Published online Aug 7, 2012. doi: 10.3748/wjg.v18.i29.3910
Revised: April 17, 2012
Accepted: April 20, 2012
Published online: August 7, 2012
AIM: To evaluate pretreatment serum carcinoembryonic antigen (CEA) as a predictor of survival for patients with locally advanced gastric cancer receiving perioperative chemotherapy.
METHODS: We retrospectively studied a cohort of 228 gastric cancer patients who underwent D2 gastrectomy combined with chemotherapy at the Sun Yat-sen University Cancer Center between January 2005 and December 2009. Among them, 168 patients received 6-12 cycles of oxaliplatin-based adjuvant (post-operative) chemotherapy, while 60 received perioperative chemotherapy (2 cycles of FOLFOX6 or XELOX before surgery and 4-10 cycles after surgery). Serum CEA was measured using an enzyme immunoassay. The follow-up lasted until December 2010.
RESULTS: In the group that had elevated serum CEA, the difference in survival time between patients receiving perioperative chemotherapy and those receiving adjuvant chemotherapy had no statistical significance (P > 0.05). However, in the group that had normal serum CEA, patients receiving perioperative chemotherapy had a longer survival time. In multivariate analysis, T staging and lymph node metastatic rate were independent prognostic factors for the patients. Perioperative chemotherapy improved the overall survival of patients who had a normal pretreatment CEA level (P = 0.070).
CONCLUSION: Normal pretreatment serum CEA is a predictor of survival for patients receiving perioperative chemotherapy.
- Citation: Chen S, Chen YB, Li YF, Feng XY, Zhou ZW, Yuan XH, Qian CN. Normal carcinoembryonic antigen indicates benefit from perioperative chemotherapy to gastric carcinoma patients. World J Gastroenterol 2012; 18(29): 3910-3916
- URL: https://www.wjgnet.com/1007-9327/full/v18/i29/3910.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i29.3910
Gastric cancer is one of the most common cancers worldwide. It is the second leading cause of cancer deaths in the world[1-3], and most of those patients are diagnosed at an advanced stage of disease[4,5]. Surgery is the main treatment for gastric cancer. Many meta-analyses have demonstrated that adjuvant (post-operative) chemotherapy can improve the prognosis for gastric cancer patients[6-8], and in some prospective clinical trials, adjuvant chemotherapy has improved the prognosis of patients with locally advanced gastric cancer[9-11]. The Cunningham trial showed for the first time that perioperative chemotherapy (treatment both before and after surgery) is superior to surgery alone in treating gastric cancers. Further studies showed that preoperative chemotherapy combined with chemoradiotherapy provided substantial responses that improved the prognosis[12-14].
Carcinoembryonic antigen (CEA) was first identified in 1965 by Gold and Freedman in human colon cancer tissue extracts[15]. In the last two decades, CEA has been widely used as a tumor marker in the diagnosis and monitoring of some malignancies[16]. Since the 1990s, tumor markers including CEA, carbohydrate antigen 19-9, and others have been widely used to monitor gastric cancer progression and even to assess the prognosis of gastric cancer patients, although their specificities have not been satisfactory[17-20]. The controversial conclusions resulting from the use of these biomarkers are therefore understandable[21]. In the present study, we retrospectively evaluated the predictive value of pretreatment serum CEA in patients with late-stage gastric cancer in China.
Inclusion criteria: (1) age: 20 to 75 years; World Health Organization performance status 0 to 1; (2) histologically proven adenocarcinoma of the stomach; T3 or T4 tumor based on endoscopic ultrasound; no evidence of distant metastases or of disease considered nonresectable by endoscopic ultrasonography, computed tomography (CT), or extended diagnostic laparoscopy; (3) no prior gastric surgery; (4) no previous radiotherapy or other treatments, including immunotherapy or Chinese traditional medicine; (5) no uncontrolled infectious or cardiac disease; adequate hepatic and renal functions; and (6) no synchronous or metachronous cancers.
Exclusion criteria: (1) age: older than 75 years or younger than 20 years; (2) hepatic, renal, pulmonary, or cardiac dysfunction; and (3) severe postoperative complications, such as anastomosis leakage or anastomosis stenosis, that may cause malnutrition or make the patients intolerant to postoperative chemotherapy.
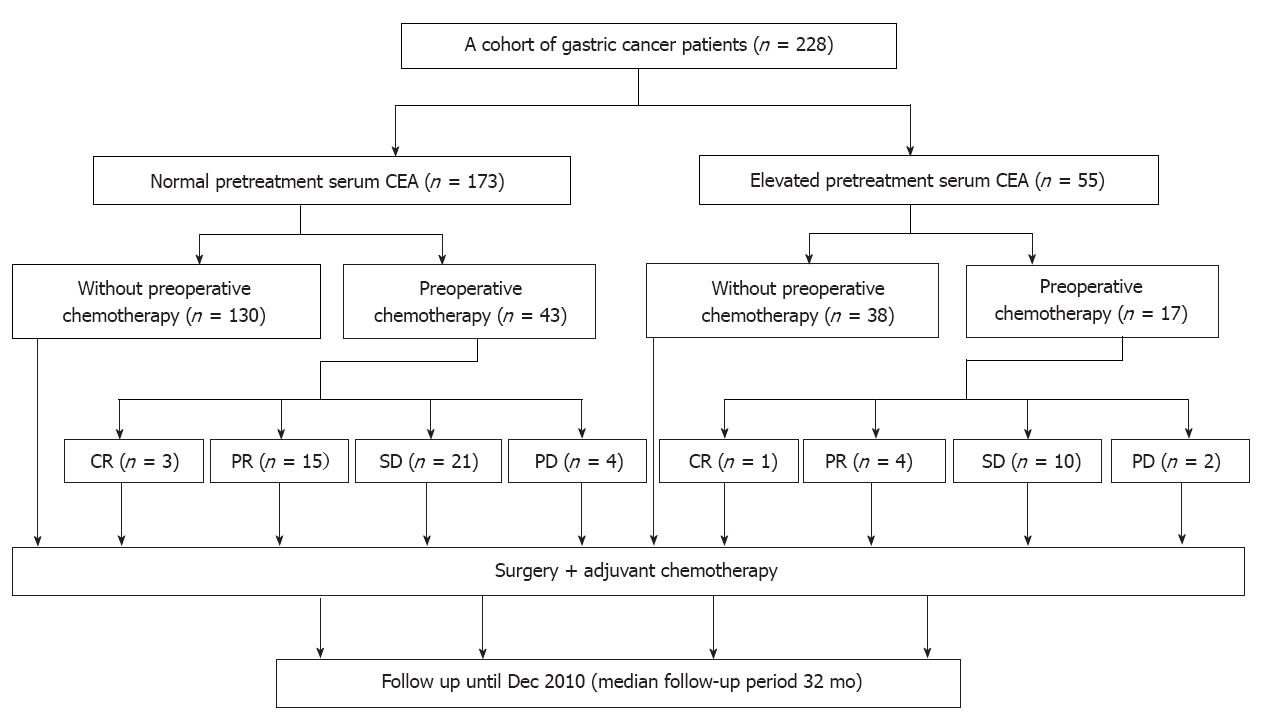
We included 228 patients who underwent D2 gastrectomy at the Sun Yat-sen University Cancer Center between January 2005 and December 2009. Among them, 173 patients had a normal pretreatment serum CEA (≤ 5 ng/mL) and 55 patients had elevated pretreatment serum CEA (> 5 ng/mL). Sixty patients among both CEA groups (43 with normal serum CEA and 17 with elevated serum CEA) received oxaliplatin-based perioperative chemotherapy, with 2 cycles before surgery and 4-10 cycles of the same regimen after surgery. The exception was 6 patients suffering from progressive disease who received second-line chemotherapy for 4-6 cycles (see Treatment section). Among both CEA groups, 168 patients received only adjuvant chemotherapy (Figure 1). The clinicopathological characteristics of all patients are presented in Table 1.
| Normal serum CEA(median 52 yr, range: 22-74 yr) | Elevated serum CEA(median 54 yr, range: 32-73 yr) | ||
| Data | n (%) | n (%) | P |
| Sex | 0.090 | ||
| Male | 116 (67.1) | 44 (80.0) | |
| Female | 57 (32.9) | 11 (20.0) | |
| Tumor location | 0.115 | ||
| Upper | 52 (30.0) | 26 (47.3) | |
| Middle | 42 (24.3) | 12 (21.8) | |
| Lower | 68 (39.3) | 15 (27.3) | |
| Total | 11 (6.4) | 2 (3.6) | |
| Histological grade | 0.128 | ||
| G1 | 1 (0.6) | 0 (0) | |
| G2 | 31 (17.9) | 18 (32.7) | |
| G3 | 112 (64.7) | 29 (52.7) | |
| G4 | 29 (16.8) | 8 (14.6) | |
| Tumor size | 0.053 | ||
| ≤ 2 cm | 23 (13.3) | 1 (1.8) | |
| 2 cm < diameter ≤ 5 cm | 98 (56.6) | 36 (65.5) | |
| > 5 cm | 52 (30.1) | 18 (32.7) | |
| Boarrman type | 0.093 | ||
| I | 3 (1.7) | 0 (0) | |
| II | 90 (52.0) | 20 (36.4) | |
| III | 69 (39.9) | 28 (50.9) | |
| IV | 11 (6.4) | 7 (12.7) | |
| Pathological T staging1 | 0.664 | ||
| T0 | 6 (3.5) | 1 (1.8) | |
| T3 | 156 (90.2) | 49 (89.1) | |
| T4 | 11 (6.4) | 5 (9.1) | |
| Lymph node metastasis rate | 0.951 | ||
| 0 | 12 (6.9) | 3 (5.5) | |
| 0 < r ≤ 0.1 | 29 (16.8) | 8 (14.5) | |
| 0.1 < r ≤ 0.3 | 50 (28.9) | 17 (30.9) | |
| r > 0.3 | 82 (47.4) | 27 (49.1) | |
| Surgery | 0.313 | ||
| Radical | 165 (95.4) | 50 (90.9) | |
| Palliative | 8 (4.6) | 5 (9.1) | |
| Chemotherapy | 0.384 | ||
| Adjuvant | 130 (75.1) | 38 (69.1) | |
| Perioperative | 43 (24.9) | 17 (30.9) |
The two cycles of preoperative chemotherapy included the XELOX and FOLFOX regimes. All the chemotherapy regimens were used under standard protocols. The XELOX regimen consisted of oxaliplatin at 130 mg/m2 (i.v. drip, day 1) and capecitabine at 1000 mg/m2 (oral, day 1-14), followed by one week of no treatment. Starting on day 22, the cycle was repeated, and surgery took place between day 43 and 47.
The FOLFOX6 regimen started on day 1 with oxaliplatin at 100 mg/m2 (i.v. drip) with folic acid at 400 mg/m2 (racemic) or 200 mg/m2 (L-form), plus 5-fluorouracil (5-FU) as a 400 mg/m2 bolus, followed by 2400 mg/m2 of 5-FU as a continuous 46 h infusion. When the infusion was completed, there was no further treatment through day 14. On day 15, the cycle was repeated, with surgery taking place between day 30 and day 33.
After surgery, all of the patients received adjuvant chemotherapy starting within 2-4 wk. The median number of cycles for each regimen was 9 for FOLFOX6 (range: 7-12) and 7 for XELOX (range: 6-8). The six progressive disease patients received paclitaxel plus 5-FU (1 patient), docetaxel plus 5-FU (2 patients), or S-1 oral administration (3 patients) chemotherapy; these patients received a median of 5 cycles (range: 4-6).
Assessment of the response to preoperative chemotherapy was based on the reduction of primary tumor size (as measured by endoscopic ultrasonography and CT scan) and the Response Evaluation Criteria in Solid Tumors criteria.
Complete response: Disappearance of all target lesions. Any pathological lymph nodes (whether target or non-target) must have a reduction of the short axis to less than 10 mm.
Partial response: At least a 30% decrease in the sum of diameters of target lesions, taking as a reference the baseline sum of diameters.
Stable disease: Neither sufficient shrinkage to qualify as a partial response nor sufficient increase to qualify as progressive disease.
Progressive disease: At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum in the study (which may include the baseline sum). The sum must also show an absolute increase of at least 5 mm.
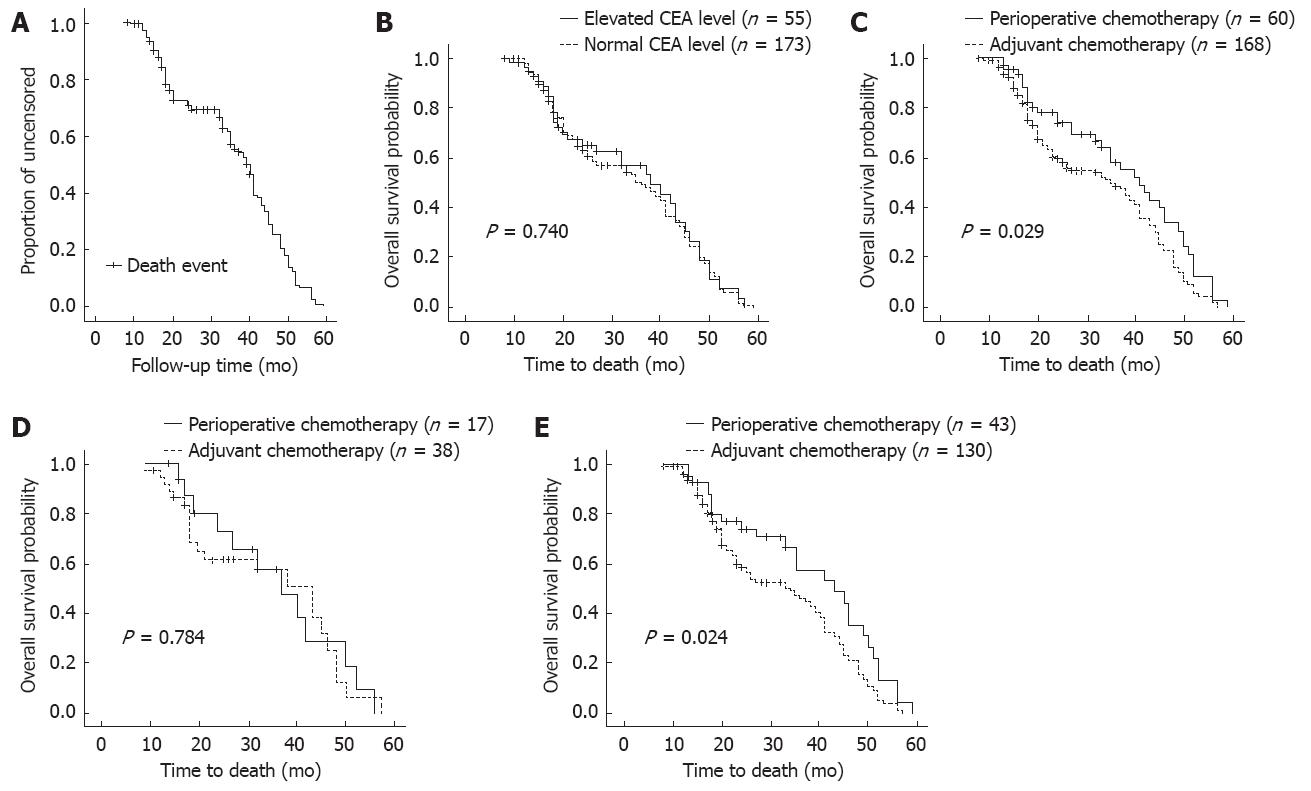
After treatment, the patients were monitored every 3 mo for the first 2 years, then every 6 mo thereafter. Telephone calls and letters were used to assess patients who could not be physically present. Complete data were collected from all 228 patients until December 2010. The follow-up period ranged from 8 mo to 59 mo (median, 32 mo). The total follow-up times are shown in Figure 2A.
The χ2 test was used to compare categorical variables between the normal and elevated serum CEA groups. Univariate survival analysis was performed using the Kaplan-Meier method. Survival curves were compared with the log-rank test. Multivariate statistical survival analysis was performed using Cox regression. Analysis were performed with SPSS software version 16.0 for Windows (SPSS, Inc., Chicago, IL, United States). Statistical significance was defined as P < 0.05.
There was no statistically significant difference in overall survival between the normal CEA group (n = 173) and the elevated CEA group (n = 55). The survival curves are shown in Figure 2B.
The efficacy of preoperative chemotherapy was evaluated. Among these 60 patients, 4 (6.67%) had complete clinical response and 19 (31.7%) had partial clinical response, yielding an overall response rate of 40.0%. The response rates of the patients who had normal pretreatment serum CEA vs those who had elevated CEA were not significantly different, although patients with normal CEA had a higher response rate (complete response + partial response) (41.9% vs 29.4%). These results are shown in Table 2.
| Response to preoperative chemotherapy | ||||
| Pretreatment serum CEA | Complete response | Partial response | Stable disease | Progressive disease |
| Normal | 3 (7.0) | 15 (34.9) | 21 (48.8) | 4 (9.3) |
| Elevated | 1 (5.9) | 4 (23.5) | 10 (58.8) | 2 (11.8) |
The 60 patients who received perioperative chemotherapy (i.e., preoperative plus adjuvant) had a significantly better overall survival rate than the 168 who received only adjuvant chemotherapy, with median survival time of 41 mo for the perioperative group vs 35 mo for the adjuvant group (P = 0.029). The survival curves are shown in Figure 2C. For the patients with elevated serum CEA, there was no significant difference in overall survival rate between perioperative chemotherapy (n = 17) and adjuvant chemotherapy (n = 38, P = 0.784, Figure 2D). For the patients with normal serum CEA, the overall survival rate was significantly better in the perioperative group (n = 43) and the median survival time was 43 mo vs 34 mo for the adjuvant group (n = 130, P = 0.024). The survival curves are shown in Figure 2E.
In univariate analyses, perioperative chemotherapy, T staging, and the lymph node metastasis rate significantly correlated with overall survival (Table 3). In multivariate analysis, T staging and the lymph node metastatic rate were independent prognostic factors. Perioperative chemotherapy improved the overall survival of patients who had normal pretreatment serum CEA (Table 4).
| Variable | No. of patients | 2-yr survival rate (%) | Median survival (mo) | P value |
| All patients | ||||
| Pathological T staging | 0.001 | |||
| T0 | 7 | 86 | 52 | |
| T3 | 205 | 52 | 37 | |
| T4 | 16 | 36 | 27 | |
| Lymph node metastasis | 0.001 | |||
| 0 | 15 | 79 | 48 | |
| 0 < r ≤ 0.1 | 37 | 68 | 31 | |
| 0.1 < r ≤ 0.3 | 67 | 42 | 32 | |
| r > 0.3 | 109 | 50 | 33 | |
| Chemotherapy | 0.029 | |||
| Adjuvant | 168 | 50 | 35 | |
| Perioperative | 60 | 58 | 41 | |
| Normal-CEA patients | ||||
| Pathological T staging | 0.001 | |||
| T0 | 6 | 83 | 50 | |
| T3 | 156 | 50 | 35 | |
| T4 | 11 | 39 | 17 | |
| Lymph node metastasis | 0.002 | |||
| 0 | 12 | 74 | 44 | |
| 0 < r ≤ 0.1 | 29 | 70 | 45 | |
| 0.1 < r ≤ 0.3 | 50 | 38 | 26 | |
| r > 0.3 | 82 | 49 | 33 | |
| Chemotherapy | 0.024 | |||
| Adjuvant | 130 | 48 | 34 | |
| Perioperative | 43 | 59 | 43 |
| Variable | Hazard ratio | 95% CI | P value |
| All patients | |||
| Perioperative chemotherapy | 0.723 | 0.501–1.044 | 0.084 |
| T staging | 1.422 | 1.067–1.896 | 0.016 |
| Lymph node metastasis rate | 1.302 | 1.101–1.539 | 0.002 |
| Normal-CEA patients | |||
| Perioperative chemotherapy | 0.670 | 0.434–1.033 | 0.070 |
| T staging | 1.443 | 1.041–2.000 | 0.028 |
| Lymph node metastasis rate | 1.274 | 1.053–1.542 | 0.013 |
Perioperative chemotherapy, although it is a large physical and psychological burden, has been proven to be effective for some gastric cancer patients. The European Organization for Research and Treatment of Cancer Randomized Trial 40 954 showed no survival benefit from preoperative chemotherapy compared with surgery alone for locally advanced cancer[22]. However, this study had low statistical power; a high number of proximal gastric cancers (which involved the gastroesophageal junction and were different from most of the cases in endemic areas); and an increased R0 resection rate, indicating a better outcome in those patients suffering from early-stage gastric cancer. In China, most gastric cancer patients are diagnosed as having locally advanced disease, suggesting that different treatments should be considered for increasing the survival of the patients. However, there is no reliable marker to determine which patients with advanced gastric cancer can benefit from perioperative chemotherapy. The goal of the present study was to determine whether the pretreatment serum CEA level could be used as a marker to select patients for this aggressive treatment.
Our study revealed that perioperative chemotherapy can improve overall survival in patients with advanced gastric cancer. Dividing the patients in two groups based on their pretreatment serum CEA, we found that perioperative chemotherapy improved the survival rate only for patients with a normal level of pretreatment serum CEA.
Although the biological functions of CEA are not fully known, the close correlation of CEA with cancer aggressiveness has been known for decades. Higher preoperative CEA correlates with more aggressive gastric cancer and a lower patient survival rate[23]. Our findings imply that patients with elevated CEA might have gastric cancers more resistant to chemotherapy, resulting in no survival benefit even from aggressive chemotherapy. CEA has been reported to have roles in homotypic adhesion and cellular aggregation[24], and it cooperates with Myc and Bcl-2 in cellular transformation[25]. In colon cancer, CEA is up-regulated in the microadenoma stage in the colon of patients with APC mutations[26], and CEA plays antiapoptotic and prometastatic roles in colon cancer cells[27]. Overexpression of CEA can protect tumor cells from apoptosis induced by loss of cell contact with the extracellular matrix (anoikis)[28].
Interestingly, those gastric cancer cells expressing alpha-fetoprotein (which is another oncofetal antigen) show P-glycoprotein overexpression and drug resistance in both animal models and human cancer[29,30]. In some case reports, drug-resistant patients always had elevated serum CEA[31,32], and CEA overexpression was observed in multidrug-resistant breast carcinoma cell lines[33]. The CEA promoter (AdCEAIacZ) can increase the IC50 of ganciclovir against gastric cancer cell lines by improving CEA production[34]. All of these pieces of evidence suggest that CEA may induce or promote drug resistance in cancer cells.
This study is retrospective, with its own weaknesses such as confounding factors and low persuasiveness. The cycles of adjuvant chemotherapy were different among the patients, and there were two main chemotherapy regimens: XELOX and FOLFOX6. We believe that the study would be more convincing if there had been a standard regimen, although most investigators report the efficacy of these two regimens as having no statistically significant difference in gastric cancer patients. Thus, more randomized controlled trials are needed to confirm that pretreatment serum CEA can be used as a marker to select patients for the aggressive perioperative treatment or to find other markers for assigning patients to an appropriate treatment.
To our knowledge, there is no strong evidence that CEA is a marker of drug resistance in gastric cancer. More experiments and clinical trials are needed to validate whether CEA levels can predict such drug resistance. In summary, our study showed that only patients with normal pretreatment serum CEA obtained a survival benefit from cytotoxic perioperative chemotherapy. The role of CEA in the drug resistance of gastric cancers warrants further exploration.
We thank David Nadziejka, Grand Rapids, Michigan, for technical editing of the manuscript.
Perioperative chemotherapy has been proven to be effective for some gastric cancer patients. However, there is no reliable marker for determining which patients with advanced gastric cancer can benefit from perioperative chemotherapy.
In the last two decades, carcinoembryonic antigen (CEA) has been widely used as a tumor marker in the diagnosis and monitoring of some malignancies. The research hotspot is to determine whether pretreatment serum CEA can be used as a marker to select patients for this aggressive perioperative treatment.
The study revealed that perioperative chemotherapy can improve overall survival in patients with advanced gastric cancer. After dividing the patients in two groups based on their pretreatment serum CEA, the authors found that perioperative chemotherapy improved the survival rate only for patients with a normal level of pretreatment serum CEA.
The study results suggest that normal pretreatment serum CEA is a predictor of survival benefit for the patients receiving perioperative chemotherapy.
This is an interesting paper aimed to evaluate the role of pretreatment serum CEA level as a predictor of survival for patients with locally advanced gastric cancer receiving neoadjuvant chemotherapy. This retrospective study is well conducted and well written.
Peer reviewers: Dr. Noriko Nakajima, School of Medicine, Nihon University, 1-8-13 Kandasurugadai, Tokyo 1018309, Japan; Dr. Marco Scarpa, Oncological Surgery Unit, Veneto Institute of Oncology, via Gattamelata 64, 35128 Padova, Italy
S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Tsai PJ. Spatial autocorrelation calculations of the nine malignant neoplasms in Taiwan in 2005-2009: a gender comparison study. Chin J Cancer. 2011;30:757-765. [PubMed] |
| 4. | Wang W, Li YF, Sun XW, Chen YB, Li W, Xu DZ, Guan XX, Huang CY, Zhan YQ, Zhou ZW. Prognosis of 980 patients with gastric cancer after surgical resection. Chin J Cancer. 2010;29:923-930. [PubMed] |
| 5. | Wei WQ, Yang CX, Lu SH, Yang J, Li BY, Lian SY, Qiao YL. Cost-benefit analysis of screening for esophageal and gastric cardiac cancer. Chin J Cancer. 2011;30:213-218. [PubMed] |
| 6. | Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441-1447. [PubMed] |
| 7. | Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059-1064. [PubMed] |
| 8. | Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, Arcangeli V, Drudi G, Canuti D, Fochessati F. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21-27. [PubMed] |
| 9. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2438] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 10. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1944] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 11. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4611] [Article Influence: 242.7] [Reference Citation Analysis (0)] |
| 12. | Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 696] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 15. | Staab HJ, Anderer FA, Brümmendorf T, Hornung A, Fischer R. Prognostic value of preoperative serum CEA level compared to clinical staging: II. Stomach cancer. Br J Cancer. 1982;45:718-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ren JQ, Liu JW, Chen ZT, Liu SJ, Huang SJ, Huang Y, Hong JS. Prognostic value of the lymph node ratio in stage III colorectal cancer. Chin J Cancer. 2012;31:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, Dimitriades M, Athanassiou A. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997;20:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ohkura H. Tumor markers in monitoring response to chemotherapy for patients with gastric cancer. Jpn J Clin Oncol. 1999;29:525-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Yamao T, Kai S, Kazami A, Koizumi K, Handa T, Takemoto N, Maruyama M. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn J Clin Oncol. 1999;29:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, Kodaira S, Okajima K, Nakazato H. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Victorzon M, Haglund C, Lundin J, Roberts PJ. A prognostic value of CA 19-9 but not of CEA in patients with gastric cancer. Eur J Surg Oncol. 1995;21:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 23. | Park SH, Ku KB, Chung HY, Yu W. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat. 2008;40:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327-334. [PubMed] |
| 25. | Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997;137:939-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest. 1997;76:703-716. [PubMed] |
| 27. | Wirth T, Soeth E, Czubayko F, Juhl H. Inhibition of endogenous carcinoembryonic antigen (CEA) increases the apoptotic rate of colon cancer cells and inhibits metastatic tumor growth. Clin Exp Metastasis. 2002;19:155-160. [PubMed] |
| 28. | Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419-3424. [PubMed] |
| 29. | Dhar DK, Nagasue N, Yoshimura H, Tachibana M, Tahara H, Matsuura H, Abe S, Chang YC, Nakamura T. Overexpression of P-glycoprotein in untreated AFP-producing gastric carcinoma. J Surg Oncol. 1995;60:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Chang YC, Nagasue N, Kohno H, Ohiwa K, Yamanoi A, Nakamura T. Xenotransplantation of alpha-fetoprotein-producing gastric cancers into nude mice. Characteristics and responses to chemotherapy. Cancer. 1992;69:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Watanabe T, Uchida M, Harada K, Homma N, Ogata N, Funada R, Hasegawa K, Soga K, Shibasaki K. [A case of advanced gastric cancer with obstructive jaundice due to multiple liver metastasis successfully treated with the following combination therapy of CPT-11 and cisplatin after combination therapy of paclitaxel and TS-1]. Gan To Kagaku Ryoho. 2007;34:605-608. [PubMed] |
| 32. | Kimura Y, Imasato M, Yano H, Taniguchi H, Danno K, Kanoh T, Ohnishi T, Tono T, Nakano Y, Monden T. Paclitaxel-resistant recurrent gastric cancer responsive to docetaxel: a case report. Gan To Kagaku Ryoho. 2011;38:643-645. [PubMed] |
| 33. | Ross DD, Gao Y, Yang W, Leszyk J, Shively J, Doyle LA. The 95-kilodalton membrane glycoprotein overexpressed in novel multidrug-resistant breast cancer cells is NCA, the nonspecific cross-reacting antigen of carcinoembryonic antigen. Cancer Res. 1997;57:5460-5464. [PubMed] |
| 34. | Tanaka T, Kanai F, Okabe S, Yoshida Y, Wakimoto H, Hamada H, Shiratori Y, Lan K, Ishitobi M, Omata M. Adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro. Cancer Res. 1996;56:1341-1345. [PubMed] |