Published online Jul 21, 2012. doi: 10.3748/wjg.v18.i27.3520
Revised: September 9, 2011
Accepted: May 12, 2012
Published online: July 21, 2012
AIM: To investigate the effect of E3-methyl-1-phenyl-2-pyrazolin-5-one (Edr) on hepatic ischemia-reperfusion (I/R) injury and liver regeneration in a porcine hepatectomy model.
METHODS: One hour ischemia was induced by occluding the vessels and the bile duct of the right and median lobes. A 40% left hepatectomy was performed after reperfusion. Six animals received Edr (3 mg/kg per hour) intravenously and six control animals received saline just before reperfusion. Remnant liver volume, hemodynamics, aspartate aminotransferase (AST), alanine aminotransferase, lactate dehydrogenase and lactic acid, were compared between the groups. The expression of transforming growth factor-β (TGF-β1) and toll-like receptor (TRL) mRNA in hepatic tissues was examined using reverse transcription polymerase chain reaction. Apoptosis was demonstrated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, respectively.
RESULTS: Serum AST (P = 0.029), and toll like receptor 4 level (P = 0.043) were significantly lower after 3 h in animals receiving Edr. In addition, TUNEL staining in Edr-treated pigs showed significantly fewer hepatocytes undergoing apoptosis compared with control pigs. After 1 mo, all factors were non-significantly different between the two groups.
CONCLUSION: Edr is considered to reduce hepatic injury in the early stage of I/R injury in a porcine model.
- Citation: Shimoda M, Iwasaki Y, Okada T, Kubota K. Edaravone inhibits apoptosis caused by ischemia/reperfusion injury in a porcine hepatectomy model. World J Gastroenterol 2012; 18(27): 3520-3526
- URL: https://www.wjgnet.com/1007-9327/full/v18/i27/3520.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i27.3520
A potent free radical scavenger, E3-methyl-1-phenyl-2-pyrazolin-5-one (Edr) has been shown to protect cardiomyocytes and brain against ischemia/reperfusion (I/R) injury[1,2]. However, this beneficial protection after I/R injury in the liver, and the effect on liver regeneration is still unknown[3].
Compression of the hepatoduodenal ligament is routinely used during partial hepatectomy for control of bleeding, but this can lead to postoperative I/R injury that may adversely affect outcome[4]. Hepatic I/R damage is caused by multiple factors, including ischemia-induced hypothermia, cytokines, coagulopathy and increased levels of cell adhesion molecules. Expression of transforming growth factor-β (TGF-β) has been demonstrated in a wide variety of diseases and in normal cells and tissues, and is known to be most abundant in platelets and bone[5]. TGF-β1 has inhibitory effects on cell growth, and acts in a stimulatory manner in fibrosis and certain mesenchymal cells[6]. It is particularly germane in the liver, as TGF-β1 is a potent inducer of apoptosis in hepatocytes, and inhibits liver cell proliferation in vitro[6]. It also has a crucial role in terminating liver regeneration after partial hepatectomy in rats[7].
Furthermore, Kupffer cells play a prominent role in I/R injury in the liver as this type of injury induces the release of high mobility group box 1 (HMGB1) from damaged liver cells, which then stimulate nonparenchymal cells, such as Kupffer cells through toll like receptor 4 (TLR4)[8]. In addition, TLR4 triggers the secretion of tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 by Kupffer cells, resulting in liver regeneration[8].
To explore the effect of Edr in liver I/R injury and to extend the application of Edr clinically, experiments using large animal models are essential. We have attempted to define the effects of Edr after I/R injury in a porcine liver resection model.
In this study, we investigated the effect of Edr on hepatic I/R injury and liver regeneration, and found that Edr significantly inhibits apoptosis in liver.
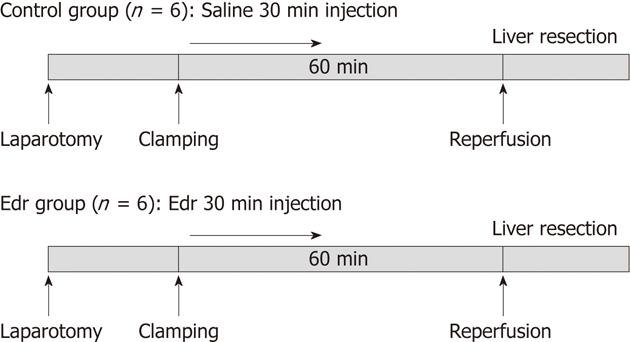
The study was performed using male pigs, weighing 23-26 kg (SEASCO, Saitama, Japan), in accordance with the Guidelines for the Care and Use of Laboratory Animals, Dokkyo Medical University. Two groups of animals were prepared: Edr group (n = 6) in which Edr (supplied by Mitsubishi-Tanabe Pharma Co., Ltd., Osaka, Japan) was administered intravenously at a dose of 3 mg/kg per hour from the commencement of clamping for 30 min, and a control group (n = 6) which received physiological saline administered intravenously for the same period. The Edr dose was the same as that used in the treatment of human brain infarction[9] (Figure 1).
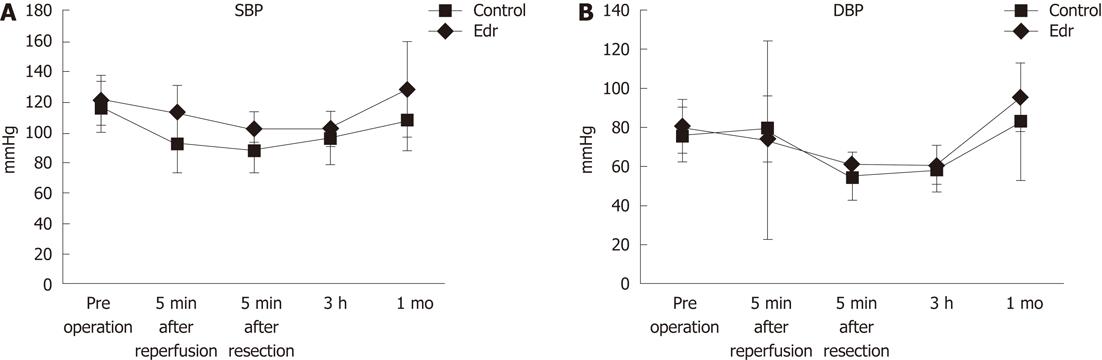
A chevron incision was made under general anesthesia, and each branch of the portal vein, hepatic artery and bile duct were carefully isolated and taped. Hemi-hepatic (approximately 60%) liver ischemia was induced by clamping the right and middle hepatic vessels and bile duct using vessel tapes and maintained for 60 min; the left portal branch was kept patent to avoid bowel congestion, and the left lobe played a role in the bypass. After declamping, the left portal vein and artery were ligated, and a left hemi-hepatectomy was performed (approximately 40%). Liver transection was achieved by the crush-clamping method using Pean forceps. During the liver transection, the exposed Glisson’s vessels were ligated with 2-0 or 3-0 silk. The hepatic vein was closed by continuous sutures using 4-0 proline. The influence of I/R injury was assumed to be on the right lobe only. During this procedure, hemodynamic parameters (systolic and diastolic arterial pressure) were monitored by a femoral arterial line. All pigs received Ringer’s solution during the procedure. After the operation, the pigs were transferred to the Dokkyo Medical University animal center and unlimited oral intake was allowed after the first postoperative day. No antibiotics were administered either orally or intravenously after surgery.
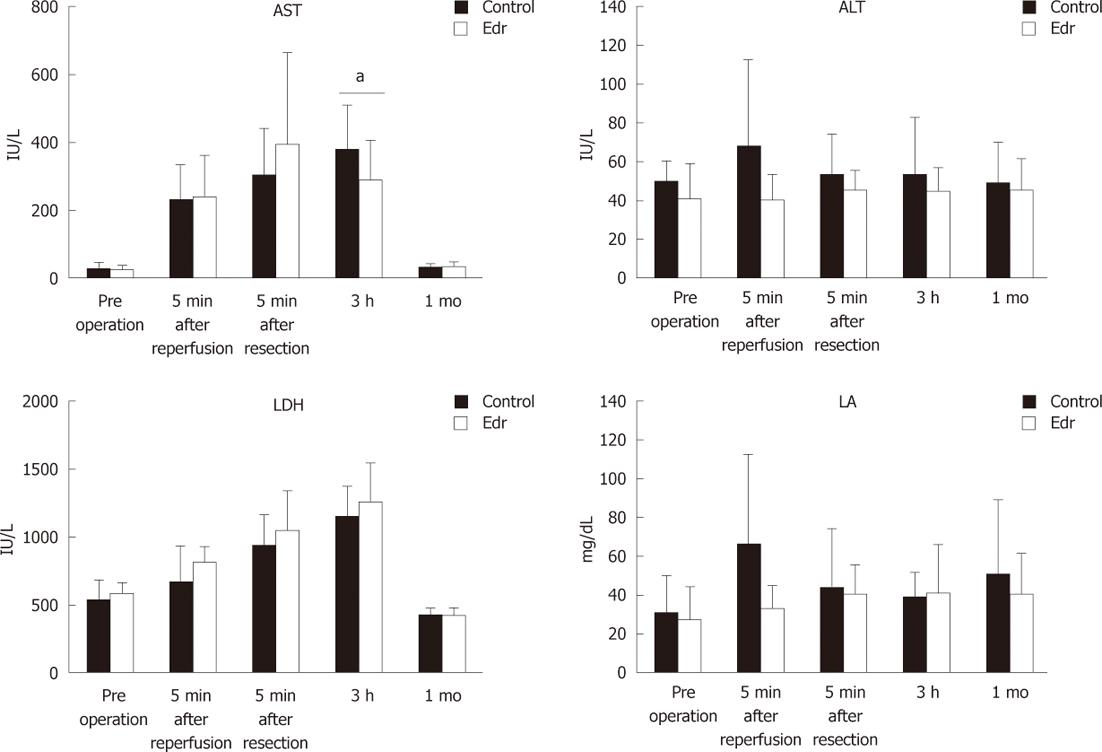
Blood samples were obtained from the arterial line immediately after laparotomy, 5 and 180 min after reperfusion, and after 1 mo. The serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and lactic acid (LA) were evaluated. Hepatic tissues were obtained from the right lobe at laparotomy, after reperfusion, and after 1 mo, and were subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, and TLR4 and TGF-β1 mRNA were determined. Serum AST, ALT, LDH and LA were measured using standard clinical methods for automated analysis (Model 7170, Hitachi, Inc., Tokyo, Japan).
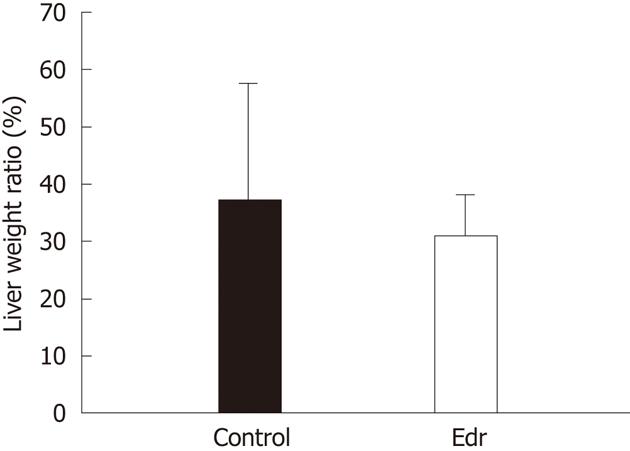
Experimental pigs were sacrificed 1 mo after operation and remnant liver was obtained. Total liver weight was calculated based on 30 pigs. We calculated the average percentage of total liver weight to body weight (2.64%), estimated the total liver weight from pre-operative body weight, and calculated the estimated remnant liver weight 1 mo after operation by subtracting the resected liver weight at the time of operation from the estimated total liver weight. Liver weight increasing ratio (%) was calculated as follows; estimated remnant liver weight at resection/remnant liver weight after 1 wk × 100.
At laparotomy, 30 mg of surgical tissue was stored in liquid nitrogen and kept at -80 °C until extraction of total RNA using a Nucleospin II kit (Macherey-Nagel, Germany). Reverse transcription reactions were performed using a SuperScript III First-strand Synthesis System for t-polymerase chain reaction (PCR) (Invitrogen, Carlsbad, CA, United States). Briefly, 1 μg of total RNA, oligo dT primer, and dNTPs were incubated at 65 °C for 5 min, and then 10 μL of cDNA synthesis mixture was added and incubated at 50 °C for 50 min. The reaction was terminated by adding 1 μL of RNase H and incubated at 37 °C for 20 min. Real-time PCR was performed using a ABI Prism 7700 sequence detector (Applied Biosystems, Warrington, United Kingdom). The PCR reaction was carried out in a final volume of 1 μL cDNA, 2 μL 10 × SYBR Green (Applied Biosystems), using 40 cycles at 95 °C for 30 s and 60 °C for 30 s. The specific primers were designed using Primer 3 software (http://frodo.wi.edu/cgi-bin/primer3/primer3_http://www.cgi) and synthesized by Sigma Genosys (Hokkaido, Japan). The sequences of each primer were as follows: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): sense 5'-CCACCCAGAAGACTGTGGAT-3', anti-sense 5'-TTCAGCTCAGGGATGACCTT-3'; TLR4 and TGF-β1: sense 5'-CCCCTGTCCATCCCTTTATT-3', anti-sense 5'-AAGCCCCAGTTCCAATTCTT-3'. For each PCR run a standard curve was constructed from serial dilutions of cDNA from the PANCI cell line. The level of expression of TLR4 and TGF-β1 were calculated using the formula: relative expression (t) = [(copy number of TLR4 + TGF-β1 number/copy number of GAPDH)] × 1000. For non-template reactions and standard cDNA dilutions from PANCI cells, liver samples were assayed in triplicate. The average and standard deviation were calculated and the t-value was determined from the averages.
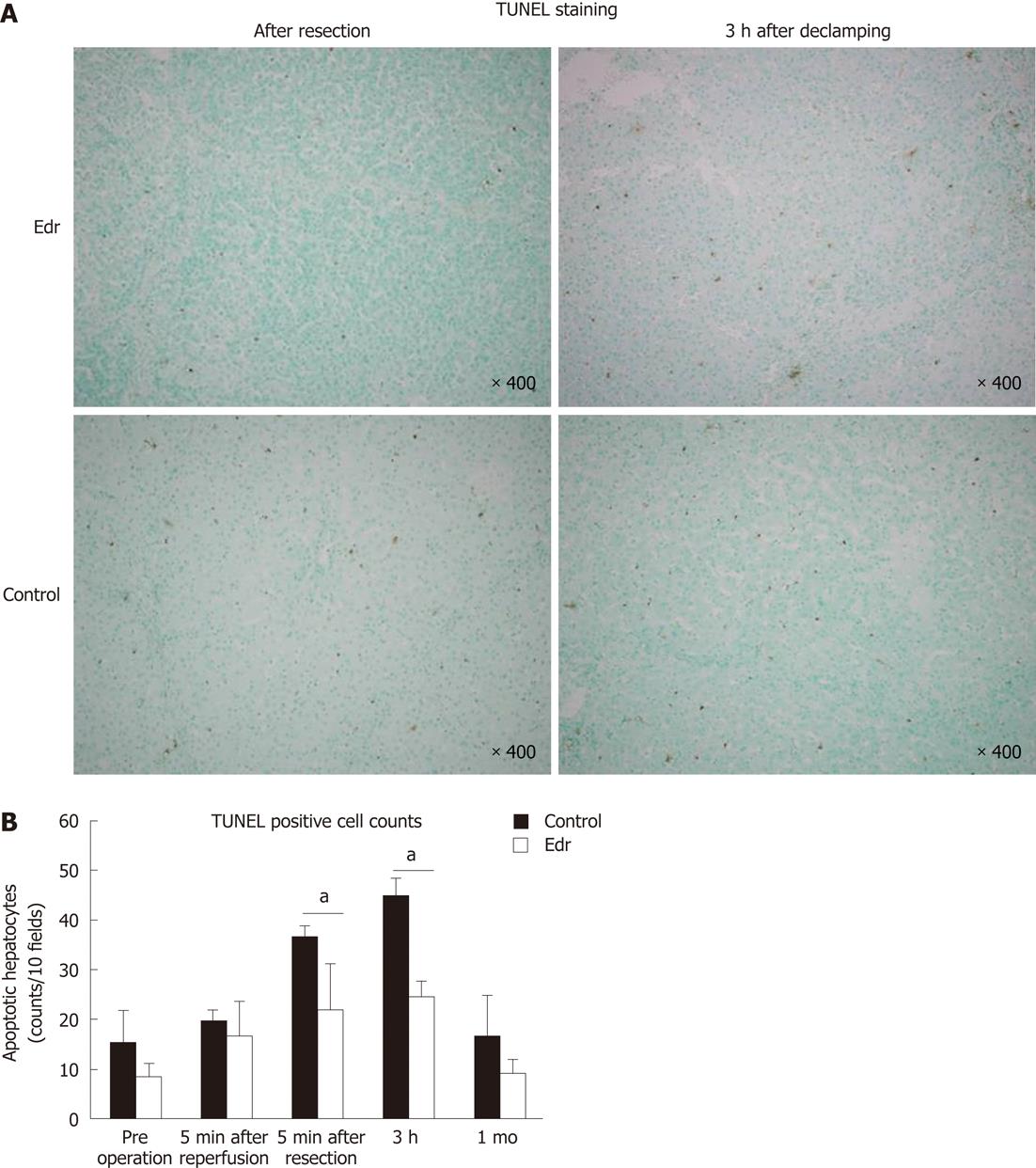
Tissue samples were obtained at the time of laparotomy, the remnant liver just after hepatectomy, 3 h after declamping of the right and middle hepatic arteries and portal vein, and after 1 mo, and were fixed with 10% formalin for 24 h and embedded in paraffin. Sections 3-μm thick were stained by the in situ terminal TUNEL method using an apoptosis in situ detection kit (Wako Pure Chemical, Inc., Osaka, Japan) according to the manufacturer’s instructions. The mean numbers of apoptotic cells per 10 random high-power fields were calculated and compared between the two groups.
All values are expressed as mean ± SD. Parameters were evaluated using the Student t test and Mann Whitney U test. Differences between the two groups were evaluated using analysis of variance with P < 0.05 considered to be significant.
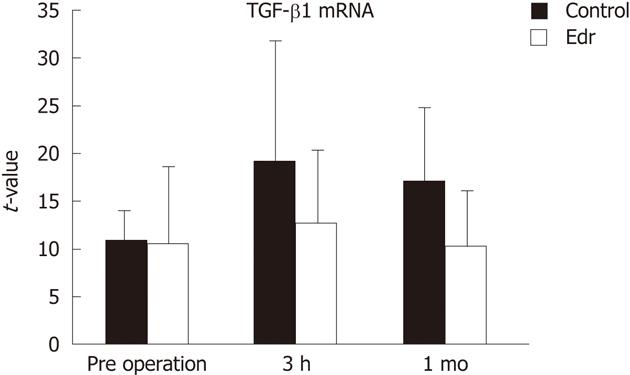
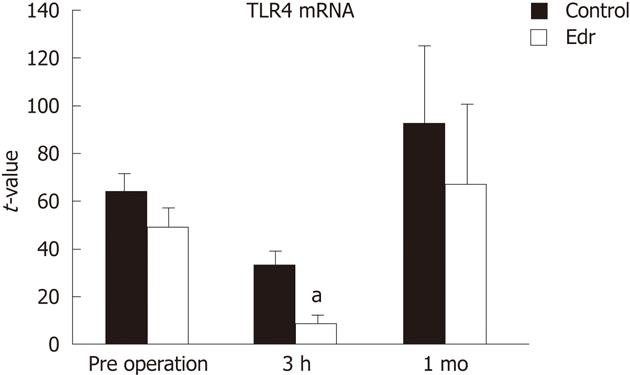
There were no significant differences in weight, amount of intraoperative hemorrhage, liver resection time, and weight of resected liver (Table 1). There was no significant difference in liver weight increasing ratio at 1 mo after operation between the two groups (P = 0.228, Figure 2). There were no significant differences in systolic and diastolic blood pressures between the two groups (Figure 3). Serum chemistry showed that the AST level at 3 h after reperfusion was significantly lower in the Edr-treated group than in the control group (P = 0.029). There were no significant differences in ALT, LDH and LA levels at any of the observation points between the two groups (Figure 4). Figure 5 shows the expression of TGF-β1-mRNA in liver tissues. Expression of TGF-β1-mRNA in the Edr-treated group tended to be lower than in the control group at 3 h (P = 0.285) and 1 h (P = 0.172) after operation. Expression of TLR4 mRNA was significantly lower in the Edr-treated group than in the control group at 3 h after operation (P = 0.043), however, there was no significant difference between the two groups 1 mo after operation (Figure 6). Figure 7A shows TUNEL staining. There were more TUNEL-positive cells in Control pigs than in Edr-treated pigs 5 min after hepatectomy and 3 h after declamping. Actual numbers of apoptotic cells in the Edr-treated and control groups were 22.16 ± 8.97 and 36.85 ± 1.83 at 5 min after hepatectomy (P = 0.015), and 24.66 ± 3.11 and 42.25 ± 3.43 at 3 h after declamping, respectively (P = 0.0001) (Figure 7B).
| Control | Edr | P value | |
| Body weight | 24.6 ± 1.1 | 24.7 ± 0.5 | 0.99 |
| Body weight (after 1 mo) | 29.4 ± 3.3 | 29.4 ± 3.1 | 0.97 |
| Bleeding (g) | 59.5 ± 44.7 | 57.4 ± 46.8 | 0.82 |
| Resection time (min) | 24.2 ± 1.6 | 24.7 ± 0.4 | 0.49 |
| Resected liver volume (g) | 295.7 ± 25.7 | 271 ± 41.0 | 0.32 |
| Liver volume (after 1 mo) | 606.3 ± 92.7 | 579.3 ± 60.0 | 0.53 |
The Pringle method is usually performed to reduce the amount of hemorrhage during hepatectomy[4]. However, this method inevitably causes I/R injury and has a risk of inducing abnormally high hepatic enzyme levels, icterus, hyperammonemia, lactacidemia, and an intractable accumulation of pleural and peritoneal effusion postoperatively. I/R injury associated with the Pringle maneuver may affect outcome when the ischemic time is long, or during hepatectomy for diseased liver, such as in cases of hepatic cirrhosis and fatty liver, despite the reduction in intraoperative hemorrhage achieved using the Pringle method[10].
I/R injury may be a causal factor in the above symptoms, and various countermeasures have been proposed. These include modified surgical methods, such as an intermittent Pringle method, partial hepatic pedicle clamping, and ischemic pre-conditioning, as well as drug therapy with steroids, prostaglandin E1, Sivelestat or Erythropoietin[10-19]. However, assessment of the effectiveness of these approaches has been limited, and this may be clarified by studies in animals of similar size to humans. In general, the process of I/R injury consists of multiple steps. Hypoxia due to cessation of blood supply impairs oxidative phosphorylation in the mitochondria, leading to profound cellular damage[20]. Furthermore, reperfusion exacerbates cellular damage by producing reactive oxygen species, activating pro-inflammatory cytokines such as IL-10 and TNF-α[21], and up-regulating cell adhesion molecules such as P-, E-, and L-selectins[22,23], resulting in tissue injury.
Edr has potent hydroxyl radical scavenging activity[24]. In various experimental models, Edr has been reported to protect several organs such as brain[25], heart[26] and liver[27-29] from free radical-mediated injuries.
Several previous studies have investigated the effect of Edr on I/R injury in the liver. Edr improves portal flow and decreases the level of hepatic enzyme[30]. Edr also decreases oxidative damage to mitochondria in liver[27]. However, these findings only indicate that Edr is effective in the acute phase of I/R injury. In the present study, we investigated the effect of Edr at both very early time points and at a late point (one mo after hepatectomy). No improvements in the markers of liver function including AST, ALT, LDH and LA, except AST at 3 h after hepatectomy, were noted at any of the observation periods. However, Edr successfully reduced apoptotic cells in the liver at 5 min and 3 h after hepatectomy. The anti-apoptotic effect of Edr was not observed at one mo after hepatectomy. Thus, the results suggest that Edr has a protective effect against I/R injury in liver by inhibiting apoptosis in the early phase after injury, which was reflected in the decrease in markers. However, prevention of I/R injury, particularly in the early phase, did not affect liver regeneration.
TGF-β is a key mediator involved in the progression of liver disease. TGF-β exerts cytotoxic effects, such as induction of apoptosis and aggravation of microcirculation disorders, due to induction of the expression of cell adhesion molecules[31]. TGF-β had antiproliferative action after partial liver resection in a rat model[7]. When administered at the time of liver resection, TGF-β clearly delayed hepatocyte proliferation[7]. In the present study, we observed that Edr tended to suppress TGF-β1-mRNA expression in liver tissue at 3 h and 1 mo after hepatectomy.
TLRs consist of 13 mammalian members containing a conserved TIR Toll/IL-1 receptor domain in their intracellular domain and an individual leucine-rich repeat domain in their extracellular domain. TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed on the cell surface and TLR3, TLR7, TLR8 and TLR9 are expressed on the endosome-lysosome membrane. TLR4 and TLR5 are the receptors for the Gram-negative bacterial cell wall components, lipopolysaccharide, and bacterial flagellin, respectively[32]. In the liver, TL4 is an important key factor for liver fibrosis, I/R injury and liver regeneration. Upon I/R injury, the TLR4 ligand, HMGB1, is released from damaged hepatocytes and subsequently stimulates Kupffer cells through TLR4[33]. Suppression of TLR4 at I/R injury, leads to the control of progress to liver regeneration by controlling liver fibrosis. Unfortunately, in this study, Edr only decreased TLR4 levels in the early phase (less than 3 h) after liver resection. This result suggested that Edr did not have any effect on liver fibrosis and regeneration.
In conclusion, our results demonstrated that Edr has a protective effect against I/R injury in the liver by inhibiting apoptosis in early period after I/R injury. Although further studies are needed to determine the dose, timing, and duration of Edr treatment to be used in the clinical setting, we have shown, for the first time in a porcine model, that Edr may be a promising agent for ameliorating I/R injury during hepatectomy.
The authors thank Hisato Hirata and Yoshifumi Machida of the laboratory animal research center at Dokkyo Medical University for their excellent technical assistance.
Peritoneal adhesions can cause intestinal obstruction and other severe clinical disorders, thus, it is very important to prevent peritoneal adhesions in abdominal surgical operations. However, to date, there are still no ideal methods to prevent peritoneal adhesions in clinical practice. Chitosan is a deacetylated derivate of chitin.
Chitosan is a natural biological material and has been processed into many forms for medical use. Chitosan has also been used in the prevention of peritoneal adhesions and various researchers are exploring how to modify chitosan using chemical and physical methods to improve its effectiveness in preventing adhesions, and simultaneously reduce its adverse reactions.
In previous reports on the application of chitosan gels to prevent peritoneal adhesions, it was found that the gel was much too fluid and did not remain in the target area for sufficient time, moreover, the gel degraded so fast that it could only maintain effectiveness for a short period. In order to delay degradation and decrease the fluidity of the gel, the authors processed pure chitosan into films, however, the film degraded too slowly and the residual film was encapsulated by surrounding tissue and the peritoneal adhesions worsened. In order to overcome these disadvantages, we mixed chitosan with gelatin and produced blended films. The blended film degraded much faster than the previous pure chitosan film, but it also created a foreign body reaction and formed a severe foreign body granuloma around the blended film. In the present study we chemically modified chitosan during gelatinization to develop a new type of chitosan film, and showed that the film is remarkably effective in preventing peritoneal adhesions induced by wounds, ischemia and infection except foreign body-induced adhesions.
The study results suggest that the gelatinized-chitosan film is a potential therapeutic material which could be used in preventing peritoneal adhesions induced by wounds, ischemia and infection.
Peritoneal adhesion: Peritoneal adhesion is a type of defensive reaction to peritoneal injury mainly wounds, infection, ischemia and foreign bodies, but it can also cause intestinal obstruction and severe clinical disorders; chitosan: Chitosan is a deacetylated derivate of chitin, and chitin is the second most abundant natural biopolymer derived from the exoskeletons of crustaceans and from the cell walls of fungi and insects.
This is a good descriptive study in which authors investigate the effect of E3-methyl-1-phenyl-2-pyrazolin-5-one (Edr) on hepatic ischemia-reperfusion (I/R) injury and liver regeneration in a porcine hepatectomy model. The results are interesting and suggest that Edr is considered to reduce hepatic injury in the early stage of I/R injury in a porcine model.
Peer reviewer: Matias A Avila, Professor, Senior Staff Scientist, Division of Hepatology and Gene Therapy, University of Navarra, Avda. Pio XII, n55, 31008 Pamplona, Spain
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Yamawaki M, Sasaki N, Shimoyama M, Miake J, Ogino K, Igawa O, Tajima F, Shigemasa C, Hisatome I. Protective effect of edaravone against hypoxia-reoxygenation injury in rabbit cardiomyocytes. Br J Pharmacol. 2004;142:618-626. [PubMed] |
| 2. | Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, Horiuchi M, Iwasaki Y. Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004;1029:200-206. [PubMed] |
| 3. | Hiranuma S, Ito K, Noda Y, Ozasa H, Koike Y, Horikawa S. Amelioration of hepatic ischemia/reperfusion injury in the remnant liver after partial hepatectomy in rats. J Gastroenterol Hepatol. 2007;22:2167-2172. [PubMed] |
| 4. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [PubMed] |
| 5. | Childs CB, Proper JA, Tucker RF, Moses HL. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci USA. 1982;79:5312-5316. [PubMed] |
| 6. | Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447-455. [PubMed] |
| 7. | Russell WE, Coffey RJ, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA. 1988;85:5126-5130. [PubMed] |
| 8. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [PubMed] |
| 9. | Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597-1604. [PubMed] |
| 10. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [PubMed] |
| 11. | Makuuchi M, Mori T, Gunvén P, Yamazaki S, Hasegawa H. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155-158. [PubMed] |
| 12. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [PubMed] |
| 13. | Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17-22. [PubMed] |
| 14. | Hanazaki K, Kajikawa S, Fujimori Y, Nakata S, Shimozawa N, Koide N, Adachi W, Amano J. Effects of prostaglandin E1 administration during hepatectomy for cirrhotic hepatocellular carcinoma. Hepatogastroenterology. 2000;47:461-464. [PubMed] |
| 15. | Uchinami M, Muraoka R, Horiuchi T, Tabo T, Kimura N, Naito Y, Yoshikawa T. Effect of intermittent hepatic pedicle clamping on free radical generation in the rat liver. Surgery. 1998;124:49-56. [PubMed] |
| 16. | Horiuchi T, Muraoka R, Tabo T, Uchinami M, Kimura N, Tanigawa N. Optimal cycles of hepatic ischemia and reperfusion for intermittent pedicle clamping during liver surgery. Arch Surg. 1995;130:754-758. [PubMed] |
| 17. | Shimoda M, Iwasaki Y, Okada T, Sawada T, Kubota K. Protective effect of Sivelestat in a porcine hepatectomy model prepared using an intermittent Pringle method. Eur J Pharmacol. 2008;587:248-252. [PubMed] |
| 18. | Shimoda M, Sawada T, Iwasaki Y, Mori S, Kijima H, Okada T, Kubota K. Erythropoietin strongly protects the liver from ischemia-reperfusion injury in a pig model. Hepatogastroenterology. 2009;56:470-475. [PubMed] |
| 19. | Kato M, Sawada T, Kita J, Shimoda M, Kubota K. Erythropoietin ameliorates early ischemia-reperfusion injury following the Pringle maneuver. World J Gastroenterol. 2010;16:4838-4845. [PubMed] |
| 21. | López-Neblina F, Toledo-Pereyra LH. Anti-ischemic effect of selectin blocker through modulation of tumor necrosis factor-alpha and interleukin-10. J Surg Res. 2007;138:275-283. [PubMed] |
| 22. | Clavien PA, Harvey PR, Sanabria JR, Cywes R, Levy GA, Strasberg SM. Lymphocyte adherence in the reperfused rat liver: mechanisms and effects. Hepatology. 1993;17:131-142. [PubMed] |
| 23. | Tsuchihashi S, Fondevila C, Shaw GD, Lorenz M, Marquette K, Benard S, Shen XD, Ke B, Busuttil RW, Kupiec-Weglinski JW. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616-624. [PubMed] |
| 24. | Watanabe K, Watanabe H, Goto Y, Yamaguchi M, Yamamoto N, Hagino K. Pharmacological Properties of Magnolol and Hōnokiol Extracted from Magnolia officinalis: Central Depressant Effects. Planta Med. 1983;49:103-108. [PubMed] |
| 25. | Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921-927. [PubMed] |
| 26. | Wu TW, Zeng LH, Wu J, Fung KP. Myocardial protection of MCI-186 in rabbit ischemia-reperfusion. Life Sci. 2002;71:2249-2255. [PubMed] |
| 27. | Okatani Y, Wakatsuki A, Enzan H, Miyahara Y. Edaravone protects against ischemia/reperfusion-induced oxidative damage to mitochondria in rat liver. Eur J Pharmacol. 2003;465:163-170. [PubMed] |
| 28. | Kono H, Asakawa M, Fujii H, Maki A, Amemiya H, Yamamoto M, Matsuda M, Matsumoto Y. Edaravone, a novel free radical scavenger, prevents liver injury and mortality in rats administered endotoxin. J Pharmacol Exp Ther. 2003;307:74-82. [PubMed] |
| 29. | Nakamura A, Akamatsu Y, Miyagi S, Fukumori T, Sekiguchi S, Satomi S. A free radical scavenger, edaravone, prevents ischemia-reperfusion injury in liver grafts from non-heart-beating donors. Transplant Proc. 2008;40:2171-2174. [PubMed] |
| 30. | Ninomiya M, Shimada M, Harada N, Shiotani S, Hiroshige S, Soejima Y, Suehiro T, Sugimachi K. Beneficial effect of MCI-186 on hepatic warm ischemia-reperfusion in the rat. Transplantation. 2002;74:1470-1472. [PubMed] |
| 31. | Ferré N, Clària J. New insights into the regulation of liver inflammation and oxidative stress. Mini Rev Med Chem. 2006;6:1321-1330. [PubMed] |