Published online Jul 21, 2012. doi: 10.3748/wjg.v18.i27.3565
Revised: March 16, 2012
Accepted: March 20, 2012
Published online: July 21, 2012
AIM: To evaluate the inhibitory effects of carbon dioxide (CO2) insufflation on pneumoperitoneum and bowel distension after percutaneous endoscopic gastrostomy (PEG).
METHODS: A total of 73 consecutive patients who were undergoing PEG were enrolled in our study. After eliminating 13 patients who fitted our exclusion criteria, 60 patients were randomly assigned to either CO2 (30 patients) or air insufflation (30 patients) groups. PEG was performed by pull-through technique after three-point fixation of the gastric wall to the abdominal wall using a gastropexy device. Arterial blood gas analysis was performed immediately before and after the procedure. Abdominal X-ray was performed at 10 min and at 24 h after PEG to assess the extent of bowel distension. Abdominal computed tomography was performed at 24 h after the procedure to detect the presence of pneumoperitoneum. The outcomes of PEG for 7 d post-procedure were also investigated.
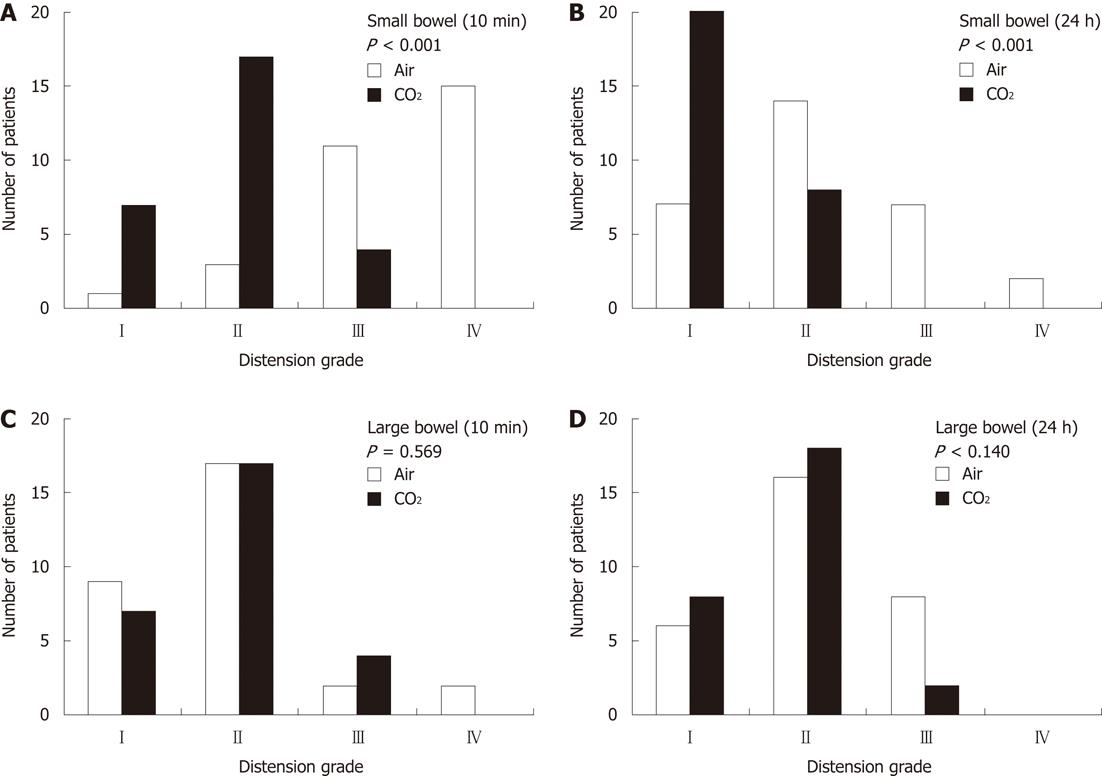
RESULTS: Among 30 patients each for the air and the CO2 groups, PEG could not be conducted in 2 patients of the CO2 group, thus they were excluded. Analyses of the remaining 58 patients showed that the patients’ backgrounds were not significantly different between the two groups. The elevation values of arterial partial pressure of CO2 in the air group and the CO2 group were 2.67 mmHg and 3.32 mmHg, respectively (P = 0.408). The evaluation of bowel distension on abdominal X ray revealed a significant decrease of small bowel distension in the CO2 group compared to the air group (P < 0.001) at 10 min and 24 h after PEG, whereas there was no significant difference in large bowel distension between the two groups. Pneumoperitoneum was observed only in the air group but not in the CO2 group (P = 0.003). There were no obvious differences in the laboratory data and clinical outcomes after PEG between the two groups.
CONCLUSION: There was no adverse event associated with CO2 insufflation. CO2 insufflation is considered to be safer and more comfortable for PEG patients because of the lower incidence of pneumoperitoneum and less distension of the small bowel.
- Citation: Nishiwaki S, Araki H, Hayashi M, Takada J, Iwashita M, Tagami A, Hatakeyama H, Hayashi T, Maeda T, Saito K. Inhibitory effects of carbon dioxide insufflation on pneumoperitoneum and bowel distension after percutaneous endoscopic gastrostomy. World J Gastroenterol 2012; 18(27): 3565-3570
- URL: https://www.wjgnet.com/1007-9327/full/v18/i27/3565.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i27.3565
Percutaneous endoscopic gastrostomy (PEG) has been widely accepted for enteral access since the introduction of the procedure in 1980[1,2]. The procedure of PEG is rapid, but it requires maximal insufflation of the stomach with air in order to tightly attach the gastric wall to the abdominal wall. Percutaneous puncture into the stomach with a needle is conducted under the fully insufflated stomach and a gastrostomy tube is placed thereafter. Abdominal distension and pneumoperitoneum are frequent symptoms after PEG[3]. Carbon dioxide (CO2) insufflation was initially introduced for colonoscopic polypectomy in the field of gastrointestinal endoscopy[4]. Applications of CO2 insufflation for endoscopic procedures have also been reported for the performance of routine colonoscopy, small bowel endoscopy, endoscopic retrograde cholangiopancreatography (ERCP), peroral cholangioscopy, and endoscopic submucosal dissection in the upper and lower gastrointestinal tracts[5-12]. These studies showed that CO2 insufflation reduces the post-procedural abdominal distension and pain without CO2 retention and adverse events. However, there has been no report on the safety and efficacy of CO2 insufflation with PEG procedures. In the present study, we evaluated the inhibitory effects of CO2 insufflation on bowel distension and pneumoperitoneum after PEG by randomized controlled trial. The safety of CO2 insufflation was also investigated.
Consecutive patients who were scheduled for PEG from November 2009 to March 2011 at our institution were recruited for this study. Exclusion criteria included any of the following: chronic obstructive pulmonary diseases (COPD), severe congestive heart failure (cardiothoracic ratio on chest radiography > 60%), previous upper gastrointestinal surgery, for the purpose of decompression via PEG, hypercapnea [arterial partial pressure of CO2 (PCO2) > 50 mmHg], and refusal to participate. Randomization was conducted individually into two treatment groups (1:1) using a computer-generated sequence. Sealed envelopes were used for the allocation of individual patients and opened by assistants at the endoscopy unit just before the procedure of PEG. The present study was approved by the Ethics Committee of our institution, and written informed consent was obtained from the patients or the patients’ family members.
CO2 was administered using a commercially available CO2 regulator system (UCR, Olympus Optical Co., Ltd., Tokyo, Japan). The flow rate of the CO2 insufflation was 1.5 L/min using the wide size of the connective tube, the volume of which was equivalent to the medium strength setting of the air insufflation system. Endoscopy assistants set up the insufflation system according to the allocation of the individual patients.
Conscious sedation was conducted by the intravenous administration of midazolam, the amount of which was previously determined by their primary doctor depending on the condition of the patient. An endoscope (GIF-H180, Olympus Optical Co., Ltd., Tokyo, Japan) was inserted up to the second portion of the duodenum to screen the upper gastrointestinal tract and pulled back to the stomach. Then, the stomach was fully inflated with air or CO2 and the site for placement was determined by transillumination of the abdominal wall and finger pressure against the stomach. The abdominal skin surface of the placement area was cleansed with povidone-iodine and local anesthesia was performed by administering 1% lidocaine. After test-puncturing with a 21-gauge needle, 3-point sutures were made using a gastropexy device (Easy Tie, Boston Scientific Japan K.K., Tokyo, Japan) to fix the stomach against the abdominal wall in order to form tight fistula formation. A Seldinger needle was then punctured at the center of the 3-point sutures and a loop wire was inserted through the outer sheath of the needle. The loop wire was grasped by a snare from the endoscope, and a 20F gastrostomy tube was placed by the pull-through technique. The materials used for PEG tube placement were a Ponsky PEG (Bard Access Systems, Inc., Salt Lake City, UT, United States) or a Safety PEG kit (Boston Scientific Co., Natick, MA, United States). The endoscope was reinserted into the stomach to secure the proper placement of the gastrostomy tube. Arterial blood gas analysis was conducted just before and immediately after the PEG procedure to measure PCO2, arterial partial pressure of O2 (PO2), pH and base excess.
The clinical pathway after PEG was determined as follows. Prophylactic intravenous administration of cefmetazole was conducted for 3 d following PEG. The gastrostomy tube was drained for 24 h and feeding was then started in those patients without serious events. Initial feeding was 100 mL of 5% glucose solution followed by 500 mL of glucose solution on the next day. Commercially available isotonic nutrients were administered from the third day and gradually increased up to 800 kcal/d for 7 d. Target calorie intake was achieved within 2 wk.
Abdominal computed tomography (CT) was conducted 24 h after PEG to detect pneumoperitoneum. In patients with pneumoperitoneum, the volume of the intraabdominal free air was estimated by CT scan. Abdominal plain radiography was conducted at 10 min and 24 h after PEG to evaluate the bowel distension. The degree of bowel distension was determined by scoring the radiographic images according to the method of Bretthauer et al[8]. In brief, grade I: No distension; grade II: Light distension; grade III: Moderate distension; and grade IV: Severe distension. The radiological estimation was conducted by a physician (Iwashita M) who had not been informed of the insufflation condition.
Evaluation of clinical symptoms after PEG was assessed by the frequency of body temperature elevation (> 38 °C), complications, and whether the scheduled clinical pathway had been pursued for 7 d after PEG. Blood examination [C-reactive protein (CRP) and leucocyte count] was conducted before, one day and 7 d after PEG. The treatments of the patients who had undergone PEG were conducted by individual primary doctors who were not informed of the patient’s allocation. Each patient was followed-up along the clinical pathway if their condition was stable. When complications or troubles occurred for the patients, treatments other than the scheduled clinical pathway were conducted by the primary attending doctor.
The continuous values were expressed as mean ± SD and analyzed using the Student’s t-test. The analysis of dichotomous categorical variables was performed using the χ2 test. The analysis of the grades of bowel distension was conducted by Fisher’s exact test. Statistical significance was defined as a P value of less than 0.05. All data were analyzed using JMP software for Windows (Version 5.1; SAS Institute Inc., Cary, NC, United States).
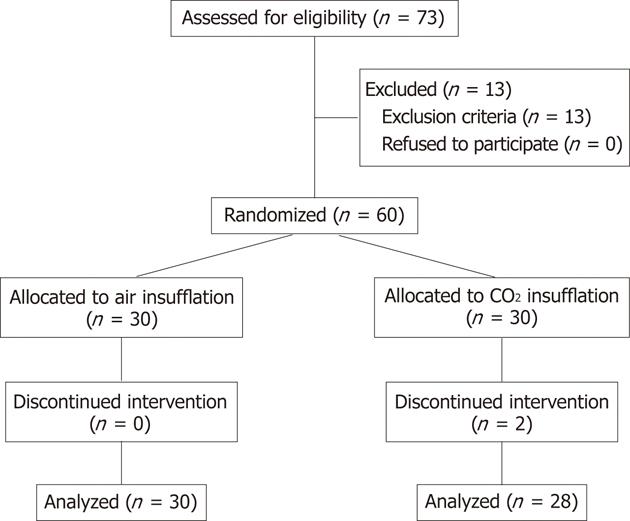
A flow diagram of this study is presented in Figure 1. A total of 73 patients were enrolled for this study. Thirteen patients were excluded by the exclusion criteria; 6 patients for COPD, 3 patients for hypercapnea, 2 patients for severe congestive heart failure, and one each for post distal gastrectomy and for the purpose of decompression. The remaining 60 patients were equally randomized and underwent PEG with air or CO2 insufflation. All of the PEG procedures in the air group were successfully performed, whereas those in the CO2 group were not completed in two patients using the above-described PEG procedure. One of the reasons for failure was inability to insert an endoscope into the stomach because of benign esophageal stenosis. The other reason was failure of gastropexy, although the PEG was performed without gastropexy. These two patients were excluded from the analyses.
The demographic data of the patients are shown in Table 1. The mean age and male/female ratio were not significantly different in the two groups. The serum albumin concentration, an indicator of nutritional status, did not differ significantly.
| Air group (n = 30) | CO2 group (n = 28) | P value | |
| Age (mean ± SD, yr) | 81.9 ± 8.8 | 82.3 ± 9.5 | 0.873 |
| Gender (male/female) | 8/22 | 5/23 | 0.421 |
| Underlying diseases | |||
| Cerebrovascular disease | 20 | 16 | |
| Dementia | 5 | 7 | |
| Neuromuscular disease | 2 | 1 | |
| Pneumonia | 1 | 4 | |
| Malignant tumor | 1 | 0 | |
| Cardiac disease | 1 | 0 | |
| Albumin (mean ± SD, g/dL) | 3.12 ± 0.46 | 3.05 ± 0.69 | 0.645 |
There were no significant differences in the amount of midazolam administered and the duration of the procedure between the two groups. The mean elevations of PCO2 in the air and CO2 groups were 2.67 mmHg and 3.32 mmHg, respectively (P = 0.408). The mean depressions of PO2 in the air and CO2 groups were 3.72 mmHg and 1.34 mmHg, respectively (P = 0.302). The mean pH after PEG of the CO2 group was significantly lower than that of the air group (P = 0.018), whereas the depression of pH during the procedure was not significantly different between the two groups (P = 0.125). There were no significant differences in base excess between the two groups (data not shown) (Table 2).
| Air group | CO2 group | P value | |
| Amount of midazolam (mg) | 2.53 ± 1.14 | 2.53 ± 1.45 | 0.995 |
| Duration of procedure (s) | 835 ± 145 | 889 ± 128 | 0.143 |
| Arterial blood gas analysis | |||
| PCO2 (mmHg) | |||
| Before procedure | 38.9 ± 4.9 | 38.6 ± 4.4 | 0.837 |
| After procedure | 41.6 ± 5.6 | 41.9 ± 4.8 | 0.778 |
| Elevation during procedure | 2.67 ± 2.82 | 3.32 ± 3.14 | 0.408 |
| PO2 (mmHg) | |||
| Before procedure | 83.0 ± 11.8 | 84.4 ± 9.3 | 0.612 |
| After procedure | 79.3 ± 10.6 | 83.1 ± 9.8 | 0.213 |
| Depression during procedure | 3.72 ± 8.95 | 1.34 ± 7.07 | 0.302 |
| pH | |||
| Before procedure | 7.479 ± 0.035 | 7.471 ± 0.023 | 0.295 |
| After procedure | 7.454 ± 0.031 | 7.435 ± 0.031 | 0.018 |
| Depression during procedure | 0.025 ± 0.031 | 0.036 ± 0.027 | 0.125 |
The most frequent distension grades of the small bowel at 10 min in the air and CO2 groups were grade IV and grade II, respectively, and those at 24 h were grade II and grade I, respectively (Figure 2) (P < 0.001). The most frequent distension grades of the large bowel in the air and CO2 groups were grade II at both 10 min and 24 h in the two groups (Figure 2).
Pneumoperitoneum was observed in 8 patients of the air group, whereas there was no patient with pneumoperitoneum in the CO2 group by abdominal CT (P = 0.003). The mean volume of the free air of the 8 patients in the air group was 36.3 mL. In addition to the 8 patients, faint extragastric air leakage around the stoma was observed in 5 patients of the air group. No such transluminal air leak was observed in any patient of the CO2 group.
The values of CRP levels and leukocyte counts were not different between the two groups at any time. The rates of the patients with fever of more than 38 °C in the air and CO2 groups were 33.3% and 17.9%, respectively (P = 0.179). The numbers of patients who did not follow the clinical pathway during the first 7 d post-procedure were 4 each for the air and CO2 groups (13.3% and 14.2%, respectively). The reasons for the discontinuation of the pathway were aspiration after feeding (3 and 2 patients for the air and CO2 groups, respectively), stomal infection (1 patient each for the two groups), and hemorrhage in 1 patient in the CO2 group. Among these complications, 1 patient in the air group and 3 patients in the CO2 group were interrupted for feeding until the complications recovered. There was no mortality within 30 d in this study (Table 3).
| Air group | CO2 group | P value | |
| CRP (mg/dL) | |||
| Before PEG | 1.58 ± 1.63 | 1.51 ± 2.71 | 0.906 |
| 1 d after PEG | 2.92 ± 3.23 | 1.66 ± 1.58 | 0.068 |
| 7 d after PEG | 2.12 ± 3.23 | 1.14 ± 1.75 | 0.158 |
| Leucocytes (/μL) | |||
| Before PEG | 6330 ± 1990 | 7330 ± 2210 | 0.077 |
| 1 d after PEG | 7760 ± 3230 | 8570 ± 2110 | 0.269 |
| 7 d after PEG | 6690 ± 2150 | 7410 ± 2400 | 0.238 |
| Fever more than 38 °C, n (%) | 10 (33.3) | 5 (17.9) | 0.179 |
| Discontinued clinical pathway, n (%) | 4 (13.3) | 4 (14.2) | 0.916 |
The present study is the first investigation of the effects of CO2 insufflation during PEG. Our results clearly indicate that the use of CO2 insufflation reduces the postprocedural abdominal distension and pneumoperitoneum compared to air insufflation. The usefulness and safety of CO2 insufflation for colonoscopy, ERCP and double balloon enteroscopy have been reported, including effects on the respective therapeutic procedures[5-12]. PEG is also an endoscopic operation, but the procedure is simple and of relatively short duration compared to the above-mentioned endoscopic procedures. However, the PEG procedure requires maximal insufflation of the stomach and penetration of the gastric wall by a needle and a gastrostomy tube, leading to postprocedural abdominal distension and pneumoperitoneum.
Pneumoperitoneum associated with PEG procedure is thought to be due to air leakage around the needle puncture site of the stomach during the period from the puncture to the placement of a gastrostomy tube[13]. The reported frequencies of pneumoperitoneum range from 8.6% to 56%[14-16]. The variation of the frequency may depend on multiple factors. Abdominal CT scan is a more sensitive modality to detect intraabdominal free air compared to plain radiography. The methods or devices used for the PEG procedure may also affect the frequency of pneumoperitoneum. The performance of gastropexy may have a preventive effect on pneumoperitoneum. We introduced the gastropexy technique in the present study using a T-fastener type fixation device just before the percutaneous needle puncture[17]. Our intention with gastropexy is to prevent peritonitis if the gastrostomy tube dislodges in the early phase after PEG. In the present study, the frequency of pneumoperitoneum in the air group was 27%, the value of which was comparable to the previous reports. However, the amount of the free air was fairly small (36.3 mL on average), which would be difficult to detect by plain abdominal radiography. Although there are no reports of the preventive effects on pneumoperitoneum by the gastropexy, the procedure is supposed to reduce the amount of the free air.
Most cases of pneumoperitoneum are considered to have no clinical significance and to require no further interventions[13,14,16]. Pneumoperitoneum after PEG usually causes no symptoms and spontaneously recovers. However, a few patients with pneumoperitoneum showed severe symptoms and underwent laparotomy in larger scale analyses. Dulabon et al[15] reported that 20% of patients with pneumoperitoneum developed peritonitis which required exploratory celiotomy. Blum et al[18] retrospectively analyzed 722 patients who had undergone PEG, and reported that pneumoperitoneum was observed in 5 out of 6 patients who had complications requiring laparotomy. They postulated that the presence of intraabdominal free fluid in addition to the free air is an indication of peritonitis requiring surgical intervention.
The inhibitory effect of CO2 insufflation on pneumoperitoneum or pneumomediastinum at the perforation of endoscopic submucosal dissection was reported by Nonaka et al[11]. They showed 3 cases of perforation with endoscopic submucosal dissection (2 cases with esophageal cancer and 1 case with gastric cancer) using CO2 insufflation but no subcutaneous or mediastinal emphysema or pneumoperitoneum developed after perforation.
CO2 insufflation has also been reported to reduce post-procedural abdominal pain as well as abdominal distension during colonoscopy and double balloon enteroscopy[5-7]. The reduction of abdominal symptoms by CO2 insufflation was also reported for ERCP[8,9], although the effects remain controversial[19]. Although the mean duration of the PEG procedure was only about 14 min in the present study, a significant reduction in small bowel distension was observed in the CO2 group compared to the air group. The evaluation of the bowel distension was analyzed by radiography at 10 min and 24 h after PEG. The result of remarkable reduction of small bowel distension at only 10 min after the procedure was consistent with the report of Nakajima et al[20]. Almost all of the patients in our study were unable to express their symptoms due to their underlying diseases and we could not use a visual analog scale for their abdominal pain and distension, but CO2 insufflation may reduce the abdominal symptoms after PEG.
There were no significant differences in clinical outcomes after PEG between the two groups. These results indicate that the reduction of pneumoperitoneum and bowel distension did not affect the outcomes after PEG. We experienced 8 out of 58 patients with complications, including 5 cases of aspiration, 2 cases of peristomal infection and 1 case of hemorrhage in this study. These complications were not derived from the CO2 or air insufflation itself.
The elevation of PCO2 using CO2 insufflation in this study was very low and the degree of elevation was comparable to that of air insufflation. These results indicate that the elevation of PCO2 is not derived from CO2 insufflation itself but derived from conscious sedation by midazolam. This assumption is consistent with previous reports[8,11,21,22]. Because the PEG procedure is of short duration, strict continuous monitoring of CO2 status by the measurement of transcutaneous PCO2 or partial pressure of end-tidal CO2 would not be necessary. Suzuki et al[23] reported that the PCO2 level increased with the duration of CO2 insufflation for endoscopic submucosal dissection under general anesthesia. They also found that patients with lower respiratory function showed a tendency toward CO2 retention compared to patients with normal respiratory function. Our study excluded patients with COPD and severe chronic heart failure, but the target patients for PEG are usually elderly and often have impaired cardiorespiratory function. Further investigation of the safety of CO2 insufflation for elderly patients will be required. In conclusion, CO2 insufflation during the PEG procedure is considered to be safe and provides comfort by reducing pneumoperitoneum and bowel distension.
Carbon dioxide (CO2) insufflation has been reported to reduce the post-procedural abdominal distension and pain in gastrointestinal endoscopy for diagnostic and therapeutic purposes. However, there has been no report on the safety and efficacy of CO2 insufflation for percutaneous endoscopic gastrostomy (PEG) procedures.
Utilizing the nature that CO2 is rapidly absorbed from the bowel, the authors investigated the effect of CO2 insufflation on patients undergoing PEG.
CO2 insufflation remarkably reduced pneumoperitoneum and small bowel distension without any adverse events.
Safety of CO2 insufflation during PEG procedure for elderly patients was demonstrated in this report. Further investigation in patients with cardiopulmonary disorders is necessary.
This is the first randomized control study of CO2 insufflation in PEG procedure and describes the effects of CO2 insufflation on pneumoperitoneum and bowel distension after PEG. The presented article is a major contribution in the research field.
Peer reviewer: Dr. Manuela Cesaretti, Department of Surgical and Diagnostic Integrated Science, University of Genoa, Ospedale San Martino, Largo Rosanna Benzi, 16100 Genova, Italy
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1299] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 2. | Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. 1980. Nutrition. 1998;14:736-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 287] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, Martin ND, Hoey BA, Stawicki SP. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007;16:407-418. [PubMed] |
| 4. | Rogers BHG. The safety of carbon dioxide insufflation during colonoscopic electrosurgical polypectomy. Gastrointest Endosc. 1974;20:115-117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Hussein AM, Bartram CI, Williams CB. Carbon dioxide insufflation for more comfortable colonoscopy. Gastrointest Endosc. 1984;30:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Stevenson GW, Wilson JA, Wilkinson J, Norman G, Goodacre RL. Pain following colonoscopy: elimination with carbon dioxide. Gastrointest Endosc. 1992;38:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Domagk D, Bretthauer M, Lenz P, Aabakken L, Ullerich H, Maaser C, Domschke W, Kucharzik T. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Maple JT, Keswani RN, Hovis RM, Saddedin EZ, Jonnalagadda S, Azar RR, Hagen C, Thompson DM, Waldbaum L, Edmundowicz SA. Carbon dioxide insufflation during ERCP for reduction of postprocedure pain: a randomized, double-blind, controlled trial. Gastrointest Endosc. 2009;70:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ueki T, Mizuno M, Ota S, Ogawa T, Matsushita H, Uchida D, Numata N, Ueda A, Morimoto Y, Kominami Y. Carbon dioxide insufflation is useful for obtaining clear images of the bile duct during peroral cholangioscopy (with video). Gastrointest Endosc. 2010;71:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Gottfried EB, Plumser AB, Clair MR. Pneumoperitoneum following percutaneous endoscopic gastrostomy. A prospective study. Gastrointest Endosc. 1986;32:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Wojtowycz MM, Arata JA, Micklos TJ, Miller FJ. CT findings after uncomplicated percutaneous gastrostomy. AJR Am J Roentgenol. 1988;151:307-309. [PubMed] |
| 15. | Dulabon GR, Abrams JE, Rutherford EJ. The incidence and significance of free air after percutaneous endoscopic gastrostomy. Am Surg. 2002;68:590-593. [PubMed] |
| 16. | Wiesen AJ, Sideridis K, Fernandes A, Hines J, Indaram A, Weinstein L, Davidoff S, Bank S. True incidence and clinical significance of pneumoperitoneum after PEG placement: a prospective study. Gastrointest Endosc. 2006;64:886-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Rogers BH, Kaminski MV, All J. Stabilizing sutures for percutaneous endoscopic gastrostomy. Gastrointest Endosc. 1989;35:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Blum CA, Selander C, Ruddy JM, Leon S. The incidence and clinical significance of pneumoperitoneum after percutaneous endoscopic gastrostomy: a review of 722 cases. Am Surg. 2009;75:39-43. [PubMed] |
| 19. | Dellon ES, Velayudham A, Clarke BW, Isaacs KL, Gangarosa LM, Galanko JA, Grimm IS. A randomized, controlled, double-blind trial of air insufflation versus carbon dioxide insufflation during ERCP. Gastrointest Endosc. 2010;72:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Nakajima K, Lee SW, Sonoda T, Milsom JW. Intraoperative carbon dioxide colonoscopy: a safe insufflation alternative for locating colonic lesions during laparoscopic surgery. Surg Endosc. 2005;19:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Nelson DB, Freeman ML, Silvis SE, Cass OW, Yakshe PN, Vennes J, Stahnke LL, Herman M, Hodges J. A randomized, controlled trial of transcutaneous carbon dioxide monitoring during ERCP. Gastrointest Endosc. 2000;51:288-295. [PubMed] |
| 22. | Kikuchi T, Fu KI, Saito Y, Uraoka T, Fukuzawa M, Fukunaga S, Sakamoto T, Nakajima T, Matsuda T. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc. 2010;24:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Suzuki T, Minami H, Komatsu T, Masusda R, Kobayashi Y, Sakamoto A, Sato Y, Inoue H, Serada K. Prolonged carbon dioxide insufflation under general anesthesia for endoscopic submucosal dissection. Endoscopy. 2010;42:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |