Jena, Germany).
After the rats were sacrificed, terminal ileum tissues were excised and fixed in Bouin’s solution and embedded in paraffin. Immunohistochemistry was performed on 5-µm thick paraffin sections. After deparaffinization and dehydration, endogenous peroxidase was blocked with 30 mL/L H2O2 for 15 min. After blocking of nonspecific binding sites with 5% normal goat serum, slides were incubated with specific primary antibody overnight at 4 °C. Primary antibodies were diluted 1:50 (rabbit polyclonal anti-human PKC, Santa Cruz Biotechnology, Inc., United States) in PBS. Next, the slides were washed three times for 5 min each with PBS and incubated with biotinylated goat anti-rabbit IgG at 37 °C for 30 min, washed as before, and developed with HRP-labeled streptavidin. The incubation and the subsequent washing were exactly the same as done before. Finally, diaminobenzidine chromogen, a peroxidase substrate, was added for color development. The reaction was stopped with a tap water rinse. The sections were counterstained with hematoxylin and mounted for examination.
Terminal ileum samples were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer [150 mmol NaCl, 50 mmol Tris·HCl (pH 7.4), 0.5 mmol phenylmethylsulfonyl fluoride, 2.4 mmol EDTA, and 1 mmol sodium orthovanadate with 1% nonidet-40 (NP-40) and Sigma protease inhibitor cocktail (1:100)] for 30 min at 4 °C. After centrifugation at 10 000 ×g for 10 min at 4 °C, the protein concentration of each sample was quantified by the Bradford method. An equal amount of total protein was separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and then transferred to a nitrocellulose membrane. After blocked overnight in tris-buffered saline (TBS) containing 0.05% tween (TBS-T) and 5% dry powdered milk, membranes were washed three times for 5 min each with TBS-T and incubated for 2 h at room temperature in primary antibody (rabbit anti-claudin-1, rabbit anti-occludin, rabbit anti-JAM-A, or rabbit anti-ZO-1). After three washes with TBS-T, the membranes were incubated for 1 h with HRP-conjugated secondary antibody. Following two washes with TBS-T and one wash with TBS, the membranes were prepared for visualization of protein by the addition of enhanced chemiluminescence (ECL) reagent (Amersham, Princeton, NJ, United States). Densitometric analysis was performed using an Alpha Imager 1220 system (Alphainotech Co., San Leandro, CA, United States).
The levels of occludin, ZO-1, claudin-1, claudin-4, JAM-A and UGT1A mRNA were measured by real-time reverse transcription-PCR (RT-PCR) using SYBR1 green[26]. Total RNA was isolated from terminal ileum samples with the TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Real-time RT-PCR was performed with an ABI prism 7000 Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). The primers were designed using the Primer Express® Program (Applied Biosystems). Their sequences are shown in Table 1. The following procedure used 2 μg of RNA. In a sterile RNase-free microcentrifuge tube, 1 μL of 20 μm oligo (dT) 15 primer was added to a total volume of 15 μL water. The tubes were heated to 70 °C for 5 min, cooled immediately on ice, and spun briefly. The following reagents were added to the annealed primer/template: 5 μL of 5 × M-MLV reaction buffer, 1.25 μL of 10 mmol dNTPs and 25 units of RNasin RNase inhibitor, and 200 units of M-MLVRT RNAse H were added to the reagent to yield a 25 μL total reaction volume. All were mixed gently and then incubated for 60 min at 42 °C before the reaction was terminated at -20 °C.
| Gene target | Genbank number (mRNA) | Oligonucleotide1 (5'- to 3'-) | Annealing temperature (°C) | Product size (bp) |
| Occludin | NM-031329 | F: GCTCAGGGAATATCCACCTATC R: TTCTCCAGCAACCAGCATC | 60 | 344 |
| ZO-1 | NM-001106266 | F: CCACAGACATCCAACCAGC R: AGCCCAAAGAACAGAAGACC | 60 | 247 |
| Claudin-1 | NM-031699 | F: GCCTCCAATGCCGTTCT R: TGCCTGCGTCCCTCTTG | 60 | 317 |
| Claudin-4 | NM-001012022 | F: GTTCCCGCCAGCAACTATG R: CCTTCAGCCCCGTATCCA | 60 | 282 |
| JAM-A | NM-053796 | F: CCTCCATCCAAGCCGACA R: CAAAGACCAAATCCCCTGAC | 60 | 211 |
| prkC | NM-001105713 | F: GATGGACGGGGTCACGA R: CGCTTGGCAGGGTGTTT | 60 | 165 |
| β-actin | NM-031144 | F: CAGGTCATCACTATCGGCAAT R: GAGGTCTTTACGGATGTCAAC | 60 | 144 |
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression was used as a house-keeping gene control. Separate PCR reactions (25 μL) were conducted for each transcript and contained 2.0 μL cDNA, 12.5 μL of 2 × SYBR Premix Ex Taq™ (TaKaRa, Ltd., Shiga, Japan), and 0.5 μL each of 10 μmol/L gene-specific forward and reverse primers. PCR conditions were optimized to 95 °C (30 s), followed by 40 cycles (45 s each) at 95 °C, 60 °C (5 s), and 72 °C (30 s), and the reaction was completed at 37 °C for 30 s. Five serial dilutions of cDNA were analyzed for each target gene and used to construct linear standard curves. To compensate for variations in the RNA input and in the efficiency of the real-time RT-PCR, we used a normalization strategy based on the house-keeping gene GAPDH.
The terminal ileum tissues were homogenized and extracted with the buffer used for Western blotting assays for 30 min at 4 °C. After centrifugation at 10 000 ×g for 10 min at 4 °C, the protein concentration of each sample was quantified by the Bradford method. The supernatant was treated with protein G plus protein A agarose beads (Sigma) and incubated overnight at 4 °C with rabbit anti-occludin antibody (Zymed) and protein G + protein A agarose beads. The beads were washed with PBS and ice-cold RIPA buffer, and immunoprecipitated proteins were separated on 10% SDS-polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose membranes (Invitrogen). The membranes were blocked with 1% BSA in PBS overnight at 4 °C and then incubated with rabbit anti-phosphoserine antibodies (Zymed) for 2 h at room temperature, followed by HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., United States). The reaction was visualized by an enzyme chemiluminescence kit from Pierce (Rockford, IL, United States). Western blotting was performed with an anti-occludin rabbit polyclonal antibody (Zymed) followed by an anti-rabbit secondary antibody coupled with peroxidase (Santa Cruz Biotechnology, Inc.) and ECL. For Western blotting of ZO-1, the same protocol was used with the rabbit polyclonal anti-ZO-1 antibody and a rabbit anti-β-actin antibody (both from Santa Cruz Biotechnology, Inc.).
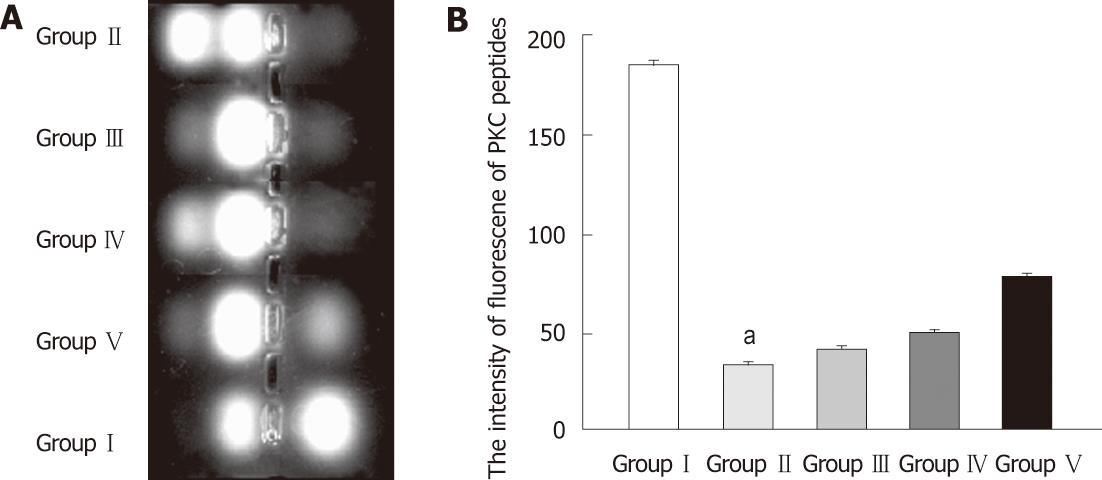
The PKC activity assay was conducted according to the instructions of the PepTag non-radioactive PKC assay kit (Promega, Madison, WI, United States). Briefly, terminal ileum tissues were homogenized and lysed in cold lysis buffer, containing 20 mmol/L tris-HCl, 0.5 mmol/L ethylene glycol tetraacetic acid, 2 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L phenylmethanesulfonyl fluoride, and 10 mg/L leupeptin (pH 7.5). Assays were then performed at 30 °C in a total volume of 25 μL containing 5 μL PKC reaction 5 × buffer, 5 μL PLSRTLSVAAK peptide, 5 μL PKC activator, 1 μL peptide protection solution, and 9 μL sample. Reactions were initiated by the addition of the 9 μL sample and terminated after 30 min by incubation of the reaction mixture at 95 °C for 10 min. After added with 1 μL of 80% glycerol, each sample was separated by 0.8% agarose gel electrophoresis at 100 V for 15 min. The intensity of fluorescence of phosphorylated peptides reflected the activity of PKC. All experiments were carried out in triplicate, with each data point representing the results from a separate culture.
Quantification of the immunohistochemical and immunofluorescence staining was performed on stored images of completely scanned tissue sections. Images were acquired with an AxioCam MRc (Carl Zeiss, Jena, Germany) connected to an Axioplan 2 fluorescence microscope (Carl Zeiss), at × 40 magnification. Each microscopic field was individually autofocused before acquisition. Five fields were selected from each slide and a total of five slides per group were examined. All image acquisition and processing were done using custom-written macros in KS400 image analysis software (version 3.0, Carl Zeiss).
Results were presented as mean ± SD of three experiments. The data were analyzed using GraphPad PRISM (GraphPad Software Inc., San Diego, CA, United States) and SPSS 11.0 (SPSS Inc., Chicago, IL, United States). All data were analyzed using one-way analysis of variance with Bonferroni/Dunnett T3 post-hoc test for multiple comparisons to determine differences between two experimental groups. P values < 0.05 were considered to be significant.
All animals survived throughout the experiment. Bile duct ligated rats were clinically jaundiced within 3 d. At reoperation on day 3, the ligation and division of the CBD were successful in all cases and resulted in significant dilatation of the CBD remnant proximal to the ligature with obvious cholestatic appearance of the liver. At reoperation on day 10, the CBD diameter had returned to a normal size in the BDL + IBD group, and the cholestatic livers also appeared improved visually (Figure 1).
Obstructive jaundice led to significantly elevated serum levels of total bilirubin [153.73 mmol/L vs 3.0 ± 1.63 mmol/L] and ALT [543.83 U/L vs 60.8 ± 5.69 U/L, P < 0.05]. Administration of L. plantarum significantly reduced levels of total bilirubin (132 ± 23.9 mmol/L vs 9.0 ± 1.87 mmol/L) and ALT (218.38 ± 91.09 U/L vs 97 ± 10.37 U/L) in the portal serum (Table 2).
| Group I | Group II | Group III | Group IV | Group V | |
| Endotoxin (ng/mL) | 0.58 ± 0.09 | 17.12 ± 1.09a | 14.25 ± 0.68 | 3.05 ± 0.78c | 1.91 ± 0.54 |
| TBIL (μmol /L) | 3.0 ± 1.63 | 153.83 ± 25.73a | 132.0 ± 23.09 | 23.75 ± 5.42c | 9.0 ± 1.87 |
| ALT (U/L) | 60.8 ± 5.69 | 543.83 ± 184.09a | 218.38 ± 91.09 | 118.63 ± 19.72c | 97.0 ± 10.37 |
| AST (U/L) | 130.9 ± 27.42 | 980.5 ± 663.25a | 512.75 ± 156.76 | 437.88 ± 42.41c | 271.0 ± 28.93 |
Group II ( BDL + L. plantarum) animals presented with significantly elevated endotoxin concentrations in portal blood compared with those in Group I ( SHAM ) (P < 0.05). Treatment with L. plantarum in the BDL + L. plantarum and BDL + IBD + L. plantarum groups significantly reduced endotoxin levels in the portal serum (Table 2).
Plasma D-lactate levels increased significantly in the BDL group compared with the SHAM group. Administration of L. plantarum significantly decreased the plasma D-lactate levels in the BDL + L. plantarum and IBD + L. plantarum groups (Table 3).
| Group I | Group II | Group III | Group IV | Group V | |
| D-lactate (mmol/L) | 1.723 ± 0.106a | 4.236 ± 0.050c | 3.599 ± 0.181 | 3.152 ± 0.123e | 2.800 ± 0.129 |
| DAO (U/L) | 2.829 ± 0.438a | 18.925 ± 1.485c | 12.928 ± 1.544 | 10.198 ± 0.584e | 7.109 ± 0.590 |
| SOD (U/mg protein) | 65.002 ± 4.397a | 26.782 ± 1.979c | 35.396 ± 1.328 | 43.916 ± 1.720e | 53.066 ± 3.203 |
| MDA (nmol/mg protein) | 0.408 ± 0.054a | 1.253 ± 0.154c | 0.914 ± 0.108 | 0.672 ± 0.054e | 0.540 ± 0.029 |
| GSH (μmol/L) | 21.091 ± 0.452a | 7.235 ± 0.479c | 8.431 ± 0.537 | 10.504 ± 0.481e | 19.082 ± 0.455 |
| GSSG (μmol/L) | 2.974 ± 0.260a | 23.753 ± 2.895c | 12.795 ± 1.360 | 4.944 ± 0.207e | 3.537 ± 0.343 |
DAO activity in the BDL group was significantly higher than that in the SHAM group. Plasma DAO activity became significantly lower after the use of probiotics in the BDL + L. plantarum and IBD + L. plantarum groups (Table 3).
Plasma GSH was significantly reduced in Group II (BDL) animals compared with those in Group I (P < 0.05). Administration of L. plantarum significantly increased the levels of GSH in the Group IV (BDL + IBD) animals, whereas GSSG was found to be significantly increased in BDL animals. Administration of L. plantarum significantly reduced the levels of GSSG in the BDL + IBD group (Table 3).
Ileum mucosal MDA was significantly increased in BDL group compared with SHAM. Administration of L. plantarum significantly decreased the levels of MDA in the BDL + IBD group. The trend in ileum mucosal SOD levels among the groups was opposite to the results of MDA (Table 3).
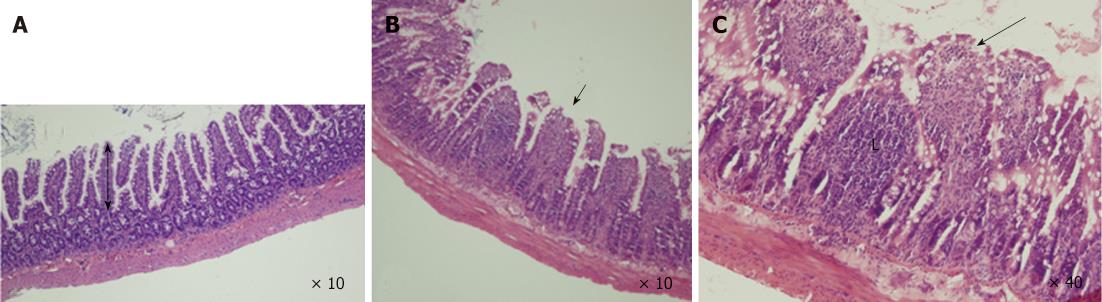
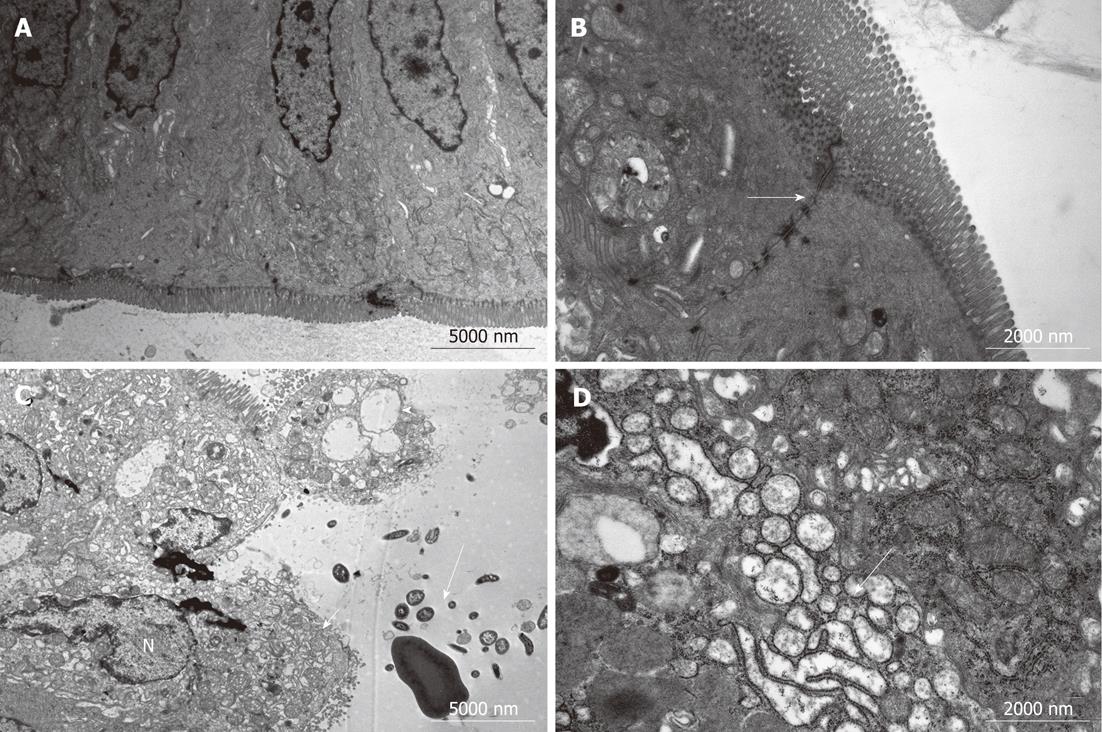
Specimens collected from the terminal ileum in the BDL group showed subepithelial edema and blunting of the villi, mostly located at the tip of the villi, with a large number of lymphocytes and plasma cells infiltrated in the intestinal mucosa (Figure 2). Under TEM, cell ultrastructure was disordered, with loss or disruption of microvilli and large dense secondary lysosomes that resembled partially degraded bacteria within the enterocyte cytoplasm. Inflated vacuolization of the cells, swollen mitochondria with partial or complete absence of interior cristae, disruption of desmosomes and formation of oedematous spaces, and expansion of endoplasmic reticulum were observed. Cells often showed serious damage to the plasma membrane and complete loss of junctional specialization between adjacent cells. Additionally, spherical and rod-shaped bacteria in the ileum were seen near enterocytes. These features are typical of prenecrotic and necrotic injury of the intestinal mucosa. However, in the BDL + L. plantarum group, cells were aligned regularly, with less swelling of mitochondria, no expansion of the endoplasmic reticulum, while the morphology was nearly normal in the IBD + L. plantarum group (Figure 3).
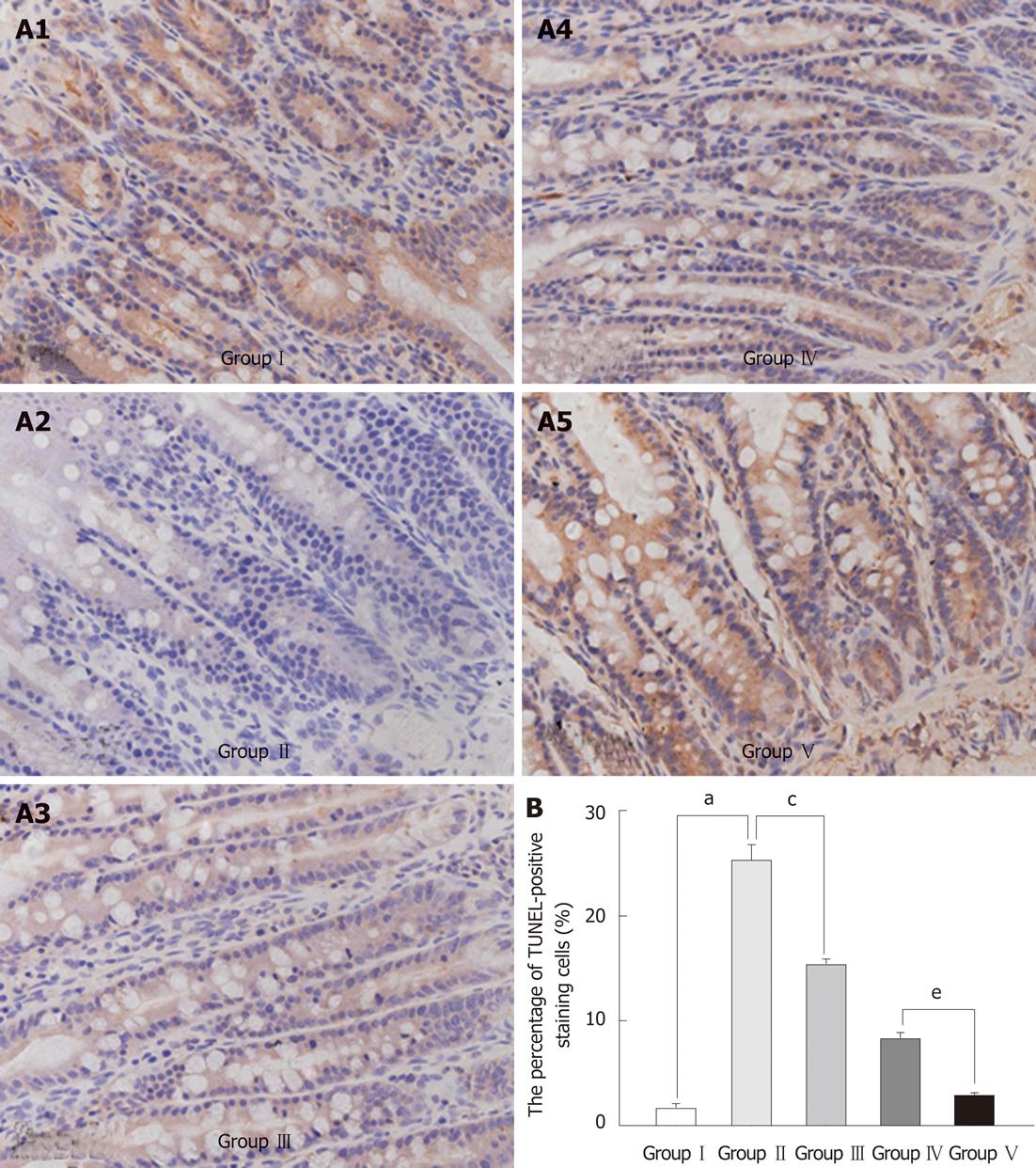
Apoptotic nuclei were significantly more abundant in the markedly atrophic villi in the BDL group than in the SHAM group. The apoptotic nuclei were mostly distributed at the top of villi (Figure 4A). Administration of L. plantarum significantly decreased the number of apoptotic nuclei in the BDL + IBD + L. plantarum group (Figure 4B).
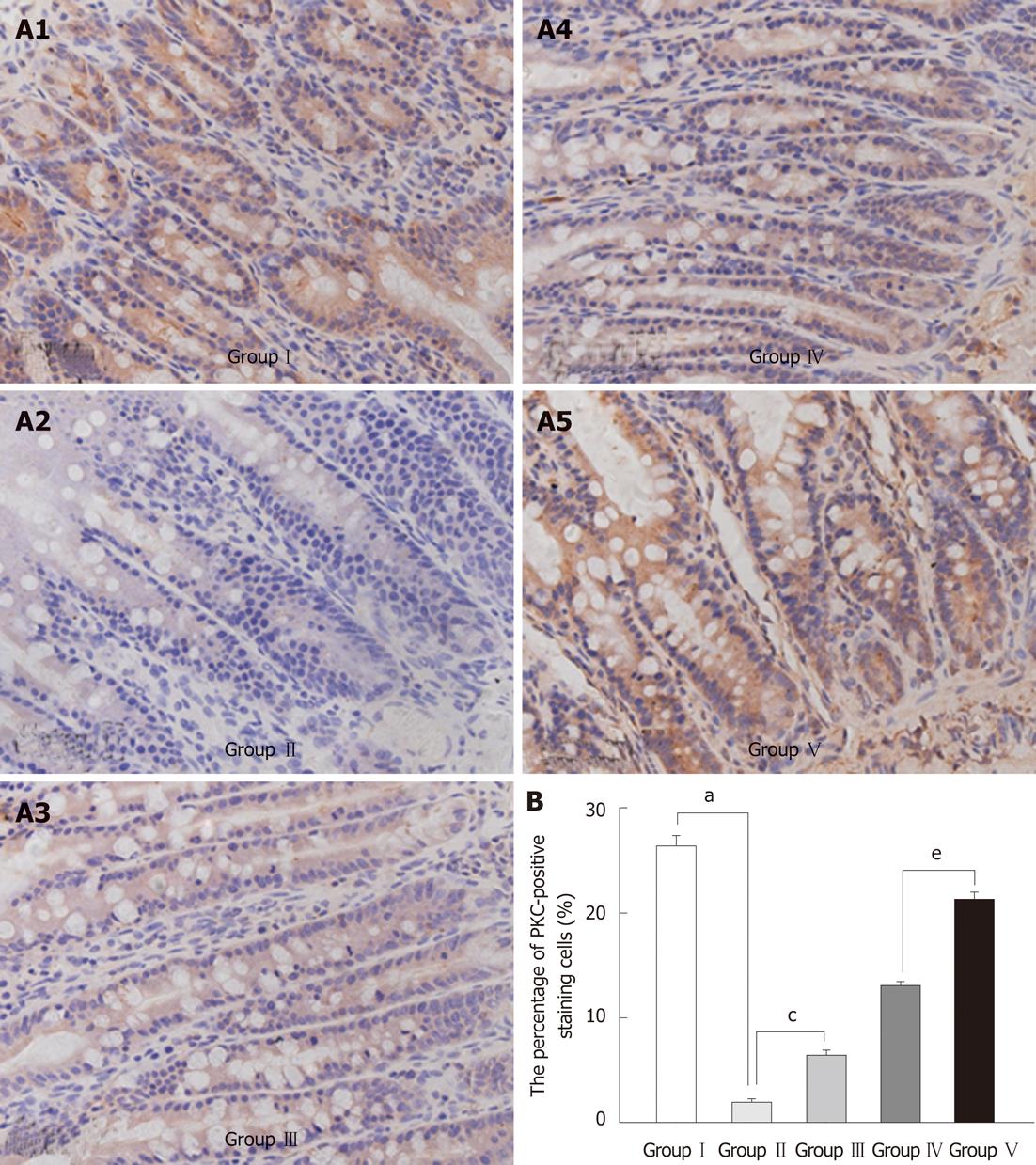
PKC appeared as brown spots in the perinuclear structure. Its expression was decreased in the BDL group compared with the SHAM group. Administration of L. plantarum significantly enhanced the expression of PKC in the BDL + L. plantarum and IBD + L. plantarum groups (Figure 5).
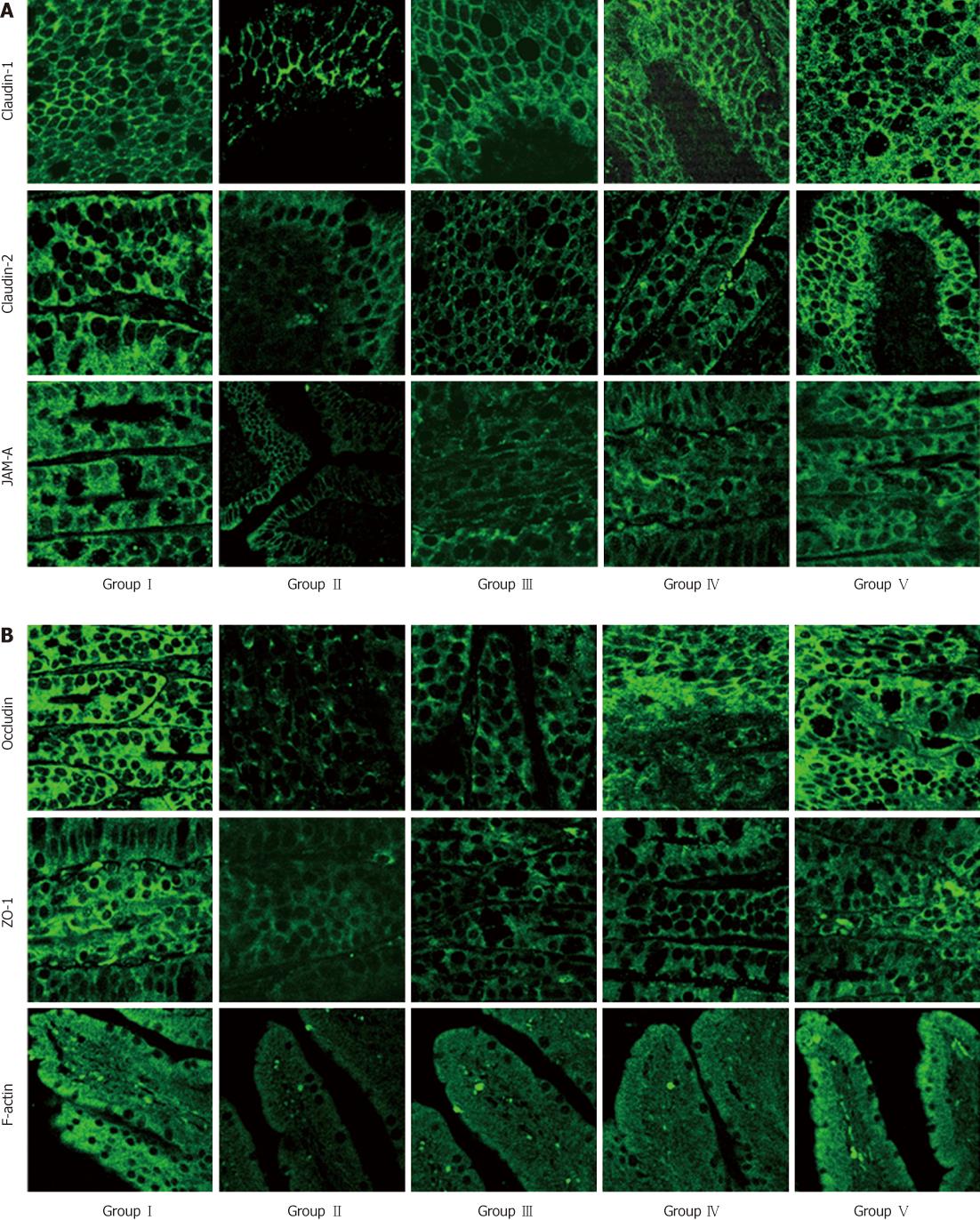
Confocal imaging was performed to assess the distribution of the TJs in each group. TJ-associated proteins were continuously distributed in bright green or red color along the membrane of the cells. The F-actin staining showed a continuous line at the cell borders and along the cytoskeleton. Their borders were very clear in the SHAM group, where TJ-associated proteins were present at the apical intercellular borders in a belt-like manner, encircling the cells and delineating the cellular borders. In the BDL group, the fluorescence was dispersed and even became punctate, with loss of membrane fluorescence as against the uniform membrane staining in controls. Administration of L. plantarum enhanced the expression of TJ-associated proteins in the BDL + L. plantarum and IBD + L. plantarum groups (Figure 6).
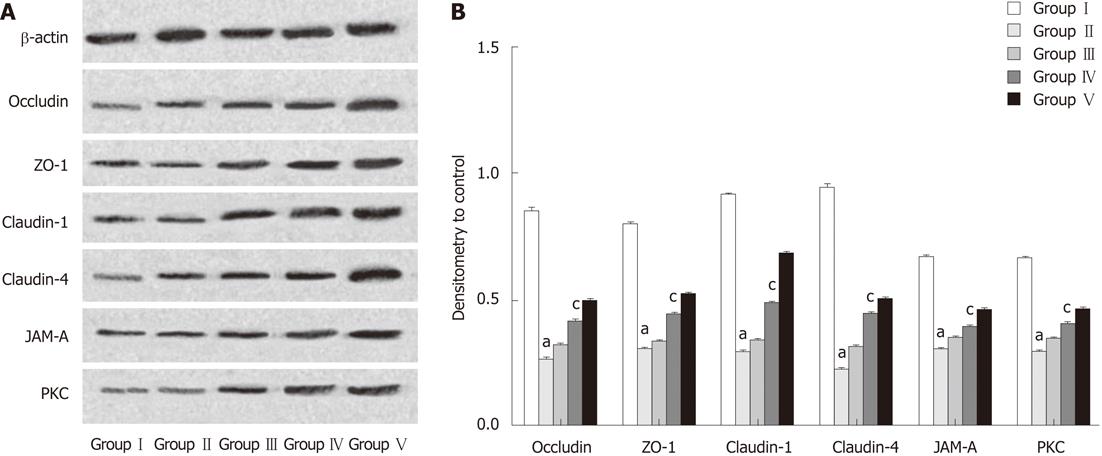
Western blotting analyses were performed to determine the relative protein levels of the target proteins occludin, claudin-1, claudin-4, JAM-A, ZO-1 and PKC in the terminal ileum. The intensity of the whole-cell proteins was determined from ratios of the integrated intensity of the target protein bands to the integrated intensities of the β-actin bands in the same sample. Compared with samples obtained from rats in the SHAM group, levels of target proteins were decreased in protein extracts from ileal mucosal scrapings of rats subjected to BDL. Administration of L. plantarum significantly enhanced the expression of TJ-associated proteins in the BDL + L. plantarum and IBD + L. plantarum groups (Figure 7).
Intragastric administration of L. plantarum resulted in changes in the levels of occludin, ZO-1, claudin-1, claudin-4, JAM-A and PKC. This result raised the question whether these altered protein levels were a consequence of changes in mRNA levels. We, therefore, used real-time RT-PCR to determine the levels of mRNA in the terminal ileum in each group. The levels of mRNA of occludin, ZO-1, claudin-1, claudin-4, JAM-A, PKC and UGT1A1 were found significantly lower in the BDL group than in the SHAM group. Administration of L. plantarum significantly increased the mRNA levels of target proteins in both the BDL + L. plantarum and IBD + L. plantarum groups (Table 4).
| Genes | Group I | Group II | Group III | Group IV | Group V |
| Occludin | 2.5458 ± 0.2260 | 0.4881 ± 0.0426a | 0.9792 ± 0.2066 | 1.4902 ± 0.0720c | 1.8976 ± 0.1049 |
| ZO-1 | 7.2420 ± 0.4025 | 0.9541 ± 0.1629a | 1.4064 ± 0.1632 | 2.8843 ± 0.1641c | 4.0727 ± 0.2059 |
| Claudin-1 | 1.9751 ± 0.0615 | 0.0546 ± 0.0336a | 0.4741 ± 0.0897 | 0.9092 ± 0.1295c | 1.4793 ± 0.2119 |
| Claudin-4 | 42.8680 ± 7.5291 | 0.3546 ± 0.0916a | 5.3245 ± 1.1801 | 8.7719 ± 1.4659c | 15.9592 ± 2.8815 |
| JAM-A | 3..3259 ± 0.3704 | 0.4712 ± 0.1107a | 0.9456 ± 0.1101 | 1.6270 ± 0.2153c | 2.1006 ± 0.1534 |
| PKC | 6.6958 ± 0.9349 | 1.7959 ± 0.2992a | 2.8281 ± 0.3287 | 3.7178 ± 0.5110c | 4.7235 ± 0.4958 |
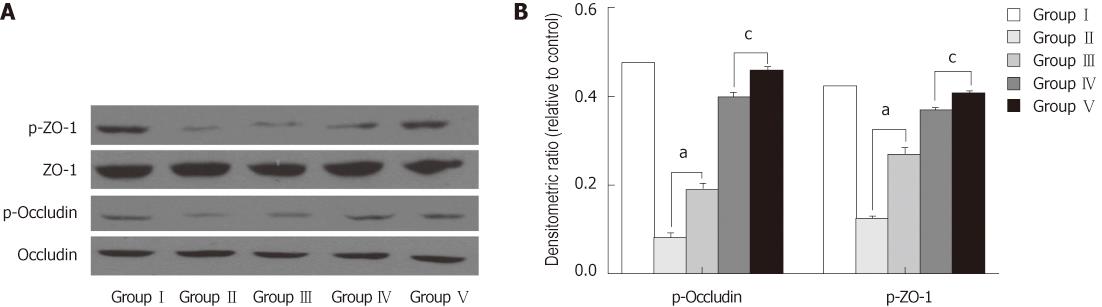
We examined the phosphorylation status of occludin and ZO-1 using immunoprecipitation and immunoblotting assays. Occludin and ZO-1 were phosphorylated at serine residues. BDL lowered p-occludin and p-ZO-1 proteins levels compared with the SHAM group. Administration of L. plantarum improved the expression of the p-occludin and p-ZO-1 proteins from the terminal ileum in the BDL + L. plantarum and BDL + IBD + L. plantarum groups (Figure 8).
As shown in Figure 9, PKC activity was significantly decreased in the BDL group compared with SHAM group. Intragastric administration of L. plantarum partly restored PKC activity in both the BDL + L. plantarum and BDL + IBD + L. plantarum groups.
The present study demonstrated that oral administration of L. plantarum significantly reduced bilirubin, ALT and endotoxin levels in the systemic circulation in an experimental obstructive jaundice animal model with internal biliary drainage for 10 d. Moreover, oral L. plantarum administration to the rats in the BDL + L. plantarum group and the BDL + IBD + L. plantarum group not only reduced the serum endotoxin levels, but also substantially improved liver function. This result is consistent with the conclusions reported in previous literature[12].
In our study, oral L. plantarum administration also significantly decreased the serum DAO activity and D-lactate level in both the BDL + L. plantarum group and the BDL + IBD + L. plantarum group. These findings indicate that L. plantarum plays an important role in intestinal integrity. Previous in vitro studies have confirmed that probiotics exert direct protective effects in intestinal epithelial cell TJs via a PKC-kinase-dependent mechanism and inhibiting epithelial cell apoptosis in cell culture experiments[11,18,10]. The current experiments focused on L. plantarum effects, while several previous studies have reported the protective effects of other lactobacilli and probiotics. For example, Moorthy et al[27] reported that pretreatment with a combination of Lactobacillus rhamnosus (L. rhamnosus) and Lactobacillus acidophilus had a significant protective effect on TJ proteins (claudin-1 and occludin) in a Shigella dysenteriae 1 infection rat model. Khailova et al[28] reported that Bifidobacterium bifidum improved intestinal integrity [composition of TJ and adherens junction (AJ) proteins] in a rat model of necrotizing enterocolitis. Mennigen et al[29] found that the probiotic mixture VSL#3 protected the epithelial barrier by maintaining TJ protein expression and preventing apoptosis in a murine model of colitis. These studies support the findings that a number of probiotic agents have the protective effects in gastrointestinal tract as described in the current work.
Previous studies, including experimental models and clinical cases of biliary obstruction, have shown that disruption of intestinal barrier integrity in obstructive jaundice is associated with high intestinal oxidative stress, as evidenced by increased lipid peroxidation and oxidation of proteins, non-protein, and protein thiols[6,30]. Increased intestinal oxidative stress, a factor in cellular injury, may also play a critical role in regulating important cellular alterations of the intestinal mucosa in obstructive jaundice, such as increased apoptosis and altered TJ expression[5,31]. The results of this study strongly suggest that obstructive jaundice induces oxidative stress in the intestine.
We also found that apoptotic nuclei were significantly more abundant in markedly atrophic villi in the BDL group. Administration of L. plantarum significantly decreased apoptosis of the terminal ileum and improved the histology of the terminal ileum, which also was affected by obstructive jaundice. The protective effect of L. plantarum may be related to amelioration of oxidative stress. Previous studies of L. rhamnosus GG, a member of the same genus as L. plantarum, showed that this bacterium attenuated the H2O2-induced disruption of barrier function[18]. Our studies also revealed that administration of L. plantarum significantly reduced the levels of GSSG, MDA and SOD in rats of the BDL + L. plantarum group and the BDL + IBD + L. plantarum group.
Intestinal epithelial TJs prevent diffusion of potential injurious factors from the gastrointestinal lumen into the tissue. TJs located at the subapical aspect of the lateral membranes contain a large number of membrane-associated proteins, including occludin, JAM and claudins, which are responsible for forming the physical connections between cells that confer the basic barrier properties. Using immunohistochemistry and immunoblotting, previous studies have demonstrated that intestinal mucosal barrier dysfunction in obstructive jaundice is associated with a regional loss of occludin expression in the intestinal epithelium[4,31]. Similarly,our study showed that levels of TJ-associated proteins such as occludin, ZO-1, claudin-1, claudin-4 and JAM-A were reduced in the intestinal epithelium, especially at the upper part of villi. These data support the conclusion that oral L. plantarum administration can enhance the expression of TJ-associated proteins.
A significant body of evidence indicates that PKC is involved in the regulation of the integrity of TJs and AJs. Recent studies have shown differences between Tyr-phosphorylation and Ser/Thr-phosphorylation of occludin. Tyr-phosphorylation of occludin is clearly associated with the disruption of TJs. Ser/Thr-phosphorylation may be required for the assembly of occludin into the TJs. PKC-ζ prevents oxidant-induced iNOS upregulation and protects microtubules and gut barrier integrity[32]. Thus, PKC-ζ appears to be an endogenous stabilizer of the microtubule cytoskeleton and of intestinal barrier function against oxidative injury[33].
The probiotic bacterium L. plantarum has been shown to improve intestinal barrier function in a range of experimental models of colitis, pancreatitis, liver injury and biliary obstruction[8,34-37]. Recent studies have shed some light on the mechanisms involved in the beneficial effects of probiotics in the gastrointestinal tract. PKC activity may be involved in epidermal growth factor-mediated protection of the intestinal epithelial barrier function against oxidative stress[38]. Seth et al[18] suggested that PKCβI activation may be one of the initial events in the probiotic-mediated protection of TJs and AJs. PKCε may play a role in the downstream events of the signaling pathway involved in this process. TJ-protein phosphorylation mediated by PKC may be related to the mechanism of protection by L. plantarum in obstructive jaundice. Previous studies have shown that phosphorylation is a key mechanism for regulating the biological function of TJ proteins. Highly phosphorylated occludin molecules are selectively concentrated at TJs, whereas non- or less phosphorylated occludin molecules are localized in the cytoplasm[39].
To determine whether PKC mediates the disruption of the intestinal barrier in obstructive jaundice, we examined the phosphorylation status of occludin and ZO-1 using Western blotting analysis with antibodies against phosphoserine. We found that obstructive jaundice decreased p-occludin and p-ZO-1 protein levels compared with sham-operation. Our study also demonstrated that obstructive jaundice resulted in a significant decrease in PKC activity. Co-incubation with L. plantarum partly restored PKC activity and increased phosphorylation of serine residues on TJ proteins in both the BDL + L. plantarum group and the BDL + IBD + L. plantarum group. Phosphorylation of these proteins occurred on Ser residues that have been described as substrates for PKC activity[40-42]. Our results suggest that the PKC pathway is involved in the process of L. plantarum-induced TJ redistribution.
In conclusion, administration of probiotics before and after operation in rats with experimental obstructive jaundice can substantially protect the gut barrier. The protective mechanisms of probiotics are associated with decreased intestinal epithelial cell apoptosis, reduction of oxidative stress, and protection of intestinal mucosal TJs. L. plantarum can prevent TJ disruption in biliary obstruction by activating the PKC pathway.
Biliary tract surgery in patients with obstructive jaundice is associated with a high morbidity and mortality rate, and obstructive jaundice increased gut permeability and bacterial translocation. Lactobacillus plantarum (L. plantarum) has been shown to have beneficial effects on intestinal barrier function. Protein kinase C (PKC) plays a crucial role in the mediation of intestinal epithelial tight junction (TJ) proteins, and L. plantarum may prevent TJ disruption in biliary obstruction by activating the PKC pathway. However, there had been few studies about the mechanism for the protective effect of probiotics on the intestinal barrier in obstructive jaundice. This study focused on the effects of L. plantarum on the intestinal mucosal barrier, oxidative stress, epithelial TJ-protein structure and phosphorylation, especially its impact on the expression and activity of PKC.
Previous in vitro studies have confirmed that probiotics could protect intestinal epithelial cell TJs via a PKC-kinase-dependent mechanism and inhibit epithelial cell apoptosis in cell culture experiments. TJ-protein phosphorylation mediated by PKC may be related to the protective effects of L. plantarum in obstructive jaundice.
The administration of L. plantarum before and after operation in rats with experimental obstructive jaundice could substantially protect the gut barrier. Protective mechanisms of L. plantarum are associated with decreased intestinal epithelial cell apoptosis, reduction of oxidative stress, and protection of intestinal mucosal TJs. L. plantarum can prevent TJ disruption in biliary obstruction by activating the PKC pathway.
By understanding the mechanism and effects of L. plantarum on the intestinal mucosal barrier, this study may represent a future strategy in the treatment of patients with obstructive jaundice.
This is a very well done experimental study for evaluating the effect of L. plantarum on the intestinal mucosal barrier, oxidative stress, epithelial TJ protein structure and phosphorylation, with special regard to its impact on the expression and activity of PKC in experimental obstructive jaundice.
Peer reviewers: Julio Mayol, MD, PhD, Department of Digestive Surgery, Hospital Clinico San Carlos, Martin-Lagos S/N, 28040 Madrid, Spain; Mathias Chamaillard, PhD, Center for Infection and Immunity of Lille, INSERM U1019-CNRS UMR 8204-Univ Lille Nord de France, Institut Pasteur de Lille, 1, rue du Professeur Calmette, 59019 Lille Cedex, France; Tamara Vorobjova, MD, PhD, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, 51014 Tartu, Estonia; Fang Yan, MD, PhD, Research Associate Professor, Division of Gastroenterology, Department of Pediatrics, Hepatology and Nutrition, Vanderbilt University Medical Center, 2215 Garland Avenue, MRB IV, Room 1035J, Nashville, TN 37232, United States
S- Editor Cheng JX L- Editor Ma JY E- Editor Xiong L
| 1. | Su CH, P'eng FK, Lui WY. Factors affecting morbidity and mortality in biliary tract surgery. World J Surg. 1992;16:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Deitch EA, Sittig K, Li M, Berg R, Specian RD. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79-84. [PubMed] |
| 3. | Van Bossuyt H, Desmaretz C, Gaeta GB, Wisse E. The role of bile acids in the development of endotoxemia during obstructive jaundice in the rat. J Hepatol. 1990;10:274-279. [PubMed] |
| 4. | Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Assimakopoulos SF, Thomopoulos KC, Patsoukis N, Georgiou CD, Scopa CD, Nikolopoulou VN, Vagianos CE. Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest. 2006;36:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Vendemiale G, Grattagliano I, Lupo L, Memeo V, Altomare E. Hepatic oxidative alterations in patients with extra-hepatic cholestasis. Effect of surgical drainage. J Hepatol. 2002;37:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lindfors K, Blomqvist T, Juuti-Uusitalo K, Stenman S, Venäläinen J, Mäki M, Kaukinen K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin Exp Immunol. 2008;152:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Ko JS, Yang HR, Chang JY, Seo JK. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-alpha. World J Gastroenterol. 2007;13:1962-1965. [PubMed] |
| 10. | Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959-50965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Qin H, Zhang Z, Hang X, Jiang Y. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | White JS, Hoper M, Parks RW, Clements WD, Diamond T, Bengmark S. The probiotic bacterium Lactobacillus plantarum species 299 reduces intestinal permeability in experimental biliary obstruction. Lett Appl Microbiol. 2006;42:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250-G254. [PubMed] |
| 15. | Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072-6076. [PubMed] |
| 16. | Rosson D, O'Brien TG, Kampherstein JA, Szallasi Z, Bogi K, Blumberg PM, Mullin JM. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J Biol Chem. 1997;272:14950-14953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Mullin JM, Kampherstein JA, Laughlin KV, Clarkin CE, Miller RD, Szallasi Z, Kachar B, Soler AP, Rosson D. Overexpression of protein kinase C-delta increases tight junction permeability in LLC-PK1 epithelia. Am J Physiol. 1998;275:C544-C554. [PubMed] |
| 18. | Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060-G1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Friberger P, Knös M, Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195-206. [PubMed] |
| 20. | Cağlayan F, Cakmak M, Cağlayan O, Cavuşoglu T. Plasma D-lactate levels in diagnosis of appendicitis. J Invest Surg. 2003;16:233-237. [PubMed] |
| 21. | Takagi K, Nakao M, Ogura Y, Nabeshima T, Kunii A. Sensitive colorimetric assay of serum diamine oxidase. Clin Chim Acta. 1994;226:67-75. [PubMed] |
| 22. | Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3140] [Cited by in RCA: 3130] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 23. | Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497-500. [PubMed] |
| 24. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [PubMed] |
| 25. | Wolf SE, Ikeda H, Matin S, Debroy MA, Rajaraman S, Herndon DN, Thompson JC. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg. 1999;188:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Jia F, Mao Q, Liang YM, Jiang JY. Effect of post-traumatic mild hypothermia on hippocampal cell death after traumatic brain injury in rats. J Neurotrauma. 2009;26:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Moorthy G, Murali MR, Devaraj SN. Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition. 2009;25:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G940-G949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140-G1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 30. | Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand. 2004;180:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Banan A, Zhang L, Fields JZ, Farhadi A, Talmage DA, Keshavarzian A. PKC-zeta prevents oxidant-induced iNOS upregulation and protects the microtubules and gut barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G909-G922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Banan A, Fields JZ, Talmage DA, Zhang L, Keshavarzian A. PKC-zeta is required in EGF protection of microtubules and intestinal barrier integrity against oxidant injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G794-G808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Lee HS, Han SY, Bae EA, Huh CS, Ahn YT, Lee JH, Kim DH. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int Immunopharmacol. 2008;8:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Mangiante G, Colucci G, Canepari P, Bassi C, Nicoli N, Casaril A, Marinello P, Signoretto C, Bengmark S. Lactobacillus plantarum reduces infection of pancreatic necrosis in experimental acute pancreatitis. Dig Surg. 2001;18:47-50. [PubMed] |
| 36. | Wang XD, Soltesz V, Molin G, Andersson R. The role of oral administration of oatmeal fermented by Lactobacillus reuteri R2LC on bacterial translocation after acute liver failure induced by subtotal liver resection in the rat. Scand J Gastroenterol. 1995;30:180-185. [PubMed] |
| 37. | Fabia R, Ar'Rajab A, Johansson ML, Willén R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155-162. [PubMed] |
| 38. | Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 436] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 40. | Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141-1149. [PubMed] |
| 41. | Avila-Flores A, Rendón-Huerta E, Moreno J, Islas S, Betanzos A, Robles-Flores M, González-Mariscal L. Tight-junction protein zonula occludens 2 is a target of phosphorylation by protein kinase C. Biochem J. 2001;360:295-304. [PubMed] |
| 42. | Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |