Published online Jul 14, 2012. doi: 10.3748/wjg.v18.i26.3435
Revised: February 6, 2012
Accepted: February 16, 2012
Published online: July 14, 2012
AIM: To survey glutathione (GSH) S-transferase (GST) isoforms in mitochondria and to reveal the isoforms’ biological significance in diabetic mice.
METHODS: The presence of GSTs in mouse liver mitochondria was systematically screened by two proteomic approaches, namely, GSH affinity chromatography/two dimensional electrophoresis (2DE/MALDI TOF/TOF MS) and SDS-PAGE/LC ESI MS/MS. The proteomic results were further confirmed by Western blotting using monoclonal antibodies against GSTs. To evaluate the liver mitochondrial GSTs quantitatively, calibration curves were generated by the loading amounts of individual recombinant GST protein vs the relative intensities elicited from the Western blotting. An extensive comparison of the liver mitochondrial GSTs was conducted between normal and db/db diabetic mice. Student’s t test was adopted for the estimation of regression and significant difference.
RESULTS: Using GSH affinity/2DE/MALDI TOF/TOF MS, three GSTs, namely, alpha3, mu1 and pi1, were identified; whereas five GSTs, alpha3, mu1, pi1, kappa1 and zeta1, were detected in mouse liver mitochondria using SDS-PAGE/LC ESI MS/MS, of these GSTs, GST kappa1 was reported as a specific mitochondrial GST. The R2 values of regression ranged between values of about 0.86 and 0.98, which were acceptable for the quantification. Based on the measurement of the GST abundances in liver mitochondria of normal and diabetic mice, the four GSTs, alpha3, kappa1, mu1 and zeta1, were found to be almost comparable between the two sets of animals, whereas, lower GST pi1 was detected in the diabetic mice compared with normal ones, the signal of Western blotting in control and db/db diabetic mice liver mitochondria is 134.61 ± 53.84 vs 99.74 ± 46.2, with P < 0.05.
CONCLUSION: Our results indicate that GSTs exist widely in mitochondria and its abundances of mitochondrial GSTs might be tissue-dependent and disease-related.
- Citation: Sun HD, Ru YW, Zhang DJ, Yin SY, Yin L, Xie YY, Guan YF, Liu SQ. Proteomic analysis of glutathione S-transferase isoforms in mouse liver mitochondria. World J Gastroenterol 2012; 18(26): 3435-3442
- URL: https://www.wjgnet.com/1007-9327/full/v18/i26/3435.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i26.3435
The glutathione (GSH) S-transferase (GST, EC2.5.1.18) superfamily contains eight subclasses classified by their properties of sequence homology, immunology, substrate specificity and isoelectric point. GSTs catalyze the reactions between reduced GSH and unsaturated aldehydes, quinines, and many other substrates, especially under conditions of oxidative stress[1-3]. These enzymes are involved in many physiological functions, such as the reduction of free radical damage, detoxification, metabolism, regulation of cell signaling and nitric oxide storage[2-7]. The abundance of GSTs is closely related with the disease status of the organism[8-12]. For instance, regarding a cancer biomarker, GST pi was found in higher abundance in several cancer cells and tissues and was believed to be involved in drug resistance[13-15]. GST alpha has main functions in detoxification in the liver, and when hepatocytes are damaged, GST alpha enters the bloodstream. Therefore, GST alpha in blood and urine is an ideal marker indicating hepatocellular impairment[16]. The accurate measurement of GSTs in tissues or body fluids is urgently required in biomedicine.
GSTs have been identified mainly in the cytoplasm, but they have also recently been detected in organelles, including the microsome, nucleus, mitochondria and peroxisomes[17-20]. Mitochondria are the primary intracellular sites of oxygen consumption and reactive oxygen species (ROS) generation. GST kappa1 was identified in mitochondria and peroxisomes but is absent in the cytoplasm, and GST kappa was reported to participate in energetic and lipid metabolism in the mitochondria[21]. In contrast to the cytoplasmic GSTs, the physiological functions of the mitochondrial GSTs have yet to be explored. Generally, it is accepted that GSTs are able to protect mitochondria from dysfunctions of catalase and superoxide dismutase by maintaining the redox balance[22-24]. A high abundance of GST pi protected the decrease of the mitochondrial membrane potential induced by rotenone. Raza group observed that under oxidative stress, GST alpha was translocated from the cytoplasm into the mitochondria, but its functions in the mitochondria were not elucidated[23]. To understand the functions of the mitochondrial GSTs fully, we must first define how many GST isoforms exist in the mitochondria. Although there were several reports regarding the distribution of GSTs in different tissue mitochondria, a systematic investigation in this field has not been undertaken.
Recently, proteomic approaches have provided a good opportunity to survey the members of a protein family, as they usually share similar properties, such as immuno-affinity, ligand binding sites, homology sequences and catalytic substrates. Using chromatography or electrophoresis based on these properties, protein family members could be separated from one another in a mixture. Moreover, mass spectrometry is able to identify the separated proteins. Such a proteomics strategy has been widely used in exploring protein isoforms[25-27].
In this study, we propose a proteomic strategy to define the GSTs in mouse tissue mitochondria. With combined separation based on size exclusion and affinity chromatography, we enriched the mitochondrial GSTs from a mitochondria preparation of mouse liver. Using MALDI TOF/TOF MS or ESI MS/MS, five GSTs were identified in the mitochondria. The presence of GSTs was further verified by Western blot using monoclonal antibodies. We constructed calibration curves of the GST quantification and employed a quantitative assay of the immunoblots to estimate the different abundances of the mitochondrial GSTs between normal and diabetic mice. For the first time, we found that GSTs are widely distributed in the mitochondria of many tissues and that mitochondrial GST pi1 is sensitive to the development of diabetes.
The C57BLKS/J db/db and control mice were provided by Peking University Diabetes Center. The heart, kidney and liver tissues of the mice were minced and homogenized in buffer (25 mmol sucrose, 0.5% protease inhibitor cocktail and 10 mmol HEPES, pH 7.4). The crude mitochondria were prepared by differential centrifugation at 1000 ×g for 30 min and at 10 000 ×g for 20 min at 4 °C. The purified mitochondria were extracted from a Nycodenz gradient at the interface of 25%-30% Nycodenz solution after centrifugation at 52 000 ×g for 90 min. The purity and integrity of the mitochondria were determined by Western blotting and transmission electron microscopy (TEM). Mitochondrial proteins were extracted using lysis buffer [7 mol/L urea, 2 mol/L thiourea, 4% CHAPS, 40 mmol/L Tris-HCl (pH 7.4) and protease inhibitor cocktail].
The animal experiments described in this article were approved by the Animal Care and Welfare Committee at the Beijing Institute of Genomics, Chinese Academy of Sciences.
We purified the GSTs using GSH-affinity chromatography with GSH-Sepharose 4B (Amersham Biosciences, United states). The GSH-Sepharose 4B was equilibrated with binding buffer [150 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 8.0), 1 mmol/L ethylene glycol tetraacetic acid, and 0.1% Triton × 100]. The mitochondria were resuspended in 500 μL binding buffer and were sonicated. After centrifugation, the supernatant was mixed with the equilibrated resin and centrifuged for 30 min 3000 r/min at 4 °C. The affinity resin was washed 3 times with binding buffer, and the proteins were eluted from the resin using 30 mmol/L reduced GSH. A sample of the elution products was retained for two-dimensional electrophoresis (2-DE) separation.
The first dimension separation was conducted using an Ettan IPGphor IEF system with 7 cm (pH 6-11) IPG strips at 20 °C. The proteins isolated by GSH-affinity chromatography were loaded onto strips, and the strips were rehydrated without voltage for 4 h and with 50 V for 8 h. The isoelectric focusing was programmed for 1 h at 500, 1000 and 4000 V, respectively, and was subsequently focused at 4000 V up to a total of 30 kVh. The focused strips were equilibrated in buffer with 6 mol/L urea, 50 mmol/L Tris-HCl, 30% glycerol, 2% SDS and trace bromophenol blue and were subsequently reduced by dithiothreitol and alkylated by iodoacetamide. The treated strips were inserted into a 15% SDS-PAGE gel running in 2.5 W (each gel) for 30 min and 15 W (each gel) thereafter until the bromophenol blue dye reached the bottom of the gels. The gels were stained by silver staining.
The proteins were identified by two mass spectrometry methods: MALDI TOF/TOF and LC ESI MS/MS.
The proteins that were separated by GSH-affinity chromatography and 2D gel electrophoresis were excised and in-gel digested with trypsin overnight and identified by MALDI TOF/TOF MS. Briefly, the tryptic digests were co-crystallized with a matrix of a-cyna-4-hydroxycinnamic acid spotted onto the AnchorChip and desalted by 0.1% trifluoroacetic acid. The AnchorChip was analyzed using an Ultraflex TOF/TOF MS mass spectrometer (Bruker Dalton, Bremen, Germany) for protein identification. Positively charged ions were analyzed in the reflector mode. Typically, 100 shots were cumulated per spectrum in the MS mode and 400 shots in the MS/MS mode. The mass spectra and tandem mass spectra obtained were processed using the FlexAnalysis 2.2 and BioTools 2.2 software tools. The protein identification was performed using the Mascot software (http://www.matrixscience.com), and the NCBInr database was searched using mouse as the taxonomy. The following parameters were used for the database searches: one incomplete cleavage, alkylation of cysteine by carbamidomethylation, oxidation of methionine, and pyro-Glu formation of the N-terminal Gln.
The 20-30 kDa proteins separated by SDS-PAGE were a mixture of many proteins, and the proteins were examined by LC ESI MS/MS after the in-gel trypsin digestion. Briefly, after capillary reversed-phase high-performance liquid chromatography, the separated peptides were analyzed using an ion-trap mass spectrometer LCQ DecaXP ion-trap mass spectrometer (Thermo Finnigan, Ringoes, NJ) with 3.2 kV of spray voltage and 150 °C at the heated desolvation capillary. A mass-to-charge (m/z) range from 400 to 2000 was scanned over 1.2 s, and the ions were detected with a high energy conversion dynode detector. The MS/MS data were converted into DTA-format files, which were further searched for proteins with Sequest software. All of the accepted results had a deltaCn of 0.1 or greater. Furthermore, a singly charged peptide must be tryptic, and the cross-correlation score (Xcorr) had to be more than 1.9. The tryptic peptides with a charge state of + 2 must have a Xcorr of more than 2.2. Triply charged tryptic peptides were accepted if the Xcorr was ≥ 3.0.
To generate the specific antibodies against these five GST isoforms, we cloned these five GST genes and expressed the recombinant protein by inserting these genes into the prokaryotic expression vector, named pET-28a. The recombinant proteins expressed from the pET-GST plasmids were 6 × His-tagged. After expression in E.coli BL21 (DE3), the proteins were purified using Ni-NTA agarose resin (Qiagen, United States) and metal chelate chromatography.
Six-weeks-old female BALB/c mice were immunized subcutaneously with the recombinant protein in Freund’s complete adjuvant. After booster injections, the mice with positive serum immunogenicity against the recombinant protein were used for the monoclonal fusion experiments. The mice’s spleens were excised, and single-spleen cell suspensions were fused with Sp2/0 myeloma cells. After several days of fusion, the hybridomas were picked and cultured in complete medium supplemented with 1% HT. Ascitic fluids were collected from the mice after an intraperitoneal injection of the hybridomas, and the antibodies were purified by protein A/G-Sepharose affinity chromatography. The protein concentrations of the purified antibodies were determined using the Bradford method, and the antibodies were diluted to 1 mg/mL and stored in 50% glycerin at -20 °C.
Proteins were separated by 15% SDS-PAGE and were electro-transferred onto polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were blocked with 5% non-fat milk dissolved in Tris-buffered saline with 0.05% Tween-20 (TTBS) at 37 °C for 90 min. The membranes were incubated with the primary antibodies at a dilution of 1:5000 in blocking reagent at room temperature for 2 h. The antibodies against the GSTs were generated by our laboratory, and the anti-ATP synthase β antibody was purchased from BD Biosciences. The membranes were incubated in goat anti-mouse/rabbit IgG conjugated with horseradish peroxidase at a 1:3000 dilution at room temperature for 1 h. The membranes were developed using the Super ECL Plus Detection Reagent kit, and the images were captured using ImageQuant ECL (GE Healthcare, United Kingdom).
To quantify the GSTs, calibration curves were constructed using the protein concentrations and immuno-signal obtained from the Western blotting. To analyze the quantities of GSTs in the diabetes mouse model statistically, the relative intensity of the Western blotting signals in the samples was quantified using ImageQuant TL software. The three pairs of samples from the control and db/db mice were then randomly paired, and the relative abundance ratios of the five GST proteins were statistically analyzed and were normalized using the levels detected in normal mice based on two parallel experiments. All of the values are expressed as the mean ± SD, and the statistical significance was set to a P < 0.05.
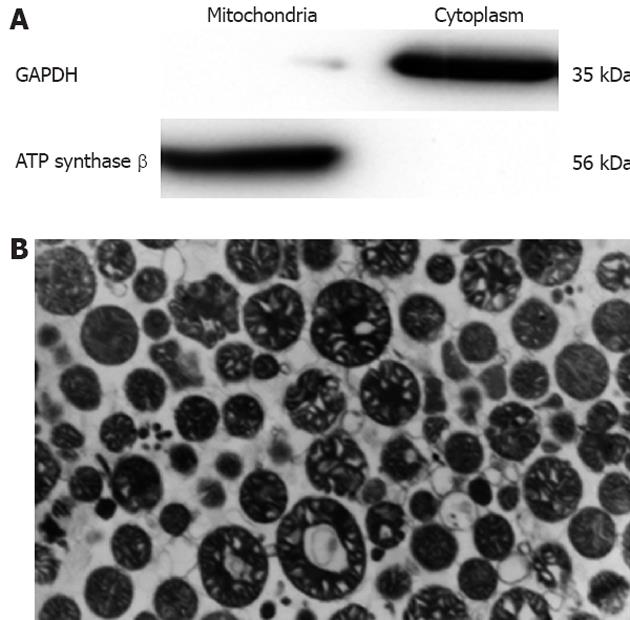
Considering that the purity of the mitochondria is a key element in studying mitochondrial components, Nycodenz gradient centrifugation was employed for the preparation of the mouse liver mitochondria. The mitochondrial integrity and purity were examined by Western blot and TEM. Our data as shown in Figure 1 indicated that the purity and integrity of the mitochondria were satisfactory for further analysis.
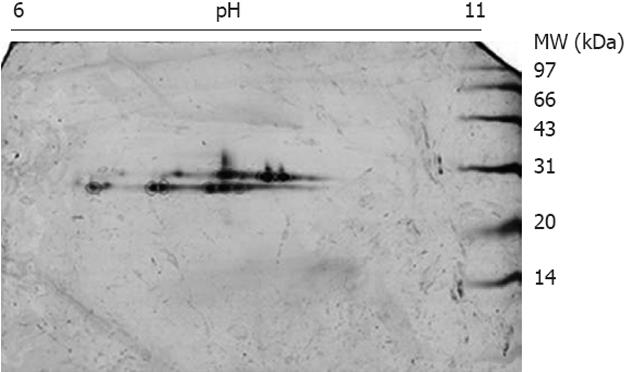
The mitochondrial lysates were loaded onto GSH affinity columns, and the fractions eluted by GSH were collected and subjected to 2DE using pH 6-11 strips and 15% SDS-PAGE. After silver staining, a string spots of approximately 28 kDa and pI 7-10 appeared on the gels (Figure 2), whereas no spots were detected in other parts of the gel, indicating that the affinity enrichment was effective to remove non-GSH-binding proteins. The silver-stained spots were excised and digested with trypsin followed by protein identification using MALDI TOF/TOF MS. In total, three GSTs, GST alpha3, GST mu1 and GST pi1, were found in the liver mitochondria. Because all of the GSTs have similar MWs (about 28 kDa), we attempted to separate the mitochondrial GSTs from the other mitochondrial proteins through size-exclusion methods. The mitochondrial lysates were loaded onto a 15% SDS-PAGE gel, and the portion corresponding to 20-30 kDa was sliced into 19 fractions. These fractions were digested with trypsin, and the digested fractions were further analyzed using LC ESI MS/MS. All of the unique peptide sequences of GSTs are listed in Table 1, indicating five GSTs: GST pi1, GST alpha3, GST mu1, GST kapp1 and GST zeta1. All of the GSTs identified by GSH/2DE/MALDI TOF/TOF MS are included in the list of the identified GSTs by SDS-PAGE/LC ESI MS/MS; therefore, the SDS-PAGE/LC ESI MS/MS approach exhibits a better sensitivity for the detection of GSTs.
| GST | Peptide sequence | MH+ | Location | XC | DeltaCn | Ions |
| Mu1 | MLLEYTDSSYDEK | 1593.699 | 19-31 | 4.062 | 0.47 | 20|24 |
| ADIVENQVMDTR | 1390.664 | 97-108 | 4.1055 | 0.5208 | 17|22 | |
| MQLIMLCYNPDFEK | 1801.811 | 109-122 | 4.8593 | 0.5987 | 20|26 | |
| Zeta1 | GIDYEIVPINLIK | 1486.852 | 28-40 | 3.0229 | 0.3943 | 15|24 |
| VITSGFNALEK | 1178.642 | 134-144 | 2.6217 | 0.4564 | 16|20 | |
| Kappa1 | FLTTVSMEQPEMLEK | 1782.866 | 102-116 | 4.944 | 0.5723 | 22|36 |
| AGMSTAQAQHFLEK | 1518.737 | 145-158 | 2.4224 | 0.2908 | 13|16 | |
| Alpha3 | SDGSLMFQQVPMVEIDGMK | 2111.982 | 46-64 | 3.9127 | 0.5378 | 21|28 |
| AILNYIASK | 992.578 | 70-78 | 3.0611 | 0.3416 | 17|26 | |
| YFPAFEK | 901.446 | 132-138 | 2.5832 | 0.3767 | 8|12 | |
| Pi1 | EAAQMDMVNDGVEDLR | 1792.785 | 86-101 | 3.9673 | 0.5391 | 22|30 |
| YVTLIYTNYENGK | 1577.785 | 104-116 | 3.6743 | 0.4268 | 18|24 |
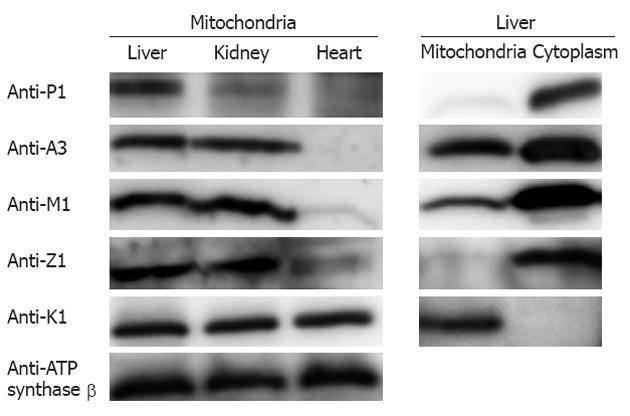
The existence of GSTs in the liver mitochondria was further confirmed by Western blotting using monoclonal antibodies against the individual GSTs (the specificity of detection is described below). As depicted in Figure 3, the five GST isoforms detected by the proteomic methods showed positive immunoreactivity; this evidence strongly supports the conclusion reached with the proteomic analysis. Furthermore, we prepared the tissue mitochondria from mouse kidney and heart and conducted Western blotting to evaluate the abundance of the GSTs in the mitochondria from different tissues. The left panel in Figure 3 reveals the wide distribution of GSTs in the mitochondria from the three tissues with relatively different abundances. As a specific GST only located in mitochondria, GST kappa1 exists in all three tissues with similar abundances. GST pi1 is clearly detected in the mitochondria of all three of the tissues, whereas GST pi1 in the liver mitochondria is in the highest abundance. The three GSTs, GST alpha1, GST mu1, and GST zeta1, are clearly observed in the liver and kidney mitochondria, whereas they are almost undetected in the heart. Furthermore, we examined the cytoplasmic GSTs and the mitochondrial GSTs in the mouse liver using Western blotting. The data illustrated in the right panel in Figure 3 indicate that GST kappa1 is the only isoform undetectable in the cytoplasm, whereas the other GST isoforms are readily detected in the two subcellular fractions. When equal amounts of proteins, either cytoplasmic or mitochondrial, are loaded onto an SDS-PAGE gel, the analysis of the relative intensities demonstrate that the abundances of GSTs in the cytoplasm are commonly higher than those in the mitochondria.
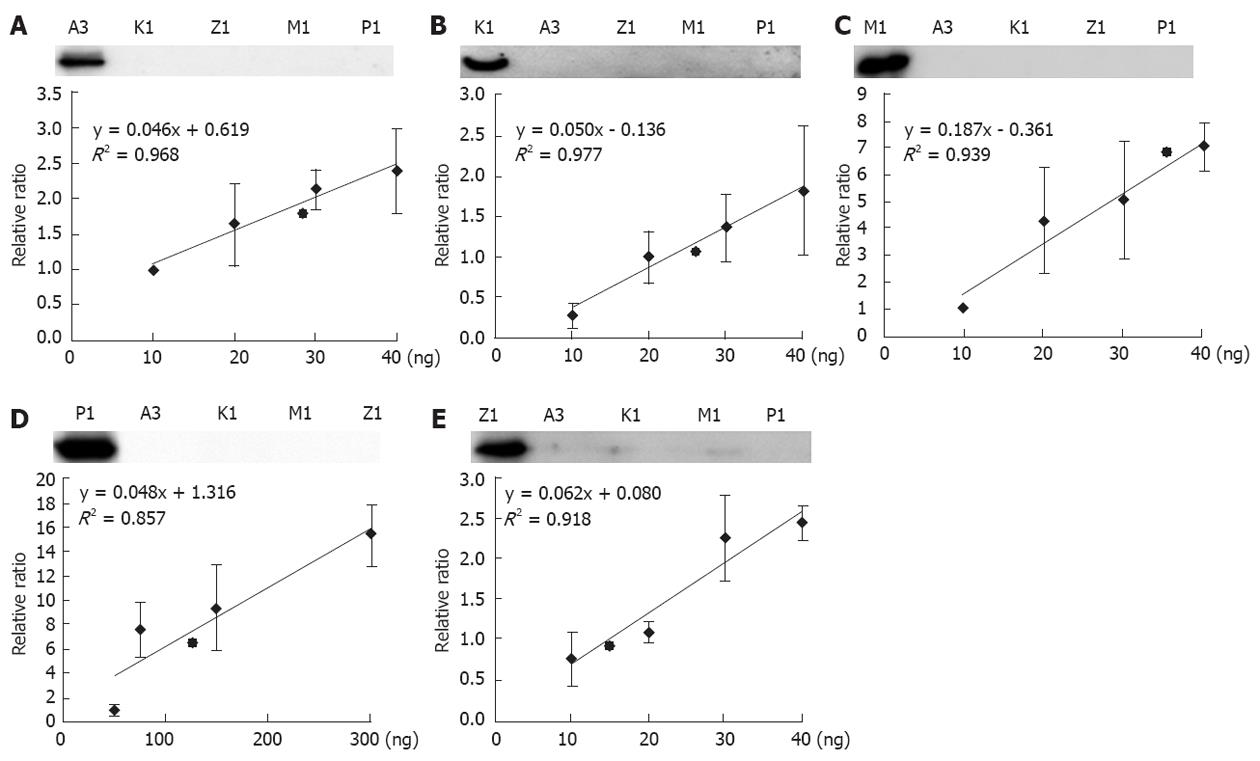
The five GSTs in liver mitochondria were first discovered by proteomic approach. As a routine assay in laboratory, mass spectrometry is not a common instrument and easy in use. Western blotting is still a widely acceptable and enough sensitive approach to detect proteins. We therefore developed a quantitative assay for the mitochondria GSTs based on immuno-blot. It is well known that specificity and sensitivity of antibody are a key factor for a successful Western blotting. To have the qualified antibodies against GSTs, we generated a set of monoclonal antibodies of GSTs, and performed a strict screening. A qualified antibody should match to criteria: (1) It can recognize 2 ng of the correspondent recombinant protein; and (2) It cannot pick up any signals to 20 ng of unrelated GSTs. The qualified GST antibodies are illustrated in the upper panel of Figure 4.
Selection of the concentration ranges for the recombinant GSTs is an important consideration for quantitative calibration. In our routine experiments, the maximum loading of mitochondrial proteins are approximately 20 μg; therefore, we loaded 20 μg of proteins and different amounts of recombinant GSTs on a relatively large scale onto the same gels and performed the Western blotting analysis. After determining the band intensities, we were able to estimate the proper amounts of the recombinant GSTs for the generation of calibration curves. As shown in Figure 4, five calibration curves are generated corresponding to the multiple assays of our Western blotting. However, Western blotting has a disadvantage in quantitative measurements because the reproducibility of the band intensities is relatively poor. The data shown in Figure 4 is in agreement with the observation, although some spots indeed display a large deviation. However, the regression calculation revealed that the values of R2 ranged from 0.85-0.98, which are generally accepted in quantitative measurements using Western blot. We further estimated the possible dynamics of the GST abundances in the liver mitochondria. With the exception of GST pi1, measuring approximately 50 ng to 300 ng, the other four GSTs were between 10 ng to 40 ng, indicating that GST pi has the highest abundance in liver mitochondria. According to the regression curves in Figure 4, the mitochondrial GST abundances were quantitatively estimated as follows: 134.61 + 53.84, 38.83 + 2.33, 29.25 + 0.29, 27.28 + 0.27, 15.84 + 0.16 ng per µg mitochondria protein for GST pi1, mu1, alpha3, kappa1, and zeta1, respectively.
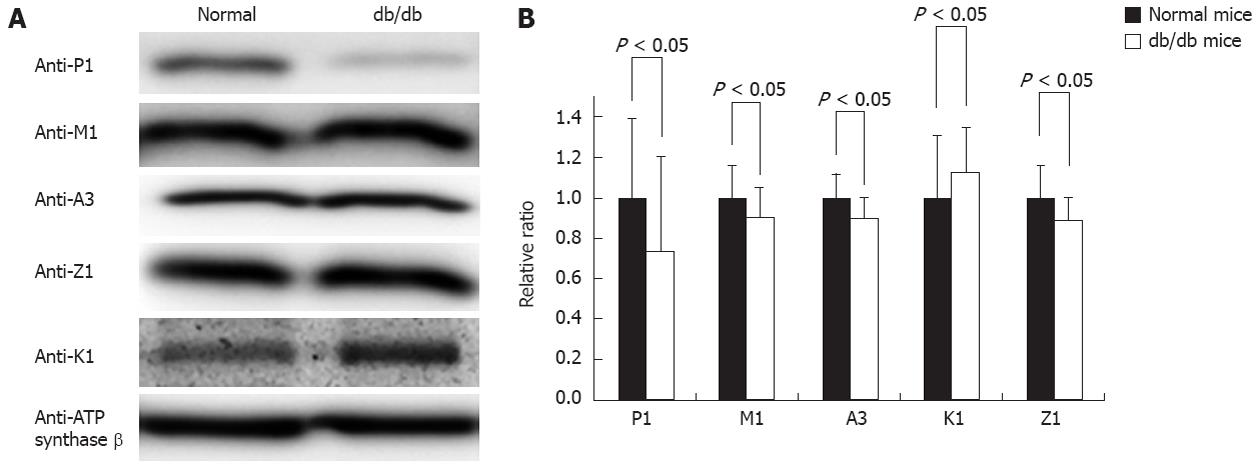
The db/db mouse is a well-accepted type II diabetes model, and previous studies have shown that free radicals were increased in most of the tissues in this model. After carefully examining the mice’s blood glucose and body weight to ensure diabetes development, we collected the livers from three normal and three db/db mice; the prepared mitochondria were analyzed by Western blot to quantify the contents of the GSTs. Figure 5A shows such a typical Western blotting, suggesting that the immunoreactivity of GST pi1 is significantly attenuated in the mitochondria of the diabetic mice, the signal of Western blotting in control and db/db diabetic mice liver mitochondria is 134.61 ± 53.84 vs 99.74 ± 46.2, with P < 0.05, whereas the other four GST isoforms are nearly comparable between the normal and diabetic mice. Statistically, we performed pairwise comparisons and estimations of the significant differences based on multiple Western blotting. The comparison data depicted in Figure 5B support the conclusion drawn from Figure 5A that the abundance of mitochondrial GST pi1 in diabetic mice is lower than in normal mice, whereas the other mitochondrial GSTs did not exhibit significant changes.
We screened the response of the GSTs to diabetes in the db/db liver mitochondria. As depicted in Figure 5A, which shows representative images of mitochondrial tissue from one pair of mice (control vs db/db), GST pi1 showed a decrease in the db/db mouse when we compared three pairs of mice. In contrast, similar expression levels of GST alpha3, mu1, and zeta1 were observed in the liver mitochondria of the db/db mice and control mice. The statistical analysis indicated in Figure 5B supported these observations.
GSTs have been detected as a group of oxidative stress proteins in mitochondria, and they were characterized as associated with the maintenance of mitochondria functions[22,28]. For example, alpha-class isozymes of GST translocated into the mitochondria under oxidative stress, and the isozymes showed glutathione peroxidase activity toward phospholipid hydroperoxide in the rat liver cytosol. Chemicals that generate reactive oxygen species, such as rotenone and antimycin A, reduced the cell viability and mitochondrial membrane potential, and the overexpression of GST pi diminished these changes.
In general, GSTs were determined by their enzymatic activities and immunological features; however, these methods had limitations in evaluating the presence of GST. The substrate specificity of purified rat liver GSTs has been investigated by a series of gamma-glutamyl-modified GSH analogues, and GST had a different ability to conjugate with the substrates. Furthermore, a product of the purification using GSH could not provide the status of GST in vivo. Therefore, the uncertainty of the type of GSTs localized in the mitochondria and the antibody specificity led to the uncertainty of an increase or decrease of GSTs under oxidative stress or in a pathological condition.
In this study, we have taken multiple approaches to identify the GSTs in mouse liver mitochondria. The mitochondrial proteins were separated by SDS-PAGE, and the 20-30 kDa proteins were analyzed by liquid chromatography and subsequently identified by ESI MS/MS. Five GST isoforms were detected in the liver mitochondria. Our strategy has two advantages: more proteins could be resolved regardless of the solubility by SDS-PAGE, and the sample complexity was reduced when the proteins distributed in the 20-30 kDa range were divided into 19 fractions for digestion and separation by liquid chromatography. Although we could not dismiss the possibility of other GSTs in the mitochondria, our multiple proteomic techniques provided new insights into analyzing GST isoforms, especially for those of low abundance.
GSH is not generated in mitochondria and is instead transferred from the cytoplasm; a GSH concentration of 5-10 mmol is maintained in mitochondria[29]. GSH is a major defense molecule against ROS, and mitochondrial GST may play an important role in responding to chemical and oxidative stress because GST is the main phase II enzyme that catalyzes the conjugation of GSH with numerous reactive electrophiles. The identification of GSTs would influence the study of the functions of mitochondrial GST.
We discovered GST zeta1 in the mitochondria. GST zeta1 is localized in the cytoplasm and plays a significant role in the catabolism of phenylalanine and tyrosine[5]. When GST zeta1 is lacking in mice, the inductions of the class alpha, mu, and pi GSTs could be detected by Western blotting and high-performance liquid chromatography analysis of glutathione affinity-purified proteins[30]. The role of GST zeta1 in the mitochondria will be investigated further.
The expression of GSTs is different in distinct tissues. Our data indicate that mitochondrial GST also has tissue-specific expression. GSTs are abundant in the liver but are scarce in heart tissue. The abundance of GST kappa1 in the heart, kidney, and liver is similar, but this GST is only detected in the mitochondria, indicating that it might perform a conserved function in the mitochondria of different cell types.
We screened these mitochondrial GSTs in the diabetes mouse model and found that the GST response to oxidative stress varies: only GST pi1 is decreased in the db/db mouse compared to normal mice, and the levels of the other four isoforms did not change. This result indicated that a decrease of GST pi1 might accelerate the pathological progress of diabetes.
The glutathione (GSH) S-transferase (GST) involve in many physiological functions. They are located mainly in the cytoplasm, but they have also been detected in suborganelles. Because mitochondria are the primary intracellular sites of oxygen consumption and reactive oxygen species generation, the mitochondrial GSTs abundances may relate with disease status. The goal was to survey GST isoforms in mitochondria and to reveal their biological significance in diabetic mice.
It is reported that GSTs are able to protect mitochondria from oxidation by maintaining the redox balance, but their functions in the mitochondria were not elucidated. To fully understand the functions of the mitochondrial GSTs, new method to monitor the mitochondrial GSTs is urgently required.
The mitochondrial GSTs are measured by the combined quantitative proteomic strategies based on mass spectrum and antibodies. The authors discovered that GSTs are widely distributed in tissue mitochondria and their responses to the diabetes physiology. It is noted the mitochondrial GST pi1 is sensitive to diabetic development.
This research showed how the proteomics produces meaningful information and offered a relatively easy and simple workflow to detect GSTs in liver mitochondria and to reveal their biological significance of normal and diabetes mouse. At the same time, the application of this strategy provides an alternative tool to analyze how isoforms of protein family response to disease in complex biological systems.
Proteomics is the protein map of a biological system and involves the systematic study of proteins in order to provide a comprehensive view of the function and regulation of proteins.
This paper provides solid data on systematic analysis of GST isoforms in mouse liver mitochondria.
Peer reviewer: You-Yong Lu, Professor, Beijing Molecular Oncology Laboratory, Peking University School of Oncology and Beijing Institute for Cancer Reaearch, 52, Fucheng Road, Haidian District, Beijing 100036, China
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Raza H, Robin MA, Fang JK, Avadhani NG. Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J. 2002;366:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol Appl Pharmacol. 2008;230:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Jakoby WB. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2571] [Cited by in RCA: 2685] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 5. | Board PG, Anders MW. Glutathione transferase zeta: discovery, polymorphic variants, catalysis, inactivation, and properties of Gstz1-/- mice. Drug Metab Rev. 2011;43:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Lok HC, Suryo Rahmanto Y, Hawkins CL, Kalinowski DS, Morrow CS, Townsend AJ, Ponka P, Richardson DR. Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes. J Biol Chem. 2012;287:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 851] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Ikeda K, Sakai K, Yamamoto R, Hareyama H, Tsumura N, Watari H, Shimizu M, Minakami H, Sakuragi N. Multivariate analysis for prognostic significance of histologic subtype, GST-pi, MDR-1, and p53 in stages II-IV ovarian cancer. Int J Gynecol Cancer. 2003;13:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ishisaki A, Hayashi H, Suzuki S, Ozawa K, Mizukoshi E, Miyakawa K, Suzuki M, Imamura T. Glutathione S-transferase Pi is a dopamine-inducible suppressor of dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:1362-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Shi M, Bradner J, Bammler TK, Eaton DL, Zhang J, Ye Z, Wilson AM, Montine TJ, Pan C, Zhang J. Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression. Am J Pathol. 2009;175:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Vlachogeorgos GS, Manali ED, Blana E, Legaki S, Karagiannidis N, Polychronopoulos VS, Roussos C. Placental isoform glutathione S-transferase and P-glycoprotein expression in advanced nonsmall cell lung cancer: association with response to treatment and survival. Cancer. 2008;114:519-526. [PubMed] |
| 12. | Arun BK, Granville LA, Yin G, Middleton LP, Dawood S, Kau SW, Kamal A, Hsu L, Hortobagyi GN, Sahin AA. Glutathione-s-transferase-pi expression in early breast cancer: association with outcome and response to chemotherapy. Cancer Invest. 2010;28:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Altinisik J, Balta ZB, Aydin G, Ulutin T, Buyru N. Investigation of glutathione S-transferase M1 and T1 deletions in lung cancer. Mol Biol Rep. 2010;37:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Bid HK, Konwar R, Saxena M, Chaudhari P, Agrawal CG, Banerjee M. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in north Indian population. J Postgrad Med. 2010;56:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Di Pietro G, Magno LA, Rios-Santos F. Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol. 2010;6:153-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Knapen MF, Peters WH, Mulder TP, Steegers EA. A marker for hepatocellular damage. Lancet. 2000;355:1463-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Goto S, Ihara Y, Urata Y, Izumi S, Abe K, Koji T, Kondo T. Doxorubicin-induced DNA intercalation and scavenging by nuclear glutathione S-transferase pi. FASEB J. 2001;15:2702-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Raza H. Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease. FEBS J. 2011;278:4243-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kawakatsu M, Goto S, Yoshida T, Urata Y, Li TS. Nuclear translocation of glutathione S-transferase π is mediated by a non-classical localization signal. Biochem Biophys Res Commun. 2011;411:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Gardner JL, Gallagher EP. Development of a peptide antibody specific to human glutathione S-transferase alpha 4-4 (hGSTA4-4) reveals preferential localization in human liver mitochondria. Arch Biochem Biophys. 2001;390:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Petit E, Michelet X, Rauch C, Bertrand-Michel J, Tercé F, Legouis R, Morel F. Glutathione transferases kappa 1 and kappa 2 localize in peroxisomes and mitochondria, respectively, and are involved in lipid metabolism and respiration in Caenorhabditis elegans. FEBS J. 2009;276:5030-5040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Goto S, Kawakatsu M, Izumi S, Urata Y, Kageyama K, Ihara Y, Koji T, Kondo T. Glutathione S-transferase pi localizes in mitochondria and protects against oxidative stress. Free Radic Biol Med. 2009;46:1392-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Thomson RE, Bigley AL, Foster JR, Jowsey IR, Elcombe CR, Orton TC, Hayes JD. Tissue-specific expression and subcellular distribution of murine glutathione S-transferase class kappa. J Histochem Cytochem. 2004;52:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Hagelin G. Mass spectrometric investigation of Maltacines E1a and E1b--two members of the Maltacine family of peptide antibiotics. Rapid Commun Mass Spectrom. 2005;19:3633-3642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Ma M, Sturm RM, Kutz-Naber KK, Fu Q, Li L. Immunoaffinity-based mass spectrometric characterization of the FMRFamide-related peptide family in the pericardial organ of Cancer borealis. Biochem Biophys Res Commun. 2009;390:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Agrawal P, Kumar S, Das HR. Mass spectrometric characterization of isoform variants of peanut (Arachis hypogaea) stem lectin (SL-I). J Proteomics. 2010;73:1573-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Zhang C, Yuan X, Mao W, Yue L, Kong X, Gao Y, Luo L, Yin Z. Inhibition of cadmium-induced apoptosis by glutathione S-transferase P1 via mitogen-activated protein kinases and mitochondrial pathways. Environ Toxicol Pharmacol. 2010;30:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci USA. 1985;82:4668-4672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 356] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Lim CE, Matthaei KI, Blackburn AC, Davis RP, Dahlstrom JE, Koina ME, Anders MW, Board PG. Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases. Am J Pathol. 2004;165:679-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |