Published online Aug 14, 2012. doi: 10.3748/wjg.v18.i30.3923
Revised: May 11, 2012
Accepted: May 26, 2012
Published online: August 14, 2012
Gastric cancer and liver cancer are among the most common malignancies and the leading causes of death worldwide, due to late detection and high recurrence rates. Today, these cancers have a heavy socioeconomic burden, for which a full understanding of their pathophysiological features is warranted to search for promising biomarkers and therapeutic targets. Osteopontin (OPN) is overexpressed in most patients with gastric and liver cancers. Over the past decade, emerging evidence has revealed a correlation of OPN level and clinicopathological features and prognosis in gastric and liver cancers, indicating its potential as an independent prognostic indicator in such patients. Functional studies have verified the potential of OPN knockdown as a therapeutic approach in vitro and in vivo. Furthermore, OPN mediates multifaceted roles in the interaction between cancer cells and the tumor microenvironment, in which many details need further exploration. OPN signaling results in various functions, including prevention of apoptosis, modulation of angiogenesis, malfunction of tumor-associated macrophages, degradation of extracellular matrix, activation of phosphoinositide 3-kinase-Akt and nuclear factor-κB pathways, which lead to tumor formation and progression, particularly in gastric and liver cancers. This editorial aims to review recent findings on alteration in OPN expression and its clinicopathological associations with tumor progression, its potential as a therapeutic target, and putative mechanisms in gastric and liver cancers. Better understanding of the implications of OPN in tumorigenesis might facilitate development of therapeutic regimens to benefit patients with these deadly malignancies.
- Citation: Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol 2012; 18(30): 3923-3930
- URL: https://www.wjgnet.com/1007-9327/full/v18/i30/3923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i30.3923
Gastric and liver cancers are among the most common malignancies and leading causes of death worldwide, which carries a heavy socioeconomic burden. Until now, surgical resection has remained the frontline treatment for patients with early stage gastric and liver cancers. Nevertheless, the majority of such patients have poor prognosis due to high rates of tumor recurrence as well as lymph node (LN) and systemic metastases. Therefore, a full understanding of gastric and liver cancers is crucial to develop useful prognostic markers and therapeutic targets. During the past decade, emerging evidence has refined the value of osteopontin (OPN) as a candidate biomarker and target for cancer therapy[1]. OPN is a secretory extracellular matrix (ECM) protein that is involved in a series of physiological and pathophysiological processes including but not limited to cell attachment, migration, invasion, proliferation, tissue remodeling, bone formation and even inflammation[1-4]. OPN is frequently overexpressed in human cancers and contributes to tumor formation and progression[5,6]. OPN belongs to the small integrin binding ligand N-linked glycoprotein family, which consists of members serving as markers of early cancer progression, due to their capabilities in modulating the activity of matrix metalloproteinases (MMPs)[7]. OPN participates in the interactions between cancer cells and tumor stroma, which plays a pivotal role in malignant cancer phenotype. A more thorough understanding of the functional role of OPN in the tumor microenvironment is warranted. There have been many reports on OPN and gastric and liver cancers, therefore, this review aims to summarize recent findings on clinical implications of OPN, its potential as a therapeutic target, and its related mechanisms in these two types of cancer. Further understanding on the role of OPN in gastric and liver cancers may facilitate development of therapeutic strategies in such patients.
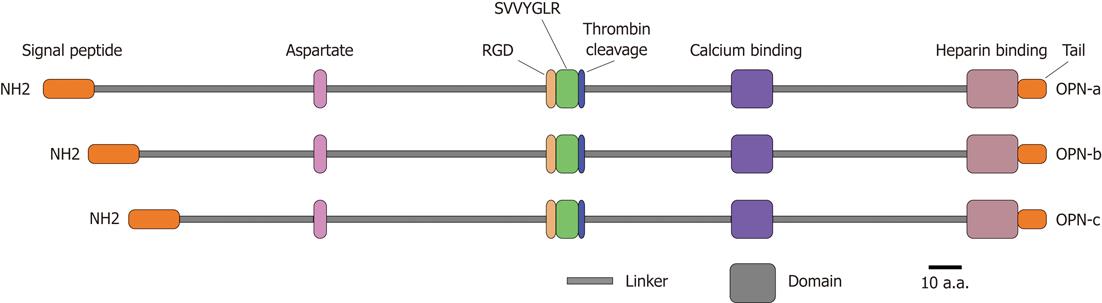
OPN is a matrix glycoprotein secreted by a variety of cell types including osteoclasts, endothelial cells, epithelial cells, and activated immune cells such as macrophages and T cells[8]. It is also known as bone sialoprotein I, early T lymphocyte activation 1 and secreted phosphoprotein 1[9-11]. Human OPN gene is located on chromosome 4q21-q25, spans approximately 11 kb, and consists of seven exons encoding the OPN protein with 314 amino acid residues[12]. It contains several highly conserved structural elements, including arginine-glycine-aspartate and Ser-Val-Val-Tyr-Gly-Leu-Arg domains for integrin binding, a calcium binding site and a heparin binding domain for CD44 receptor binding[13] (Figure 1). Alternative splicing produces three OPN isoforms, OPN-a, OPN-b and OPN-c, which probably display different expression profiles and functional heterogeneity in a tumor-specific manner[14,15]. Moreover, OPN protein is subjected to a series of post-translational modifications including serine/threonine phosphorylation, glycosylation and tyrosine sulfation, resulting in molecular variants ranging from 25 to 75 kDa[16]. These modifications are cell type specific and depend on physiological and pathophysiological factors, which likely affect both OPN structure and functions[17].
OPN expression is significantly elevated in most gastric cancer patients at both transcriptional and translational levels[18-24]. OPN protein is overexpressed in both primary gastric cancer and metastatic lesions, mildly expressed in the epithelial cells in chronic atrophic gastritis that is a precancerous lesion for gastric cancer, and negatively in normal gastric mucosa, which indicates that OPN may play a role and serve as a potential biomarker in the formation and progression of gastric cancer[18-20]. Moreover, Wu et al[19] have found higher OPN plasma level in gastric cancer patients as compared with healthy individuals, suggesting that OPN plasma level may also be a biomarker for gastric cancer, and is of particular clinical interest because plasma-derived biomarkers are more convenient in clinical application than biomarkers from tissues. In gastric cancer tissues, OPN protein is diffusely located in the cytoplasm of tumor cells as well as tumor-associated macrophages (TAMs), which is in line with its implications in the interactions between cancer cells and tumor stroma.
Until now, the diagnostic and prognostic values of OPN have been implicated in gastric cancer patients. Microarray studies have identified gene signatures including OPN in gastric cancer patients[18]. OPN overexpression is significantly associated with clinicopathological parameters in gastric cancer such as low apoptotic index, high proliferative index, low grade, high stage, LN and vascular invasion, and distant metastasis[20-24]. In addition, OPN overexpression is an independent predictor of poor prognosis and tumor recurrence in patients with gastric cancer[21,22]. Dai et al[22] have suggested that patients with OPN-positive gastric cancer have poorer outcome than OPN-negative cases. Multivariate analysis has revealed OPN expression as an independent prognostic indicator of poor disease-free and overall survival in patients with gastric cancer, particularly for survival in cases in tumor, node, metastasis (TNM) stage II and III. The prognostic value of the marker combinations of OPN with conventional biomarkers has also been explored in gastric cancer patients. Zhang et al[24] have found the combination of OPN and caudal-related homeobox gene 2 (CDX2) as a survival predictor of advanced gastric cancer patients. OPN plasma level is commonly elevated in patients with gastric cancer, and is significantly associated with the clinicopathological features including late stage, serosal invasion, LN and vascular invasion, and liver metastasis[19]. High OPN plasma level is inversely correlated with poor prognosis in gastric cancer patients, especially in those with invasive phenotypes. Thus, elevated OPN plasma level may serve as an independent risk factor for poor survival in gastric cancer patients.
OPN is positive in most hepatocellular carcinoma (HCC) patients at both transcriptional and translational levels[25-35]. Yuan et al[28] demonstrated OPN mRNA overexpression in 79 (51%) of 156 primary HCC patients. Kim et al[35] disclosed that OPN protein was expressed in 92 (32.3%) of 285 tumors. The expressions of OPN mRNA and protein display a positive correlation[29]. In HCC, OPN is secreted by both cancer cells and TAMs, and secreted by bile duct epithelium and stellate cells, but not by normal hepatocytes or Kupffer cells, in normal liver conditions[34,35]. OPN+ cancer cells are often dispersed in the periphery of cancer nodules and are adjacent to stromal cells[34,35]. In addition, OPN plasma level is also significantly elevated in HCC patients, especially in those with cirrhosis or in advanced stages[35-39]. Kim et al[35] determined that OPN plasma level in HCC patients was significantly higher than in patients with chronic liver diseases or healthy controls (954 ng/mL vs 381 ng/mL; 954 ng/mL vs 155 ng/mL). Zhang et al[36] also found that OPN plasma level of HCC patients was significantly higher than that of healthy controls (176.90 ng/mL vs 63.74 ng/mL). These data propose that elevated OPN plasma level can serve as a potential biomarker for HCC.
Meanwhile, several microarray studies have identified OPN-containing gene signatures of HCC patients[30-32]. Ye et al[31] have identified OPN as a leading gene in the gene signature that was relevant to tumor metastasis and patient survival. Luo et al[32] have found that overexpressed OPN gene belongs to a specific gene signature in HCC. In addition, many studies have established a significant correlation between OPN overexpression and clinicopathological features of HCC, including the severity of liver damage according to Child-Pugh class, high grade, late stage, LN/vascular/bile duct/capsular invasion, and intrahepatic or distant metastases[26-30,40-44]. Until now, OPN overexpression has been revealed as an independent prognostic factor for poor overall and disease-free survival in HCC patients[25-28,33,42-47]. In 2010, Weber et al[42] performed a meta-analysis and found that OPN level correlated with poor overall and disease-/relapse-free survival, and as a biomarker for stage, grade, and early tumor progression in HCC. Chen et al[25] disclosed that OPN expression was a prognostic marker for HCC patients at TNM stage I. Furthermore, novel biomarker combinations are evaluated to predict patient outcome in HCC, since classical parameters cannot provide exact information. The biomarker combinations, OPN and α-fetoprotein (AFP), or OPN and CD44s, are revealed to have better prognostic value than the classical diagnostic biomarkers[29,44]. Huang et al[48] have suggested that the combination of OPN and caspase-3 can be an effective indicator for HCC patients after curative resection. However, because the published data are conflicting in many cases, further large-scale studies are necessary to confirm their clinical value[49].
Tumor recurrence is a persistent issue after surgical resection. A number of studies have suggested OPN as a useful marker for predicting early recurrence in HCC patients[25-27,33,44-46,50]. OPN polymorphisms and the combination of OPN and CD44 are potential predictors of tumor recurrence in HCC[45,46]. OPN overexpression is associated with early recurrence of hepatitis C virus (HCV)-related HCC[50]. Chen et al[25] found that OPN expression was correlated with early postoperative recurrence in patients at stage I. Sieghart et al[33] have revealed that OPN is an independent predictor of tumor recurrence and survival in HCC patients beyond Milan criteria undergoing orthotopic liver transplantation. Thus, OPN may be able to help determine the patients who need adjuvant therapy to prevent early recurrence after surgical resection.
At present, many serum biomarkers are under evaluation for the detection of HCC, but none of them has sufficient sensitivity and specificity to be considered in the guidelines. OPN plasma level increases significantly with advanced Child-Pugh class, large tumor size, high grade, and late stage[35,38]. OPN plasma level is suggested as an adverse prognostic factor for both overall survival, disease-free survival and relapse-free survival in hepatitis B virus (HBV)- or HCV-related HCC patients[36,38,51]. In addition, OPN plasma level may be a potential diagnostic biomarker for HCC in the surveillance of patients with HBV or HCV infection. Sun et al[51] have suggested that preoperative plasma level of OPN and AFP can be used as a prognostic marker for early stage HCC. A recent study conducted by Shang et al[52] has also identified serum OPN as a novel marker for early HCC diagnosis because OPN was found clearly elevated 1 year before diagnosis in a pilot prospective study including 22 patients. In another two studies, a greater area under curve value of OPN than AFP was observed, suggesting superior diagnostic accuracy of OPN for HCC[38]. HCC patients whose pretreatment OPN serum level is low and declines following transarterial chemoembolization exhibit better tumor response and longer survival[37]. These data suggest that OPN plasma level can be used, either independently or coupled with AFP, for predicting clinical outcome in HCC patients.
OPN as a therapeutic target has been explored in various tumors including cancers of breast, lung, head and neck, stomach, colon and liver. Promising results have been achieved in a series of studies[53-57]. The strategies often utilize OPN antibody to block its binding to receptors so as to inhibit the downstream signal transduction related to tumor growth and invasion, and deliver the small interfering RNA (siRNA) targeting OPN to tumor cells to decrease directly the expression of OPN to abrogate the effects triggered by elevated OPN.
At present, OPN-knockdown-induced tumor suppression in gastric cancer has been shown through RNA interference (RNAi)[58-60]. In vitro and in vivo studies have demonstrated OPN-RNAi-induced inhibition of tumor growth, migration and invasion in gastric cancer[58,59]. Moreover, Wang et al[60] silenced OPN expression in gastric cancer cell line SGC7901 using lentiviral-OPN siRNA technology, and found reduced detectable tumors, fewer metastases, and longer survival time in mice implanted with OPN-SGC7901 cells. These data suggest that targeting OPN and its related signaling network is likely to provide an effective therapeutic approach for gastric cancer (Table 1).
| Cell lines | Mouse model | Method of study | Resultant effects | Possible mechanisms |
| Gastric cancer | ||||
| SGC7901 | Nude mice | siRNA knockdown | Reduced angiogenesis in vitro and in vivo | Decreasing microvessel density |
| BGC-823 | Nude mice | Transient/stable siRNA knockdown | Inhibited cell growth, anchorage-independent growth, migration and invasion in vitro, and suppressed tumor growth and prolonged survival in vivo | Inhibition of MMP-2 and uPA expression, NF-κB DNA binding activity, and Akt phosphorylation |
| SGC7901 | Nude mice implanted with SGC-OPN-cells | Lentivirus-mediated stable depletion | Suppressed metastases and prolonged survival time in vivo | Reducing expression of VEGF |
| HCC | ||||
| MHCC97-L, MHCC97-H, HCC-LM3 | siRNA knockdown | Decreased cell invasion and cell cloning number in vitro | ||
| HuH1/4/7, MHCC97, SMMC7721, SK-Hep-1, Hep3B, CCL13, HCCLM3 | Nude mice of lung metastasis | OPN-neutralizing antibody | Blocked invasion of SK-Hep-1 and Hep3B cells in vitro, inhibited pulmonary metastasis of HCC-LM3 cells in vivo | |
| HCC-LM6 | Nude mice implanted with HCC-LM6 | Antisense knockdown | Suppressed migration and invasion in vitro, decreased lung metastases in vivo | Inhibiting MMP-2 and uPA expression |
| HCC-LM3 | Nude mice implanted with Lenti OPNi- transfected HCC-LM3 cells | Stable depletion using lentiviral vectors encoding miRNA against OPN | Inhibited both in vitro proliferation, invasion and in vivo tumor growth and lung metastasis | Inhibiting MAPK and NF-κB pathways, and MMP-2 and suppressing uPA expression |
| HCC-LM3 HepG2 | Nude mice | shRNA gene silencing | Inhibited HCC cell growth, adhesion and invasion in vitro, and suppressed tumorigenicity and lung metastasis in vivo, enhanced sensitivity of HCC cells to chemotherapeutic drugs | Suppressing αv, β1, β3 integrin expression, blocking NF-κB activation, inhibiting apoptosis |
In recent years, efforts have also been made to inhibit HCC progression and metastasis by interfering OPN[27,31,61-63]. OPN knockdown significantly suppresses migration and invasion of HCC cells in vitro and decreases lung metastases in vivo, which is associated with decreased angiogenesis in HCC cells[61,62]. Besides, OPN-specific antibody can effectively block HCC cell invasion in vitro and inhibit lung metastasis of HCC cells in vivo[31]. In addition, Zhao et al[63] have demonstrated that short hairpin RNA-mediated OPN depletion enhances sensitivity of HCC cells to chemotherapeutic drugs through blockade of nuclear factor (NF)-κB activation. Thus, targeting OPN and its related signaling network is likely to help develop novel therapeutic regimens for HCC (Table 1).
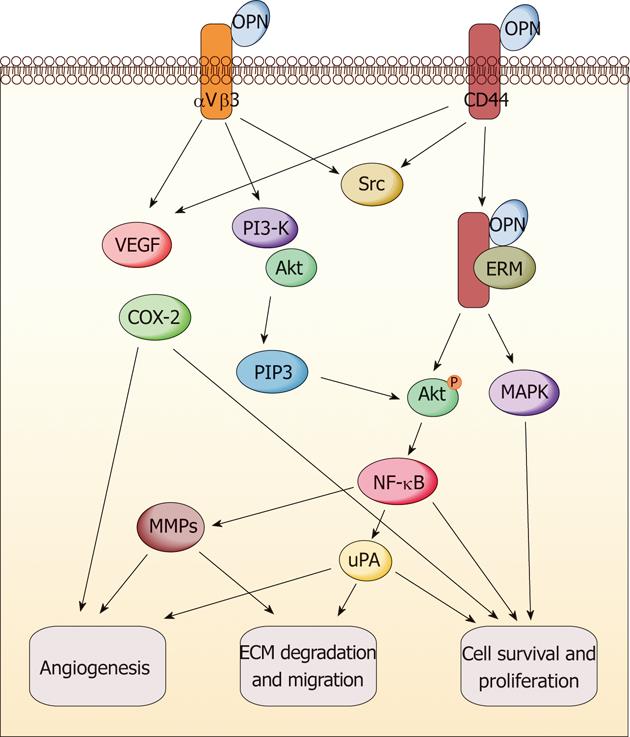
The multifunction of OPN has been revealed in promoting tumor formation and progression (Figure 2). It exerts these functions through direct binding to integrin and/or CD44. The subsequent activation of various pathways leads to increased malignant phenotype[64,65]. Various signaling transduction pathways triggered by OPN molecule have been reported in different cancer models such as breast cancer, melanoma, lung cancer, myeloma, prostate cancer and gastrointestinal cancers. The results indicated that OPN exerts the tumor-related functions through a complicated signaling network[65]. Here, we only summarize the reported signaling pathways of OPN relevant to gastric and liver cancers; some of which are commonly overlapped with other cancers, but some are specific in these two types of cancers. It has been suggested that phosphoinositide 3-kinase (PI3-K)/protein kinase B (Akt) pathway and hypoxia-inducible factor-1 are involved in the tumor-promoting function of OPN, which induces pro-survival and anti-apoptosis signaling in gastric and liver cancers after the survival pathway is activated[63,66]. Mitogen-activated protein kinase pathway (MEK/ERK1/2) can also be triggered by OPN protein in liver cancer to promote tumor growth and metastasis, while the effect can be reversed through OPN knockdown[62]. The NF-κB pathway is crucial to keep cell survival through initiating the gene expression of antiapoptotic proteins, and is often induced by chemotherapeutic drugs and contributes to resistance to chemotherapy[59,62]. Relevant tumor-promoting functions of OPN are found to be highly associated with NF-κB pathway activation in gastric and liver cancers[59,62,63]. The MMP family is responsible for ECM degradation and remodeling, which play an important role in tumor invasion and metastasis. OPN-induced metastasis of gastrointestinal cancers is also involved in several MMP members such as MMP-2, MMP-9, MMP-7 and other famous invasion-related proteins such as vascular endothelial growth factor (VEGF) and urokinase-type plasminogen activator (uPA)[54,59,62,67,68]. Recently, Lee et al[66] illustrated that OPN can enhance the survival of gastric cancer through the interaction with CD44 variant isoforms. The underlying mechanism involves Src kinase signaling upon OPN binding to CD44, followed by “inside-out” integrin activation. In addition, there may be a positive correlation between OPN and cyclooxygenase-2 (COX-2). OPN, VEGF and COX-2 could synergistically induce angiogenesis and metastasis in gastric cancer[69]. On the other hand, the antitumor activity of COX-2 inhibitors in intestinal cancer is probably mediated through downregulation of OPN, which results from blockade of nuclear receptor subfamily 4, group A, member 2 (NR4A2) and Wnt/β-catenin signaling, two important components of the OPN regulatory network[70].
Several mechanisms regulating OPN gene expression have been revealed, but many details remain to be elucidated. OPN is a transcriptional target of aberrant Wnt/β-catenin signaling[70-72], and is also regulated by other molecules including specificity protein 1, v-ets erythroblastosis virus E26 oncogene homolog 1, runt-related transcription factor 2, v-myb myeloblastosis viral oncogene homolog, CDX2, deleted in liver cancer 1, late SV40 factor (LSF), epidermal growth factor (EGF), NR4A2 and NO[24,73-79]. Interestingly, the activation of several downstream targets of OPN, such as Akt, LSF, NO, EGF and thrombin, can enhance OPN expression in turn, suggesting a positive feedback regulation of OPN gene expression[68,73,78-82]. Moreover, the modulation of OPN mRNA stability also influences OPN expression in HCC[83,84]. In addition, miRNA-181a decreased OPN expression in HCC cell lines, suggesting that miRNA is involved in the regulation of OPN gene expression[85]. Furthermore, the expression of OPN is also affected by COX-2 and 30-kDa Tat-interacting protein[70,86,87].
In short, OPN signaling could result in the activation of anti-apoptosis and pro-survival pathways via PI3-K-Akt and NF-κB signaling molecules, angiogenesis modulation via VEGF induction, ECM degradation via MMPs and uPA secretion, leading to tumor growth and metastasis in gastric and liver cancers.
OPN overexpression occurs frequently in patients with gastric cancer and liver cancer. Previous studies have revealed its clinicopathological correlation with tumor formation and progression in these two types of cancer, indicating its potential as an independent indicator for predicting outcome in such patients. Functional studies have shown the potential of OPN as a therapeutic target in gastric and liver cancers both in vitro and in vivo. OPN mediates multifaceted roles in the interaction between cancer cells and tumor microenvironment, in which many details need to be further explored. The various mechanisms of OPN signaling in gastric and liver cancers including evasion of apoptosis, modulation of angiogenesis, ECM degradation, activation of PI3-K-Akt and NF-κB pathways, might induce the development and progression of gastric and liver cancers. However, no clinical trial targeting OPN is in progress for tumor treatment, although the importance of OPN has been widely investigated and demonstrated in various cancers, and many patents including antibodies or peptides against OPN have been filed to treat different tumors. OPN is an important cytokine to mediate normal physiological functions. Blocking OPN possibly results in severe adverse effects due to interference with normal OPN roles. Therefore, further understanding of the implications and roles of OPN in various tumors including gastric and liver cancers could help develop better therapeutic strategies for such patients. On the other hand, OPN as a secreted plasma protein seems to have a greater potential to be utilized as a diagnostic or prognostic marker for in relevant cancers in combination with other biomarkers or alone.
Peer reviewers: Dr. Fernando J Corrales, Division of Hepatology and Gene Therapy, Center for Applied Medical Research, University of Navarra, Av. Pío XII 55, 31008 Pamplona, Spain; Wei Jia, Professor, Nutrition Unit, University of North Carolina at Greensboro, 500 Laureate Way, Suite 4226, Greensboro, KA 28081, United States
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552:61-85. [PubMed] |
| 2. | El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276-4284. [PubMed] |
| 3. | Johnston NI, Gunasekharan VK, Ravindranath A, O'Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361-4372. [PubMed] |
| 4. | Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10:71-81. [PubMed] |
| 6. | Servais EL, Suzuki K, Colovos C, Rodriguez L, Sima C, Fleisher M, Rusch VW, Sadelain M, Adusumilli PS. An in vivo platform for tumor biomarker assessment. PLoS One. 2011;6:e26722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501-8511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Cheng J, Huo DH, Kuang DM, Yang J, Zheng L, Zhuang SM. Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res. 2007;67:5141-5147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Franzén A, Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985;232:715-724. [PubMed] |
| 10. | Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, Regnier DC, Kozak CA, Mock BA, Morse HC. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145-161. [PubMed] |
| 11. | Senger DR, Perruzzi CA, Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989;9:1291-1299. [PubMed] |
| 12. | Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990;7:491-502. [PubMed] |
| 13. | Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med. 2010;14:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Takafuji V, Forgues M, Unsworth E, Goldsmith P, Wang XW. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361-6371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Chae S, Jun HO, Lee EG, Yang SJ, Lee DC, Jung JK, Park KC, Yeom YI, Kim KW. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. Int J Oncol. 2009;35:1409-1416. [PubMed] |
| 16. | Christensen B, Petersen TE, Sørensen ES. Post-translational modification and proteolytic processing of urinary osteopontin. Biochem J. 2008;411:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sørensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282:19463-19472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Junnila S, Kokkola A, Mizuguchi T, Hirata K, Karjalainen-Lindsberg ML, Puolakkainen P, Monni O. Gene expression analysis identifies over-expression of CXCL1, SPARC, SPP1, and SULF1 in gastric cancer. Genes Chromosomes Cancer. 2010;49:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79:127-132. [PubMed] |
| 21. | Higashiyama M, Ito T, Tanaka E, Shimada Y. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007;14:3419-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Dai N, Bao Q, Lu A, Li J. Protein expression of osteopontin in tumor tissues is an independent prognostic indicator in gastric cancer. Oncology. 2007;72:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Imano M, Satou T, Itoh T, Sakai K, Ishimaru E, Yasuda A, Peng YF, Shinkai M, Akai F, Yasuda T. Immunohistochemical expression of osteopontin in gastric cancer. J Gastrointest Surg. 2009;13:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Tsukamoto T, Mizoshita T, Ban H, Suzuki H, Toyoda T, Tatematsu M. Expression of osteopontin and CDX2: indications of phenotypes and prognosis in advanced gastric cancer. Oncol Rep. 2009;21:609-613. [PubMed] |
| 25. | Chen RX, Xia YH, Cui JF, Xue TC, Ye SL. Osteopontin, a single marker for predicting the prognosis of patients with tumor-node-metastasis stage I hepatocellular carcinoma after surgical resection. J Gastroenterol Hepatol. 2010;25:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Pan HW, Ou YH, Peng SY, Liu SH, Lai PL, Lee PH, Sheu JC, Chen CL, Hsu HC. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G, Du H, Liang Y. Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Mol Biol Rep. 2011;38:5205-5210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yuan RH, Jeng YM, Chen HL, Lai PL, Pan HW, Hsieh FJ, Lin CY, Lee PH, Hsu HC. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol. 2006;209:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Beckebaum S, Chen X, Sotiropoulos GC, Radtke A, Daoudaki M, Baba HA, Wohlschlaeger J, Broelsch CE, Gerken G, Cicinnati VR. Role of osteopontin and CD44s expression for patients with hepatocellular carcinoma undergoing liver transplantation or resection. Transplant Proc. 2008;40:3182-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Hua Z, Chen J, Sun B, Zhao G, Zhang Y, Fong Y, Jia Z, Yao L. Specific expression of osteopontin and S100A6 in hepatocellular carcinoma. Surgery. 2011;149:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 643] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 32. | Luo JH, Ren B, Keryanov S, Tseng GC, Rao UN, Monga SP, Strom S, Demetris AJ, Nalesnik M, Yu YP. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Sieghart W, Wang X, Schmid K, Pinter M, König F, Bodingbauer M, Wrba F, Rasoul-Rockenschaub S, Peck-Radosavljevic M. Osteopontin expression predicts overall survival after liver transplantation for hepatocellular carcinoma in patients beyond the Milan criteria. J Hepatol. 2011;54:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19-24. [PubMed] |
| 35. | Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, Cho SY, Hong YJ, Park HY, Lee M. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:2051-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Zhang H, Ye QH, Ren N, Zhao L, Wang YF, Wu X, Sun HC, Wang L, Zhang BH, Liu YK. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Kim SH, Chung YH, Yang SH, Kim JA, Jang MK, Kim SE, Lee D, Lee SH, Lee D, Kim KM. Prognostic value of serum osteopontin in hepatocellular carcinoma patients treated with transarterial chemoembolization. Korean J Hepatol. 2009;15:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | El-Din Bessa SS, Elwan NM, Suliman GA, El-Shourbagy SH. Clinical significance of plasma osteopontin level in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. Arch Med Res. 2010;41:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, Wang J, Han Y, Liu Z. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract. 2008;62:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Wu JC, Sun BS, Ren N, Ye QH, Qin LX. Genomic aberrations in hepatocellular carcinoma related to osteopontin expression detected by array-CGH. J Cancer Res Clin Oncol. 2010;136:595-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Chen HJ, Xiao JR, Yuan W. Loss of p16INK4, alone and with overexpression of osteopontin, correlates with survival of patients with spinal metastasis from hepatocellular carcinoma. Med Oncol. 2010;27:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103:861-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Xie H, Song J, Du R, Liu K, Wang J, Tang H, Bai F, Liang J, Lin T, Liu J. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis. 2007;39:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Peng SY, Ou YH, Chen WJ, Li HY, Liu SH, Pan HW, Lai PL, Jeng YM, Chen DC, Hsu HC. Aberrant expressions of annexin A10 short isoform, osteopontin and alpha-fetoprotein at chromosome 4q cooperatively contribute to progression and poor prognosis of hepatocellular carcinoma. Int J Oncol. 2005;26:1053-1061. [PubMed] |
| 45. | Yang GH, Fan J, Xu Y, Qiu SJ, Yang XR, Shi GM, Wu B, Dai Z, Liu YK, Tang ZY. Osteopontin combined with CD44, a novel prognostic biomarker for patients with hepatocellular carcinoma undergoing curative resection. Oncologist. 2008;13:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Shin HD, Park BL, Cheong HS, Yoon JH, Kim YJ, Lee HS. SPP1 polymorphisms associated with HBV clearance and HCC occurrence. Int J Epidemiol. 2007;36:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Korita PV, Wakai T, Shirai Y, Matsuda Y, Sakata J, Cui X, Ajioka Y, Hatakeyama K. Overexpression of osteopontin independently correlates with vascular invasion and poor prognosis in patients with hepatocellular carcinoma. Hum Pathol. 2008;39:1777-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Huang H, Zhang XF, Zhou HJ, Xue YH, Dong QZ, Ye QH, Qin LX. Expression and prognostic significance of osteopontin and caspase-3 in hepatocellular carcinoma patients after curative resection. Cancer Sci. 2010;101:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Weber GF. The cancer biomarker osteopontin: combination with other markers. Cancer Genomics Proteomics. 2011;8:263-288. [PubMed] |
| 50. | Iso Y, Sawada T, Okada T, Kubota K. Loss of E-cadherin mRNA and gain of osteopontin mRNA are useful markers for detecting early recurrence of HCV-related hepatocellular carcinoma. J Surg Oncol. 2005;92:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Sun J, Xu HM, Zhou HJ, Dong QZ, Zhao Y, Fu LY, Hei ZY, Ye QH, Ren N, Jia HL. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage-I of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 53. | Zhao B, Sun T, Meng F, Qu A, Li C, Shen H, Jin Y, Li W. Osteopontin as a potential biomarker of proliferation and invasiveness for lung cancer. J Cancer Res Clin Oncol. 2011;137:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Likui W, Hong W, Shuwen Z, Yuangang Y, Yan W. The potential of osteopontin as a therapeutic target for human colorectal cancer. J Gastrointest Surg. 2011;15:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Talbot LJ, Mi Z, Bhattacharya SD, Kim V, Guo H, Kuo PC. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells. Surgery. 2011;150:224-230. [PubMed] |
| 56. | Dai J, Li B, Shi J, Peng L, Zhang D, Qian W, Hou S, Zhao L, Gao J, Cao Z. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Minai-Tehrani A, Jiang HL, Kim YK, Chung YS, Yu KN, Kim JE, Shin JY, Hong SH, Lee JH, Kim HJ. Suppression of tumor growth in xenograft model mice by small interfering RNA targeting osteopontin delivery using biocompatible poly(amino ester). Int J Pharm. 2012;431:197-203. [PubMed] |
| 58. | Tang H, Wang J, Bai F, Hong L, Liang J, Gao J, Zhai H, Lan M, Zhang F, Wu K. Inhibition of osteopontin would suppress angiogenesis in gastric cancer. Biochem Cell Biol. 2007;85:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Gong M, Lu Z, Fang G, Bi J, Xue X. A small interfering RNA targeting osteopontin as gastric cancer therapeutics. Cancer Lett. 2008;272:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Wang ZM, Cui YH, Li W, Chen SY, Liu TS. Lentiviral-mediated siRNA targeted against osteopontin suppresses the growth and metastasis of gastric cancer cells. Oncol Rep. 2011;25:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 61. | Chen RX, Xia YH, Xue TC, Zhang H, Ye SL. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol Rep. 2011;25:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL, Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Zhao J, Dong L, Lu B, Wu G, Xu D, Chen J, Li K, Tong X, Dai J, Yao S. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135:956-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004;21:515-523. [PubMed] |
| 65. | Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 66. | Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Wai PY, Mi Z, Guo H, Sarraf-Yazdi S, Gao C, Wei J, Marroquin CE, Clary B, Kuo PC. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005;26:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Georges R, Adwan H, Zhivkova M, Eyol E, Bergmann F, Berger MR. Regulation of osteopontin and related proteins in rat CC531 colorectal cancer cells. Int J Oncol. 2010;37:249-256. [PubMed] |
| 69. | Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, Xie H, Zhang F, Lan M, Yao W. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Zagani R, Hamzaoui N, Cacheux W, de Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C, Lamarque D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology. 2009;137:1358-66.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Rohde F, Rimkus C, Friederichs J, Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR, Janssen KP. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int J Cancer. 2007;121:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Mitra A, Menezes ME, Pannell LK, Mulekar MS, Honkanen RE, Shevde LA, Samant RS. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3β to downregulate β-catenin transcription target, osteopontin. Oncogene. 2012;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Guo H, Marroquin CE, Wai PY, Kuo PC. Nitric oxide-dependent osteopontin expression induces metastatic behavior in HepG2 cells. Dig Dis Sci. 2005;50:1288-1298. [PubMed] |
| 74. | Takami Y, Russell MB, Gao C, Mi Z, Guo H, Mantyh CR, Kuo PC. Sp1 regulates osteopontin expression in SW480 human colon adenocarcinoma cells. Surgery. 2007;142:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Wai PY, Mi Z, Gao C, Guo H, Marroquin C, Kuo PC. Ets-1 and runx2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J Biol Chem. 2006;281:18973-18982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Mol Biol Rep. 2011;38:3671-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Zhou X, Zimonjic DB, Park SW, Yang XY, Durkin ME, Popescu NC. DLC1 suppresses distant dissemination of human hepatocellular carcinoma cells in nude mice through reduction of RhoA GTPase activity, actin cytoskeletal disruption and down-regulation of genes involved in metastasis. Int J Oncol. 2008;32:1285-1291. [PubMed] |
| 78. | Zhang G, Huang Z, Shi R, Lin Y, Hao B. Osteopontin regulation by protein kinase B (Akt) in HepG2 cells. Exp Oncol. 2006;28:36-39. [PubMed] |
| 79. | Yoo BK, Emdad L, Gredler R, Fuller C, Dumur CI, Jones KH, Jackson-Cook C, Su ZZ, Chen D, Saxena UH. Transcription factor Late SV40 Factor (LSF) functions as an oncogene in hepatocellular carcinoma. Proc Natl Acad Sci USA. 2010;107:8357-8362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Shao J, Washington MK, Saxena R, Sheng H. Heterozygous disruption of the PTEN promotes intestinal neoplasia in APCmin/+ mouse: roles of osteopontin. Carcinogenesis. 2007;28:2476-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Zhang GX, Zhao ZQ, Wang HD, Hao B. Enhancement of osteopontin expression in HepG2 cells by epidermal growth factor via phosphatidylinositol 3-kinase signaling pathway. World J Gastroenterol. 2004;10:205-208. [PubMed] |
| 82. | Xue YH, Zhang XF, Dong QZ, Sun J, Dai C, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX. Thrombin is a therapeutic target for metastatic osteopontin-positive hepatocellular carcinoma. Hepatology. 2010;52:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Emani S, Zhang J, Guo L, Guo H, Kuo PC. RNA stability regulates differential expression of the metastasis protein, osteopontin, in hepatocellular cancer. Surgery. 2008;143:803-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Zhang J, Guo H, Mi Z, Gao C, Bhattacharya S, Li J, Kuo PC. EF1A1-actin interactions alter mRNA stability to determine differential osteopontin expression in HepG2 and Hep3B cells. Exp Cell Res. 2009;315:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Bhattacharya SD, Garrison J, Guo H, Mi Z, Markovic J, Kim VM, Kuo PC. Micro-RNA-181a regulates osteopontin-dependent metastatic function in hepatocellular cancer cell lines. Surgery. 2010;148:291-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Jahns F, Wilhelm A, Jablonowski N, Mothes H, Radeva M, Wölfert A, Greulich KO, Glei M. Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis. 2011;32:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Zhao J, Lu B, Xu H, Tong X, Wu G, Zhang X, Liang A, Cong W, Dai J, Wang H. Thirty-kilodalton Tat-interacting protein suppresses tumor metastasis by inhibition of osteopontin transcription in human hepatocellular carcinoma. Hepatology. 2008;48:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |