Published online Jul 21, 2012. doi: 10.3748/wjg.v18.i27.3511
Revised: October 15, 2011
Accepted: May 12, 2012
Published online: July 21, 2012
AIM: To evaluate the presence of progenitor cells in healthy adult rat liver displaying the equivalent advanced hepatogenic profile as that obtained in human.
METHODS: Rat fibroblastic-like liver derived cells (rFLDC) were obtained from collagenase-isolated liver cell suspensions and characterized and their phenotype profile determined using flow cytometry, immunocytochemistry, reverse transcription polymerase chain reaction and functional assays.
RESULTS: rFLDC exhibit fibroblastoid morphology, express mesenchymal (CD73, CD90, vimentin, α-smooth muscle actin), hepatocyte (UGT1A1, CK8) and biliary (CK19) markers. Moreover, these cells are able to store glycogen, and have glucose 6 phosphatase activity, but not UGT1A1 activity. Under the hepatogenic differentiation protocol, rFLDC display an up-regulation of hepatocyte markers expression (albumin, tryptophan 2,3-dioxygenase, G6Pase) correlated to a down-regulation of the expression of the biliary marker CK19.
CONCLUSION: Advanced hepatic features observed in human liver progenitor cells could not be demonstrated in rFLDC. However, we demonstrated the presence of an original rodent hepato-biliary cell type.
- Citation: Maerckx C, Scheers I, Tondreau T, Campard D, Nyabi O, Najimi M, Sokal E. Hepato-biliary profile of potential candidate liver progenitor cells from healthy rat liver. World J Gastroenterol 2012; 18(27): 3511-3519
- URL: https://www.wjgnet.com/1007-9327/full/v18/i27/3511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i27.3511
Liver transplantation is considered to be the standard treatment for end-stage liver diseases. Unfortunately, clinical applications are restricted by the scarcity of organs and uncertainty about the very long-term success of the procedure.
In recent years, liver cell transplantation using hepatocytes was successfully performed in patients with inborn errors of metabolism as an alternative, or at least as a bridge to orthotopic liver transplantation[1-5]. However, the success of such a therapeutic approach remains limited by the quality of transplanted cells. In fact, cryopreservation procedures induce significant alterations at morphological and functional levels of the thawed hepatocytes[6,7].
To overcome these problems, several approaches to isolate and propagate liver stem or progenitor cells have been developed. In our laboratory, Najimi et al[8] isolated adult derived human liver stem/progenitor cells (ADHLSC) with hepato-mesenchymal profile. Under specific hepatogenic conditions, these cells exhibit hepato-specific functions like glycogen storage, gluconeogenesis, urea synthesis, glucuronoconjugation, and pharmacologic properties such as activity of phase I and II enzymes[9]. These cells are also able to specifically engraft and differentiate into mature human hepatocytes in mouse liver parenchyma[8].
Preclinical studies using homologous animal models of human liver metabolic diseases are attractive. It is therefore a prerequisite to obtain homologous cells from syngenic animals to perform such studies. The relevance of using human progenitor cells in immunosuppressed animal models is indeed questionable.
In this context, we evaluated the presence of a liver progenitor cell in adult rat liver that would express the same specifications as the previously reported human progenitor cell, referred to as ADHLSC.
In the current study we isolated and characterized rat fibroblastic-like liver derived cells (rFLDC) from healthy adult rats. Characterization included proliferation rate, phenotype, genotype and hepatic-specific functional assays.
Five male Wistar rats weighing ± 200 g were purchased from UCL Animalerie Centrale (Brussels, Belgium) and treated in accordance with the internal Animal Ethic and Welfare Committees (UCL/MD/2009/003).
We isolated rat liver parenchymal cells in a two-step collagenase A (1100 units/L) (Roche, Mannheim, Germany) perfusion procedure according to the Seglen method[10]. We then obtained a hepatocyte enriched cell fraction following low-speed centrifugation (160 r/min for 3 min).
Viable hepatocytes, 1.5 million (> 75%, trypan blue exclusion), were seeded onto rat tail collagen I-coated plates (Greiner, Wemmel, Belgium) in Williams’ E medium (Invitrogen, Merelbeke, Belgium) supplemented with 10% fetal bovine serum (FBS) (AE Scientific, Marcq, Belgium), 25 μg/L human epidermal growth factor (EGF) (Peprotech, London, United Kingdom), 10 mg/L human insulin (Lilly, Brussels, Belgium), 1 μmol/L dexamethasone (Sigma, Bronem, Belgium), and 1% penicillin/streptomycin (P/S) (Invitrogen) at 37 °C in a fully humidified atmosphere containing 5% CO2. After 24 h we changed the medium in order to eliminate the non-adherent cells and thereafter we renewed it every 3 d. On days 7-12, hepatocytes died and small colonies of spindle-shaped fibroblastic cells emerged and proliferated. At this time, we switched the culture medium to Dulbecco’s modified Eagle’s medium (DMEM medium) [DMEM high glucose (Invitrogen) supplemented with 10% FBS and 1% P/S] in order to accelerate the proliferation of emerging cells. When cell cultures reached 90% confluence, we trypsinized them with 0.05% trypsin-1 mmol EDTA solution (Invitrogen) and replated them on a collagen-coated plate at a density of 104 cells/cm2. The medium was refreshed every 3 d.
Population doubling (PD) was evaluated after each passage using the following equation: [log (harvested cells)/log (seeded cells)]/log 2. Cumulative population doubling (CPD) was calculated with the sum of PD at all passages.
At passages 2, 4 and 8, cells were analyzed using reverse transcription polymerase chain reaction (RT-PCR), immunocytochemistry and flow cytometry.
We obtained bone marrow from Wistar rats by flushing the femur and tibia with ice cold phosphate-buffered saline (PBS) (Lonza, Verviers, Belgium) and isolated the cell fraction using Ficoll (GE Healthcare, Uppsala, Sweden) density gradient centrifugation at 340 r/min for 30 min.
Cells were then resuspended in α-MEM (Invitrogen) supplemented with 10% FBS (Perbio, Erembodegem, Belgium) and 1% P/S (Invitrogen) and seeded in 75 cm² culture flasks. We removed non-adherent cells after 1 d and then refreshed the medium every 3-4 d. When cultures had reached 80%-90% confluence, we harvested the cells with 0.05% trypsin-1 mmol EDTA solution and replated them at a density of 7 × 103 cells/cm2. These cells were used as the internal control in mesodermal differentiation studies.
Flow cytometry: Cells from the initial parenchymal fraction or after passaging were suspended at a concentration of 1000 cells/μL in PBS and 0.5% bovine serum albumin (BSA, Sigma) and then incubated for 25 min at room temperature with the following antibodies: CD29-PE (rabbit monoclonal, 1/70), CD44-FITC (mouse monoclonal, 1/20), CD45-FITC (mouse monoclonal, 1/20) (Abcam, Belgium), CD73-FITC (mouse monoclonal, 1/20), CD90-PE (mouse monoclonal, 1/20) (BD, Erembodegem, Belgium). Unspecific binding of antibodies, was evaluated using mouse IgG1 FITC and the PE isotypes control (BD).
We then washed and fixed them in cytofix/cytoperm (BD) until analysis with a FACSCantoII flow cytometer (BD).
Immunocytochemistry: We fixed rFLDC cultured on 24 well rat collagen type-1 coated plates with 3.5 % formaldehyde (v/v, VWR, Leuven, Belgium) for 15 min at room temperature. After rinsing in PBS, we permeabilized cells with 1% Triton × 100 (w/v Roche) in PBS for 10 min. Before incubation with specific rat antibodies, endogenous peroxidase activity was inhibited with PBS supplemented with 3% H2O2 (VWR) solution for 3 min. Non-specific immunostaining was prevented by incubation with 3% BSA solution (Sigma) for 1 h. Cells were then incubated for 1 h with 0.3% BSA containing the following antibodies: fibronectin (rabbit polyclonal, 1/50) (Dako, Heverlee, Belgium), vimentin (mouse monoclonal, 1/100), and α-smooth muscle actin (ASMA) (rabbit polyclonal, 1/100) (BD). After rinsing with PBS, cells were finally incubated for 30 min with Envision®, a secondary antibody against mouse or rabbit (Dako). Immunostaining results were evidenced by the addition of diaminobenzidine and urea reagents (Sigma) and counterstained with Mayer hematoxylin solution.
RT-PCR analysis: We extracted total RNA from expanded or differentiated rFLDC using the TriPure isolation reagent (Roche) and carried out cDNA with the Thermoscript RT-PCR system (Invitrogen) using 1 μg total RNA, according to the manufacturer’s instructions. Rat specific primers used for gene amplification are listed in Table 1. We thereafter electrophoresed amplified cDNA on a 1% agarose gel (Invitrogen) followed by 0.01% ethidium bromide (Sigma) staining.
| Gene | Amplicon size (bp) | Primers |
| Vim | 241 | F: AAGCAGGAGTCAAACGAATA |
| R: GAGCCATCTTTACATTGAGC | ||
| Fn | 392 | F: ACCGTGGAGTATGTGGTTAG |
| R: GGTGACACCTGAGTGAACTT | ||
| ASMA | 385 | F: ATGCTTCTGGACGTACAACT |
| R: GACTCCATTCCAATGAAAGA | ||
| CK8 | 378 | F: TGGAGAATGAGTTTGTCCTC |
| R: TGATGTTACGGTTCATCTCA | ||
| CK19 | 287 | F: GCCAGTACTTCAAGACCATC |
| R: ACTAATTTCCTCCTCGTGGT | ||
| HNF4 | 332 | F: CGGATGTGTGTGAGTCTATG |
| R: AAAGAAGATGATGGCTTTGA | ||
| TAT | 275 | F: CTGGACAAAACATCCTCATT |
| R: GATCTCGTCAGCTAAGATGG | ||
| TDO | 366 | F: CTCCTGGTACAGCAGTTCTC |
| R: CTTTTTCGCCTGAATCTTTA | ||
| aFP | 289 | F: AACGTAGCTACCATTGTCGT |
| R: CAGTTTCTGGAAAGTGGAAG | ||
| Alb | 365 | F: TTTACGAGAAGCTTGGAGAG |
| R: TGTGCAGATATCAGAGTGGA | ||
| UGT1A1 | 369 | F: CCATGTTGCTTTTATTAGGG |
| R: ACAAAACATGAGCACAGTGA | ||
| G6Pase | 386 | F: GAAGATGTTTCCCTGATGAA |
| R: AGTCACCATTACCATTCAGG | ||
| GAPDH | 315 | F: CCACTCAGAAGACTGTGGAT |
| R: TGTTGAAGTCACAGGAGACA | ||
| CD29 | 321 | F: TTCAATGAACTTGTTGGTCA |
| R: AGTGACTGCAAAAATCGTCT | ||
| CD44 | 383 | F: AGGATTTCCCCAGAACTTAG |
| R: ACAGGTCAAGATGGAAGATG | ||
| CD45 | 321 | F: TGAACATACGGATTGTGAAA |
| R: TTTGTTCGGACTGTAAGGTT | ||
| CD73 | 341 | F: ATAGTCACCTCTGACGATGG |
| R: ATTTCATCTGGGTGTCTGAG | ||
| CD90 | 380 | F: AAGGAGAAACAGGAAACCTC |
| R: ACAGACACAGTCCAACTTCC | ||
| CD105 | 386 | F: TACCTCCAAGACACAGATCC |
| R: TCTGCATATTGTGGTTGGTA |
Hepatogenic differentiation: rFLDC from passage four were seeded at a density of 104/cm2 into 6 wells and 24 wells rat tail type I collagen-coated plates in the presence of expansion medium. When cell cultures reached 90% confluence, we switched the medium to Iscove’s modified Dulbecco’s medium (IMDM, Invitrogen) supplemented with 20 μg/L human EGF (Peprotech, London, United Kingdom) and 10 μg/L human basic fibroblast growth factor-2 (FGF2) (Peprotech) for 2 d. Thereafter, we subjected cells to differentiation induction for 10 d with IMDM containing 20 μg/L human hepatocyte growth factor (HGF) (Peprotech), 10 μg/L FGF2, nicotinamide 0.61 g/L (Sigma), and 1% insulin-transferrin-selenium (ITS) (Invitrogen). An intermediate step of differentiation/maturation in IMDM containing 20 μg/L HGF, 20 μg/L human oncostatin M (Peprotech), nicotinamide 0.61 g/L and 1% ITS was performed over 10 d. The subsequent maturation step consisted of treatment with IMDM containing 20 mg/L oncostatin M, 1 μmol/L dexamethasone (Sigma), and 1% ITS premix for 10 d. For each step, we changed the medium every 3-4 d.
Mesodermal differentiation: At passages 0, 2 4 and 8, rFLDC were plated at 1.5 × 104 cells/cm2 on six-well rat tail collagen I-coated plates. At confluency, we performed osteogenic differentiation with complete DMEM medium containing 0.1 μm dexamethasone, 0.1 mmol/L ascorbate and 10 mmol/L β-glycerophosphate (Sigma). After 4 wk, calcium deposition was evidenced using alizarin red staining. For adipogenic differentiation, we incubated cells with expansion medium complete DMEM containing 1 μm dexamethasone, 0.5 mmol/L isobutyl-methylxanthine, 0.2 mmol/L indomethacin (Sigma) and 10 μg/mL insulin (Lilly). Medium change was carried out twice a week. After 4 wk, oil red O staining revealed the presence of lipid vesicles. As a control of mesodermal differentiation capacity, the differentiation procedure was validated with rat bone marrow mesenchymal stem cells using α-MEM complete medium.
Glycogen storage: Undifferentiated and differentiated rFLDC fixed with 3.5% formaldehyde (Sigma) were incubated for 10 min in 1% periodic acid (Sigma). After washing with distilled water, the cells were incubated with Schiff’s reagent (Sigma) for 15 min. The preparations were then washed and mounted.
Glucose-6-phosphatase activity: We investigated glucose-6-phosphatase (G6Pase) activity in undifferentiated and differentiated rFLDC. After washing with PBS, cells were incubated for 4 h at 37 °C in 1.5 mL 50 mmol/L Tris (Sigma) and 50 mmol/L maleate (Sigma) buffer (pH 6.7) solution containing 5 mmol/L glucose-6-phosphate (G6Pate, Sigma) and 0.03 g lead nitrate (Acros, Geel, Belgium). We obtained brownish precipitates of lead sulfate following incubation of cells in a solution containing 0.1% ammonium sulfide (Sigma)[11]. Cells were then mounted and viewed by light microscopy (Leica DM IL, Groot-Bijgaarden, Belgium).
Undifferentiated and differentiated rFLDC were incubated in William’s medium and 1% FBS containing unconjugated bilirubin (Sigma) for 24 h and 48 h. Afterwards, we harvested the supernatant and added 2 μg/mL xantobilirubinic acid (use as internal standard: IS). We then submitted the product obtained in this reaction to an alkaline methanolysis followed by nitrogen evaporation as described by Muraca et al[12]. Precipitates were resuspended with 10 μL chloroform (Sigma) and 100 μL dimethyl sulfoxide (Sigma). We then injected ten microliters of this solution into the liquid chromatograph (Waters 515 HPLC pump) and eluted it with a C18 column (Macherey-Nagel, Düren, Germany). Elutriation flow started at 1 mL/min with methanol/water/tetrabutylammonium (solvent A) and ended after 11 min with methanol/ethanol/water/tetrabutylammonium (solvent B). Elution was continued for 6 min with solvent B, and the column was re-equilibrated with solvent A. The absorbance of the eluted pigments was monitored at 436 nm using a 996 photodiode array detector (Waters, Zellik, Belgium) and the area under peak was integrated electronically (Millennium software, Waters). We calculated the concentration, in micromoles per liter, of each bilirubin fraction in samples using the following equation: (Areapigment/areaIS) × (IS/IV) × RF.
In which IS corresponds to micrograms of internal standard added to the sample, SV to the volume of sample (mL), and RF to the response factor.
Conjugation rate (CR) was evaluated using the equation: (Conjugated bilirubin concentration)/(total bilirubin concentration) × 100.
In which total bilirubin concentration was the sum of unconjugated and conjugated bilirubin.
An enriched population of hepatocytes obtained after collagenase A digestion and low speed centrifugation was plated on type I collagen-coated 6-well plates.
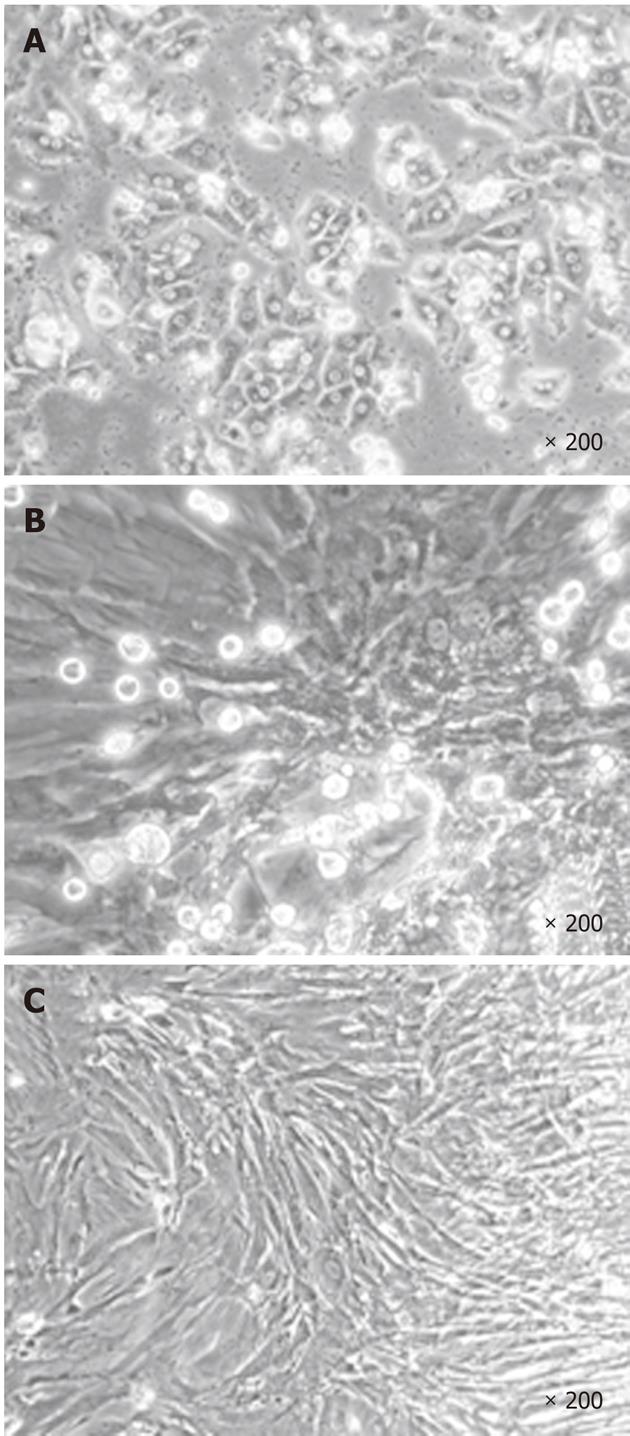
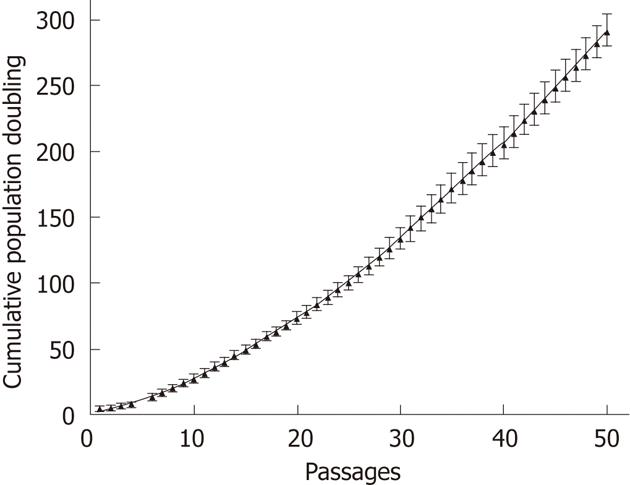
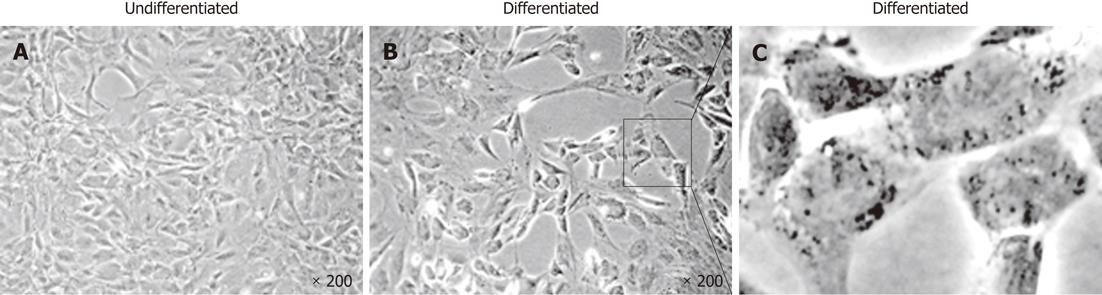
During the first step of culture, mature hepatic cells present in the culture died due to their inability to proliferate (Figure 1). After 7 to 12 d, cells with a fibroblastic-like shape emerged and proliferated (Figure 1B and C). These cells demonstrated a high proliferative potential with a CPD of 294.55 ± 20.91 after 50 passages (Figure 2).
rFLDCs were reproducibly isolated from at least five different liver cell suspensions.
All isolated rFLDC were analyzed and characterized after passages 2, 4 and 8 using FACS analysis and RT-PCR. Furthermore, a stable expression profile was observed up to P50 (data not shown).
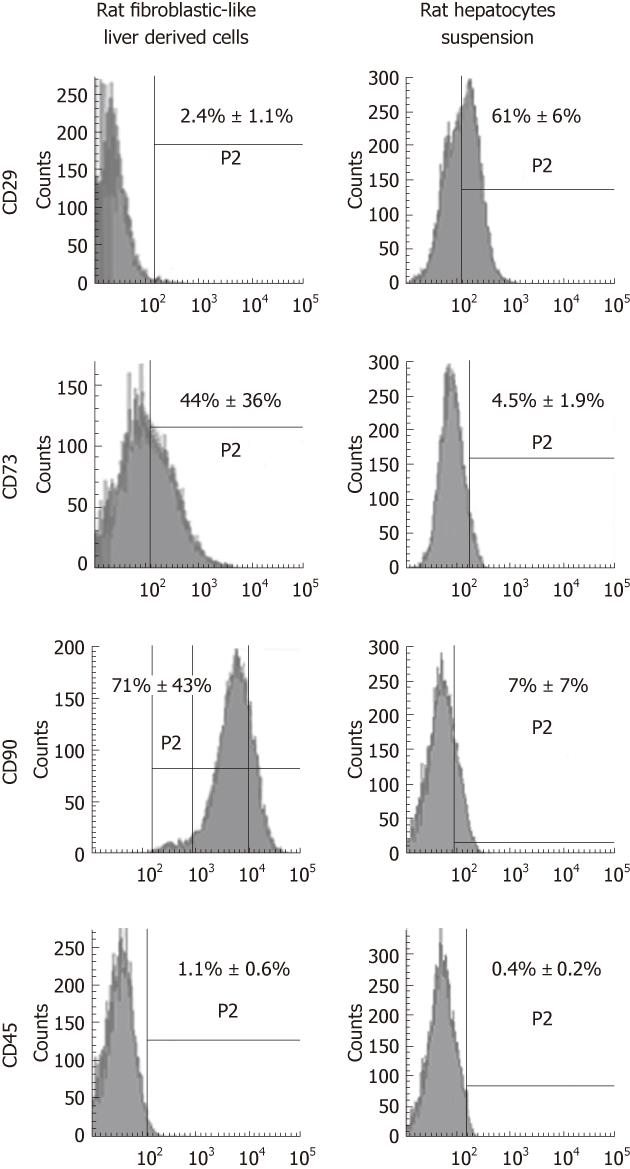
Representative flow cytometry data at passage 4 demonstrated that the most described mesenchymal markers, CD73 and CD90 constituted 44% ± 36% and 71% ± 43% of the cell population, respectively (Figure 3), whereas the expression of CD29 protein was only detected in 2.4% ± 1.1%. The hematopoietic marker, CD45 (1.1% ± 0.6%) was almost undetectable.
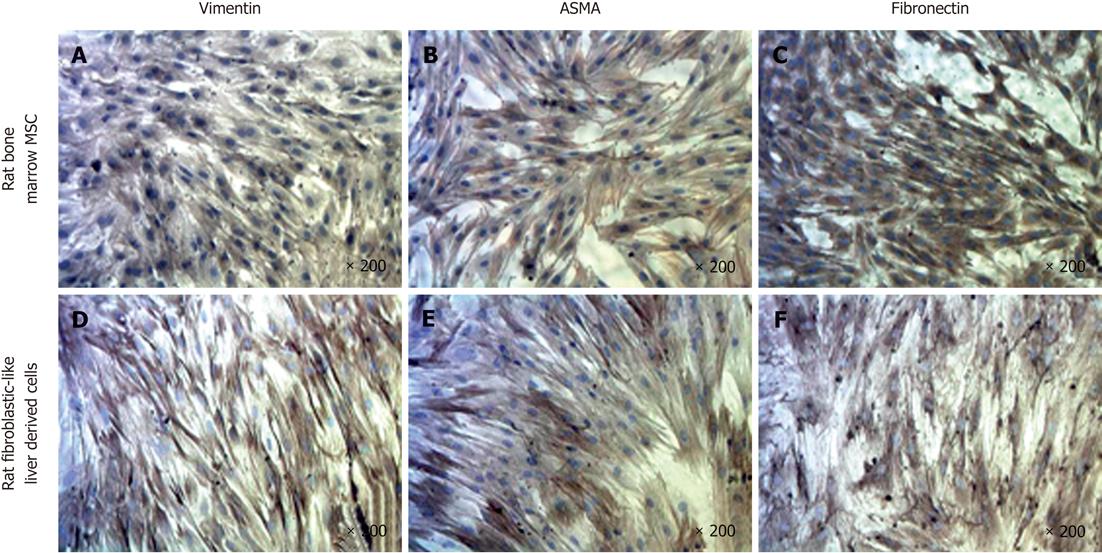
To further characterize our cell population, we performed immunocytochemistry (ICC) for vimentin, fibronectin and ASMA proteins and compared the findings with rat bone marrow-derived mesenchymal stem cells (rBM-MSC) (Figure 4). The results indicated positive staining for ASMA, vimentin and fibronectin as observed with rBM-MSC.
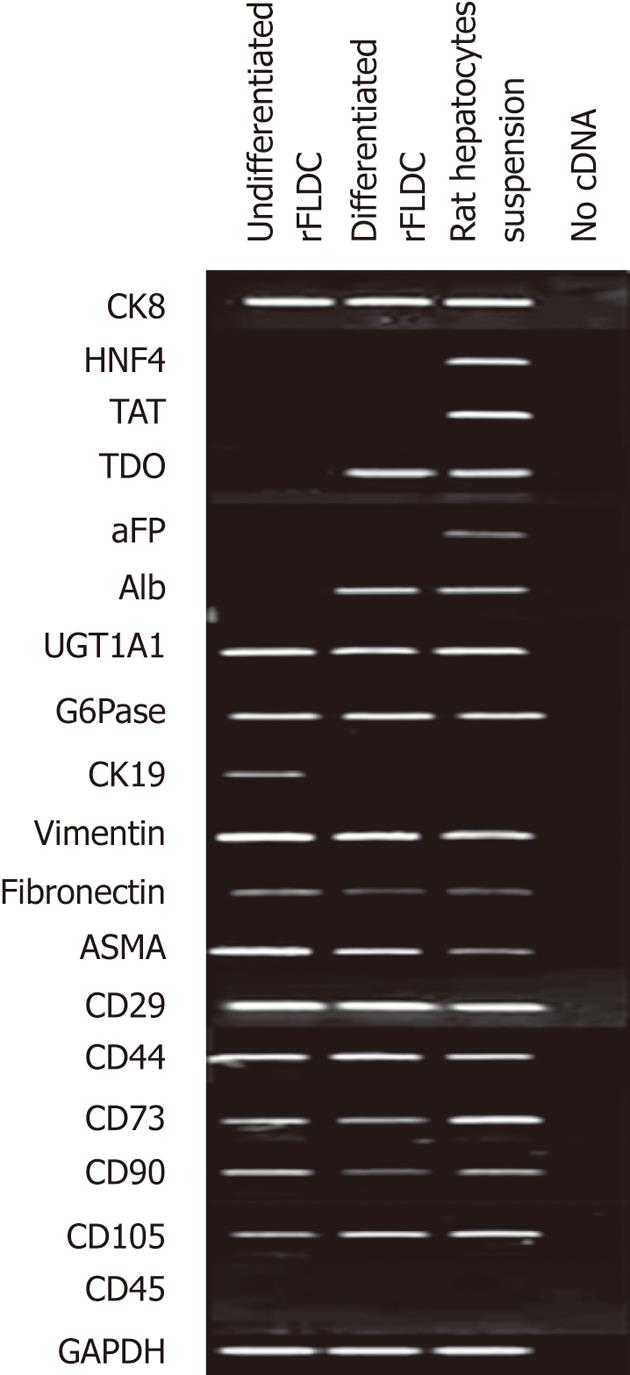
To confirm the phenotypic profile of isolated rFLDC we performed RT-PCR analysis using specific mesodermal, hepatocyte and cholangiocyte markers at passage 4 (Figure 5).
The mesenchymal expression profile was confirmed by the detection of vimentin, fibronectin, ASMA, integrin β-1 (CD29), hyaluronic acid receptor (CD44), ecto 5’-nucléotidase (CD73), Thy-1 (CD90) and endoglin (CD105) mRNAs. RT-PCR also confirmed the absence of CD45 expression (Figure 5).
Because of their liver origin, rFLDC were also studied for their expression of hepatocyte and cholangiocyte markers. mRNA analysis revealed the expression of UDP-glucuronosyl transferase 1A1 (UGT1A1), cytokeratin 8 (CK8) and G6Pase (Figure 5). However, tyrosine aminotransferase (TAT), tryptophan 2,3-dioxygenase (TDO), albumin (Alb), α-fetoprotein (αFP) and hepatocyte nuclear factor 4 (HNF4) transcripts were not detected, all expressed by fully differentiated hepatocytes. mRNA analysis also revealed the expression of cytokeratin 19, a biliary marker.
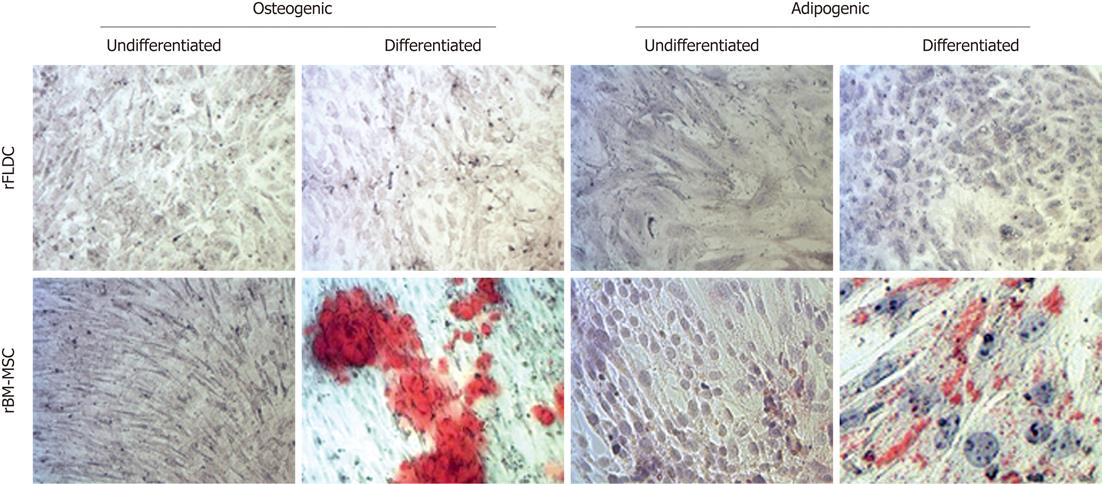
First, we checked the ability of rFLDC to differentiate into adipocytes in the presence of specific media supplemented with dexamethasone, isobutyl-methylxanthine, indomethacin and insulin (Figure 6). We noticed that at early passages (P0-P2) two out of five rat fibroblastic-like liver derived cell cultures demonstrated a weak localized adipocytic differentiation. This ability was lost in further passages. Under osteogenic induction, no calcium deposit was noted (Figure 6).
In order to demonstrate their potential to differentiate into mature hepatocytes we seeded 104 cells/cm² from passage 4 in serum free-medium in the presence of several “hepatogenic” factors, as described in the Materials and Methods section. After 32 d, cells showed a slight morphology change and few cells adopted a polygonal shape (Figure 7). Using RT-PCR, we compared the expression of immature and mature hepatocytic/biliary mRNA on undifferentiated and differentiated rFLDC (Figure 5). Despite a variation in serum concentration (10% vs 2%) between the expansion medium and hepatogenic control medium, respectively, no differences in mRNA expression were noted (data not shown). Interestingly, differentiated rFLDC lost the expression of CK19, a biliary marker. Moreover, differentiated rFLDC acquired the expression of mature hepatocyte lineage markers including TDO and albumin.
To test their liver metabolic activity, we explored their ability to store glycogen and to perform glucogenesis (G6Pase activity) and their potential to conjugate bilirubin.
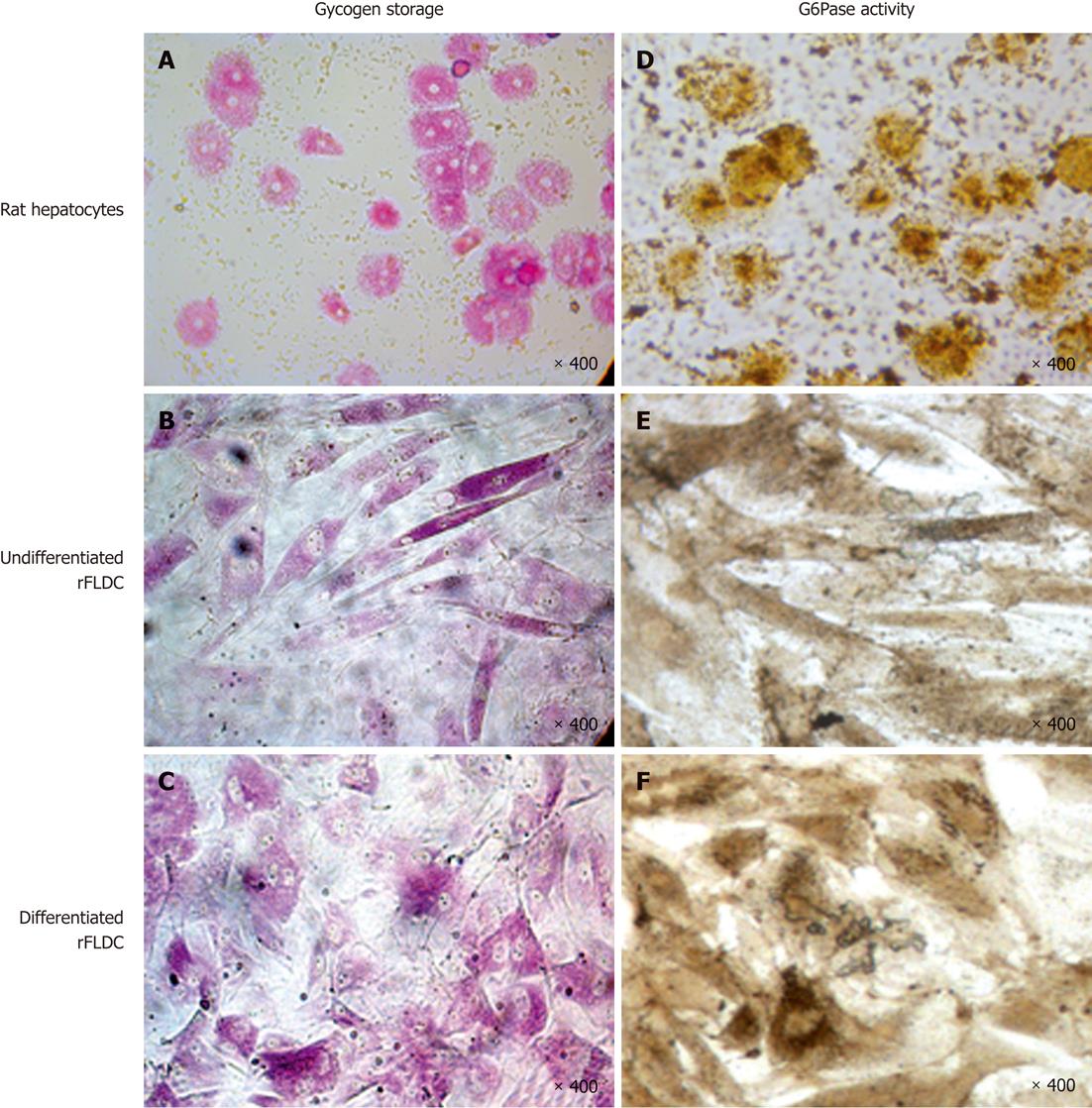
Glycogen storage, evidenced by periodic-acid shift staining, showed that, like rat hepatocytes (Figure 8A), undifferentiated and differentiated cells can store glycogen (Figure 8B and C).
As shown at Figure 8D-F, rat hepatocytes and rFLDC cells also revealed a basal G6Pase activity. These results were corroborated by the expression of G6Pase at the mRNA level.
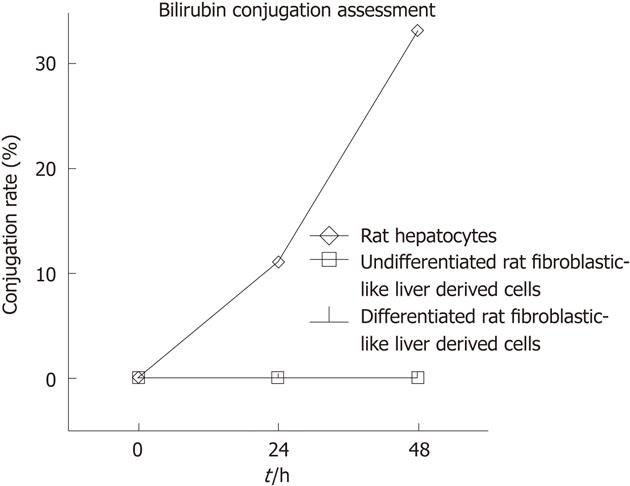
In addition to glycogen storage and G6Pase activity we assessed the ability of differentiated and undifferentiated rFLDC to conjugate bilirubin. Therefore, we incubated 104 cells/cm² with William’s medium-1% serum containing unconjugated bilirubin. After 24 h and 48 h, no bilirubin conjugate was observed in the culture medium in comparison with freshly isolated hepatocytes used as positive controls. For this late population the bilirubin CR reached 11% and 33% after 24 h and 48 h, respectively (Figure 9).
Because preclinical studies use animal models mimicking human diseases, we tried to isolate from rodent liver a liver progenitor cell that would display characteristics reported for ADHLSC. The use of human derived cells in animal models is considered irrelevant, as they may not engraft and function similarly in a xenogenic rodent environment.
Like human cells, rFLDC were isolated and emerged in vitro after culture of liver cell suspension following enzymatic-mediated disaggregation of liver. However, many differences were observed: rFLDC demonstrated a higher proliferative potential and did not reach senescence after at least 50 passages in contrast to human cells which stopped proliferating after 10-12 passages[13]. rFLDC were able, at early passages, to differentiate into adipocytes, in contrast to ADHLSC.
Like human cells, rFLDC displayed a mesenchymal profile as evidenced by the expression of CD44, CD73, CD90 and CD105. The cell population was not contaminated by hematopoietic stem cells as evidenced by the absence of CD45 expression. These results confirmed the presence of a new enriched cell population different from the freshly isolated hepatic cells. RT-PCR also revealed expression of CK8, UGT1A1 and G6P underlining their hepatic-like profile. Undifferentiated rFLDCs have the capacity to store glycogen and show G6Pase activity as mature hepatocytes.
In a hepatogenic differentiation medium, low numbers of rFLDC display the polygonal morphology of mature hepatocytes. Differentiated rFLDC express both albumin and TDO. However, we did not observe the expression of more specific hepatic markers such as HNF4 or TAT.
Candidate progenitor cells reported in this study were isolated from healthy rat livers, in contrast to other progenitor liver cells described elsewhere, such as oval cells, obtained after submitting animals to carcinogenic agents or toxic injuries[14-19]. Stellate cells[20] and rFLDC share common features like the expression of vimentin, ASMA, CK19, and CD90, although no expression of αFP was detected in rFLDC.
Differentiated rFLDC do not express CK19 or αFP, and differ therefore from small hepatocytes and epithelial cells also recovered from normal livers[17-19].
Recently, Sahin et al[19], using a 2-step collagenase protocol, reported a cell population derived from adult rat liver and called them LDPCs (liver-derived progenitor cells). Regarding their oval morphology and expression of HNF3β, CD45, CD34 and CD90, these cells seem to be closely related to oval cells despite the absence of CK7, CK8 and CK19 expression.
In conclusion our results showed that rodent progenitor cells homologous to ADHLSC can not easily be obtained even when the same isolation and culture protocolwas applied using a rat model. However, this protocol allowed the isolation of a novel type of liver progenitor cell population with both hepatic and biliary phenotype including G6Pase activity, glycogen storage, CK8, UGT1A1 and CK19 expression.
In the presence of a hepatogenic differentiation medium, rFLDC lose the CK19 biliary marker, but do not acquire a more mature hepatic status possibly due to the use of human c ytokines and growth factors, which may not be appropriate for rodent precursors, stressing again the difficulty in generating homologous models.
Further characterization and in vitro hepatogenic differentiation improvement are required before their relevant use in preclinical studies.
Liver cell transplantation using hepatocytes was successfully performed in patients with inborn errors of metabolism. However, the success of such a therapeutic approach remains limited by the quality of transplanted cells. To overcome these problems several approaches to isolate and propagate liver stem or progenitor cells have been developed. The capacity of those cells to restore a liver metabolic function must be demonstrated.
Preclinical studies using homologous animal models of human liver metabolic diseases are attractive. It is therefore a prerequisite to isolate and propagate human homologous liver stem or progenitor cells from syngenic animals to perform such studies. In this study, the authors showed that rodent progenitor cells homologous to human adult-derived liver stem/progenitor cells can not easily be obtained even when the same protocol was applied.
In this study, the authors reported the isolation of novel potential candidate liver progenitor cells isolated from healthy rat liver called rat fibroblastic-like liver derived cells (rFLDC). These cells express both hepatic and biliary phenotype and are able to acquire some hepatic characteristics in the presence of hepatogenic differentiation medium.
Isolation and characterization of progenitor/stem cells would be very useful to assay the in-vivo efficacy of liver mesenchymal progenitor cells in syngeneic animal models of liver metabolic diseases, particularly in the Gunn rat, a model of hyperbilirubinemia.
This study shows that the advanced hepatic features of human liver progenitor cells have not been demonstrated in rFLDC. Although it strengthens the unique specificity of these human liver progenitor cells, it also shows that homologous models for cell therapy can not easily be developed even when the same isolation and culture protocols are applied. The authors should make a comparison of their cells with human established liver cells.
Peer reviewer: Dr. Abdel-Majid Khatib, PhD, INSERM, UMRS 940, Equipe Avenir, Cibles Thérapeutiques, IGM 27 rue Juliette Dodu, 75010 Paris, France
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [PubMed] |
| 2. | Najimi M, Sokal E. Update on liver cell transplantation. J Pediatr Gastroenterol Nutr. 2004;39:311-319. [PubMed] |
| 3. | Najimi M, Sokal E. Liver cell transplantation. Minerva Pediatr. 2005;57:243-257. [PubMed] |
| 4. | Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735-738. [PubMed] |
| 5. | Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559-569. [PubMed] |
| 6. | Stéphenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy? World J Gastroenterol. 2010;16:1-14. [PubMed] |
| 7. | Stéphenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409-419. [PubMed] |
| 8. | Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007;16:717-728. [PubMed] |
| 9. | Khuu DN, Scheers I, Ehnert S, Jazouli N, Nyabi O, Buc-Calderon P, Meulemans A, Nussler A, Sokal E, Najimi M. In vitro differentiated adult human liver progenitor cells display mature hepatic metabolic functions: a potential tool for in vitro pharmacotoxicological testing. Cell Transplant. 2011;20:287-302. [PubMed] |
| 10. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [PubMed] |
| 11. | Sokal EM, Trivedi P, Portmann B, Mowat AP. Developmental changes in the intra-acinar distribution of succinate dehydrogenase, glutamate dehydrogenase, glucose-6-phosphatase, and NADPH dehydrogenase in the rat liver. J Pediatr Gastroenterol Nutr. 1989;8:522-527. [PubMed] |
| 12. | Muraca M, Blanckaert N. Liquid-chromatographic assay and identification of mono- and diester conjugates of bilirubin in normal serum. Clin Chem. 1983;29:1767-1771. [PubMed] |
| 13. | Scheers I, Maerckx C, Khuu N, Marcelle M, Decottignies A, Najimi M, Sokal E. Human liver progenitor cells in long term culture maintain appropriate gatekeepers mechanisms against transformation. In press. |
| 14. | Qin AL, Zhou XQ, Zhang W, Yu H, Xie Q. Characterization and enrichment of hepatic progenitor cells in adult rat liver. World J Gastroenterol. 2004;10:1480-1486. [PubMed] |
| 15. | Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315-324. [PubMed] |
| 16. | Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636-647. [PubMed] |
| 17. | Nagai H, Terada K, Watanabe G, Ueno Y, Aiba N, Shibuya T, Kawagoe M, Kameda T, Sato M, Senoo H. Differentiation of liver epithelial (stem-like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem Biophys Res Commun. 2002;293:1420-1425. [PubMed] |
| 18. | Tateno C, Yoshizato K. Growth and differentiation in culture of clonogenic hepatocytes that express both phenotypes of hepatocytes and biliary epithelial cells. Am J Pathol. 1996;149:1593-1605. [PubMed] |
| 19. | Sahin MB, Schwartz RE, Buckley SM, Heremans Y, Chase L, Hu WS, Verfaillie CM. Isolation and characterization of a novel population of progenitor cells from unmanipulated rat liver. Liver Transpl. 2008;14:333-345. [PubMed] |
| 20. | Geerts A, Niki T, Hellemans K, De Craemer D, Van Den Berg K, Lazou JM, Stange G, Van De Winkel M, De Bleser P. Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatology. 1998;27:590-598. [PubMed] |