Published online Jul 14, 2012. doi: 10.3748/wjg.v18.i26.3426
Revised: February 20, 2012
Accepted: February 26, 2012
Published online: July 14, 2012
AIM: To investigate the efficacy of hepatic arterial infusion chemotherapy (HAIC) using floxuridine (FUDR) in patients with advanced hepatocellular carcinoma (HCC) confined to the liver.
METHODS: Thirty-four patients who had advanced HCC with unresectability or unsuccessful previous therapy in the absence of extrahepatic metastasis were treated with intra-arterial FUDR chemotherapy at our hospital between March 2005 and May 2008. Among the 34 patients, 9 patients were classified as Child class C, and 18 patients had portal vein tumor thrombus (PVTT). One course of chemotherapy consisted of continuous infusion of FUDR (0.3 mg/kg during day 1-14) and dexamethasone (10 mg on day 1, 4, 7 and 11), and this treatment was repeated every 28 d.
RESULTS: Two patients (5.9%) displayed a complete response, and 12 patients (35.3%) had a partial response. The tumor control rate was 61.8%. The median overall survival times were 15.3 mo, 12.4 mo and 4.3 mo for the patients who were classified as Child class A, Child class B and Child class C, respectively (P = 0.0392). The progression-free survival was 12.9 mo, 7.7 mo and 2.6 mo for the patients who were classified as Child class A, Child class B and Child class C, respectively (P = 0.0443). The cumulative survival differed significantly according to the Child-Pugh classification and the presence of PVTT. In addition to hepatic reserve capacity and PVTT, the extent of HCC was an independent factor in determining a poor prognosis. The most common adverse reactions to HAIC were mucositis, diarrhea and peptic ulcer disease, but most of these complications were improved by medical treatment and/or a delay of HAIC.
CONCLUSION: The present study demonstrates that intra-arterial FUDR chemotherapy is a safe and effective treatment for advanced HCC that is recalcitrant to other therapeutic modalities, even in patients with advanced cirrhosis.
- Citation: Baek YH, Kim KT, Lee SW, Jeong JS, Park BH, Nam KJ, Cho JH, Kim YH, Roh YH, Lee HS, Choi YM, Han SY. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol 2012; 18(26): 3426-3434
- URL: https://www.wjgnet.com/1007-9327/full/v18/i26/3426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i26.3426
Hepatocellular carcinoma (HCC), which is one of the most common malignancies worldwide, causes over 600 000 deaths per year and is the third most common cancer in South Korea[1,2]. Although surgical resection or liver transplantation can be curative for HCC, most patients are not candidates for surgery at the time of diagnosis because it is difficult to detect HCC at an early stage. In addition, some patients have advanced cirrhosis by the time of diagnosis[3].
Although recent advances in therapeutic modalities, such as hepatic resection, percutaneous ethanol injection, radiofrequency ablation (RFA), transarterial chemoembolization (TACE), radiotherapy and liver transplantation, have improved the treatment of HCC, the prognosis for patients with advanced HCC remains poor. In addition, these current therapies have many limitations, and recurrence and metastasis are relatively common[4-7]. Among the currently available therapies, TACE is the current standard of care for patients who are not candidates for curative therapy. The survival benefit conferred by TACE was reported in a randomized controlled trial[8], which showed that the median survival of the patients in the TACE group was approximately 14 mo. However, TACE is contraindicated in advanced HCC with main portal vein tumor thrombus (PVTT), massive or diffuse infiltration, poor liver function with Child class C, and severe hepatic arterioportal shunt.
Regional hepatic arterial infusion chemotherapy (HAIC) has also been used in patients with advanced HCC because liver cancers receive most of their blood supply from the hepatic artery, whereas normal liver tissue has a dual vascular supply (i.e., 20% of the blood supply comes from the hepatic artery, and the remaining 80% comes from the portal vein). Thus, HAIC may be used, albeit with caution, in cases in which TACE is not indicated or is ineffective[9]. HAIC may provide higher concentrations of chemotherapeutic agents directly to the HCC and produces minimal systemic concentrations of chemotherapeutic agents, which can minimize systemic toxicity[10].
Floxuridine (FUDR) is an active metabolite of 5-fluorouracil (5-FU) that has the advantage of being rapidly metabolized, with a 94%-99% extraction rate within the liver via first-pass metabolism. FUDR is maintained at an intrahepatic concentration that is more than tenfold greater than that of 5-FU, cisplatin, mitomycin or doxorubicin, which permits maximal tumor cell death while preventing systemic toxicity[11,12] .
Most previous studies have reported the efficacy of HAIC using 5-FU and cisplatin in advanced HCC or HAIC using FUDR in patients with hepatic metastasis from colorectal cancer[10,13,14].
In the present study, we evaluated the efficacy and toxicity profile of HAIC using FUDR in patients with advanced HCC confined to the liver who failed to respond to previous therapy or who were unable to receive other therapeutic modalities.
Thirty-four patients with advanced HCC that was unresectable or resistant to previous therapy in the absence of extrahepatic metastasis were treated with intra-arterial FUDR chemotherapy at our hospital between March 2005 and May 2008. The criteria for unresectability included bilobar disease with 4 or more lesions, large tumors occupying more than 50% of the liver, and invasion of the tumor into major vascular structures. Previous therapies included RFA, TACE and radiotherapy. All of the patients belonged to tumor node metastasis stage IIIA or IIIB. To assess the eligibility for inclusion, each patient received a computed tomography (CT) scan of the abdomen and pelvis. Each patient provided a full medical history and underwent a physical examination, chest X-ray, and laboratory tests, including a test for alpha-fetoprotein. Additional examinations, such as magnetic resonance imaging (MRI) scans, liver biopsy and angiography, were performed if the CT scan and tumor marker analyses were insufficient for diagnosis. Among the 34 patients, disease was histologically confirmed in 5 patients. When metastasis was suspected, positron emission tomography scans and bone scans and/or CT scans of suspicious areas were conducted. Patients with distant metastases were excluded. Informed consent was obtained from all of the patients.
A pump catheter was inserted at the proper hepatic artery from the superior mesenteric artery. The distal gastroduodenal artery, the right gastric artery, the small branches supplying the stomach or duodenum, and all of the accessory hepatic arteries received ligations to prevent gastrointestinal toxicity. The patients with hepatitis B received prophylactic or therapeutic antiviral agents before HAIC. Some of the patients with hepatitis C had previously been treated with pegylated-interferon/interferon and ribavirin. The remaining hepatitis C patients were carefully monitored because antiviral treatment was deferred because of underlying bone marrow and immune suppression complications.
The patient responses were classified according to the Response Evaluation Criteria In Solid Tumors guidelines. Complete response (CR) was defined as the disappearance of all evidence of disease and the normalization of tumor markers for at least 4 wk. Partial response (PR) was defined as a ≥ 30% reduction in unidimensional tumor measurements without the appearance of any new lesions or the progression of any existing lesion. Progressive disease (PD) was defined as any of the following: a 20% increase in the sum of the diameters of five measurable lesions, the appearance of any new lesions, or the reappearance of any lesion that had previously disappeared. Stable disease (SD) was defined as a tumor response that did not fulfill the criteria for CR, PR or PD. CT scans or MRIs of the measurable lesions were carried out within 4 wk before the start of treatment and repeated every 2 cycles (2 mo). Responses were confirmed by subsequent CT or MRI scans after the documentation of the initial response.
Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria. The FUDR dose was modified when grade 3-4 toxicity was observed.
Hepatic toxicity was defined as a significant increase over baseline values (3- to 4-fold for aspartate transaminase or alanine transaminase and greater than 1.5-fold for bilirubin), and the increases in hepatic enzyme levels caused by the disease varied across patients. If a patient complained of epigastric pain, an evaluation that included an upper gastrointestinal endoscopy was performed. If an ulcer or gastroduodenitis was identified, then chemotherapy was stopped until recovery. If a patient had severe diarrhea or abdominal pain, chemotherapy was stopped until recovery. In addition, angiography was performed to block collateral vessels when extrahepatic perfusion was suspected.
Local chemotherapy was started between 3 and 5 d after pump insertion. The patients received FUDR (0.3 mg/kg per day for 14 d) and dexamethasone (10 mg on day 1, 4, 7 and 11) via an intra-arterial pump. FUDR was synthesized by APP Pharmaceuticals, LLC (Schaumburg, IL, United states). After the 14 d of treatment with FUDR, the pump was emptied and refilled with 30 000 units of heparin in 0.9% saline for 14 d. This treatment was repeated every 28 d. FUDR was given indefinitely until the disease progressed or the therapy was discontinued due to toxicity or patient death.
The objective of the present study was to estimate the efficacy and toxicity of continuous HAIC with FUDR via an implantable pump. All of the analysis were performed using SPSS version 18.0.
Survival times were calculated from the start of the study treatment until patient death or the final follow-up. Progression-free survival (PFS) was calculated from the first day of chemotherapy until the date of progression. PFS and overall survival (OS) curves were obtained using the Kaplan-Meier method, and comparisons were made using the log rank test. Multivariate analysis to evaluate the influence of prognostic factors on survival was performed using Cox proportional hazard methods. Statistical significance was established as P < 0.05.
A total of 34 patients, 27 men and 7 women, with a median age of 62.2 years received intra-arterial FUDR chemotherapy between March 2005 and May 2008. The patient baseline characteristics are shown in Table 1. The major etiology of the patients’ liver disease was hepatitis B virus (27 of 34, 79.4%). Eighteen patients had PVTT, whereas 16 patients did not have PVTT. The majority of patients had received TACE as the previous therapeutic modality (20 of 34, 58.8%).
| Characteristics | IA-FUDR (n = 34) |
| Age (yr) | 62.2 ± 7.4 |
| Sex | |
| Male | 27 (79.4) |
| Female | 7 (20.6) |
| Cause of HCC | |
| Hepatitis B virus | 27 (79.4) |
| Hepatitis C virus | 2 (5.9) |
| Alcoholism | 5 (14.7) |
| Child-Pugh classification | |
| Child class A | 7 (20.6) |
| Child class B | 18 (52.9) |
| Child class C | 9 (26.5) |
| Portal vein thrombosis | |
| Yes | 18 (52.9) |
| No | 16 (47.1) |
| Tumor morphology | |
| Multinodular | 16 (47.1) |
| Huge, massive > 50% of liver | 18 (52.9) |
| Bilirubin (mg/dL) | 2.6 ± 2.3 |
| Albumin (g/dL) | 2.8 ± 1.1 |
| Prothrombin time (INR) | 2.0 ± 0.7 |
| AFP (ng/mL) | 5136.03 (median) |
| Treatment prior to chemotherapy or supportive care | |
| Surgery | 0 (0) |
| RFA | 3 (8.8) |
| TACE | 20 (58.8) |
| RFA and TACE | 1 (2.9) |
| Radiation | 1 (2.9) |
| No treatment | 9 (26.5) |
Patients received 2-10 (the median was 3.5) cycles of chemotherapy. All of the patients received at least 2 cycles of intra-arterial FUDR chemotherapy, and 17 (50%) patients received more than 4 cycles.
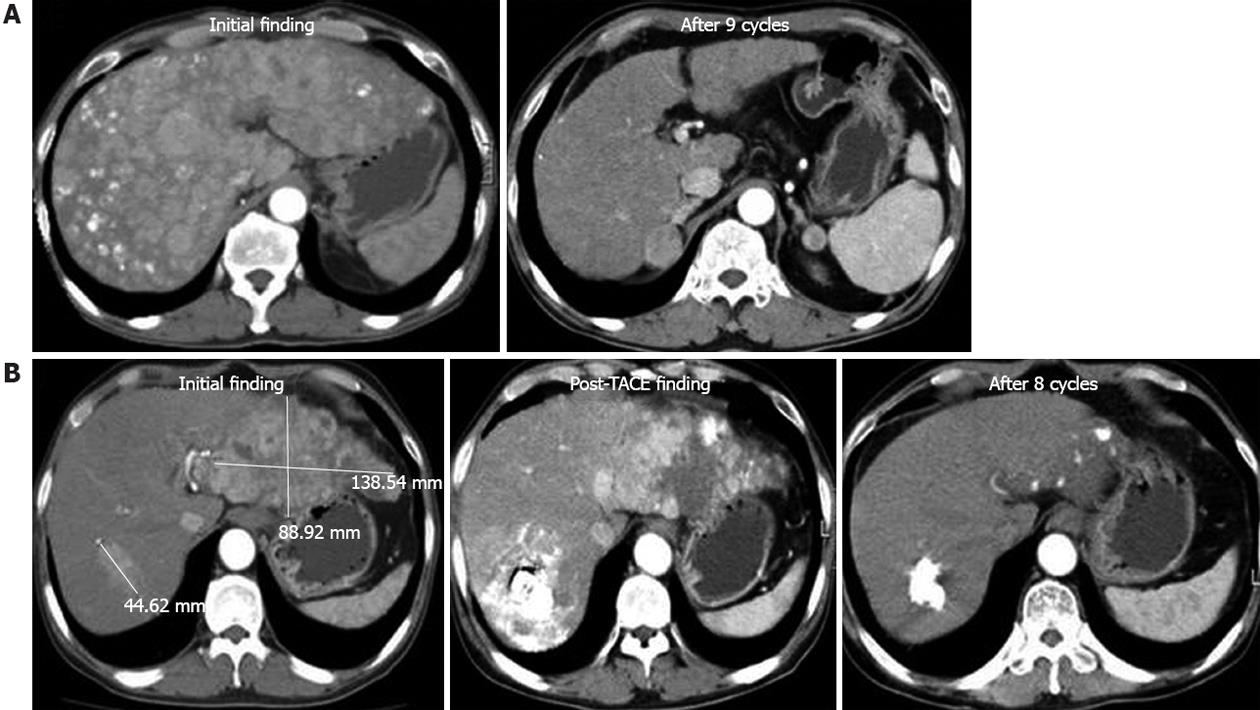
The patients’ responses to treatment are summarized in Table 2. Two patients (5.9%) had CR, 12 (35.3%) had PR, seven (20.6%) had SD, and 13 (38.2%) had PD. The CT findings for the two patients with CR (Figure 1). Among the patients who were classified as Child class A or B, 2 (8%) had CR and 11 (44%) had PR. The patients who were classified as Child class A or B had a response rate of 52% and a disease control rate of 72%.
| Tumor response | Intra-arterial FUDR chemotherapy | |||
| Total | Child class A | Child class B | Child class C | |
| CR | 2 (5.9) | 1 (14.3) | 1 (5.6) | 0 (0) |
| PR | 12 (35.3) | 2 (28.6) | 9 (50) | 1 (11.1) |
| SD | 7 (20.6) | 2 (28.6) | 3 (16.7) | 2 (22.2) |
| PD | 13 (38.2) | 2 (28.6) | 5 (27.8) | 6 (66.7) |
| Response rate | 14 (41.2) | 3 (42.9) | 10 (55.6) | 1 (11.1) |
| Disease control rate | 21 (61.8) | 5 (71.4) | 13 (72.2) | 3 (33.3) |
| Total | 34 (100) | 7 (100) | 18 (100) | 9 (100) |
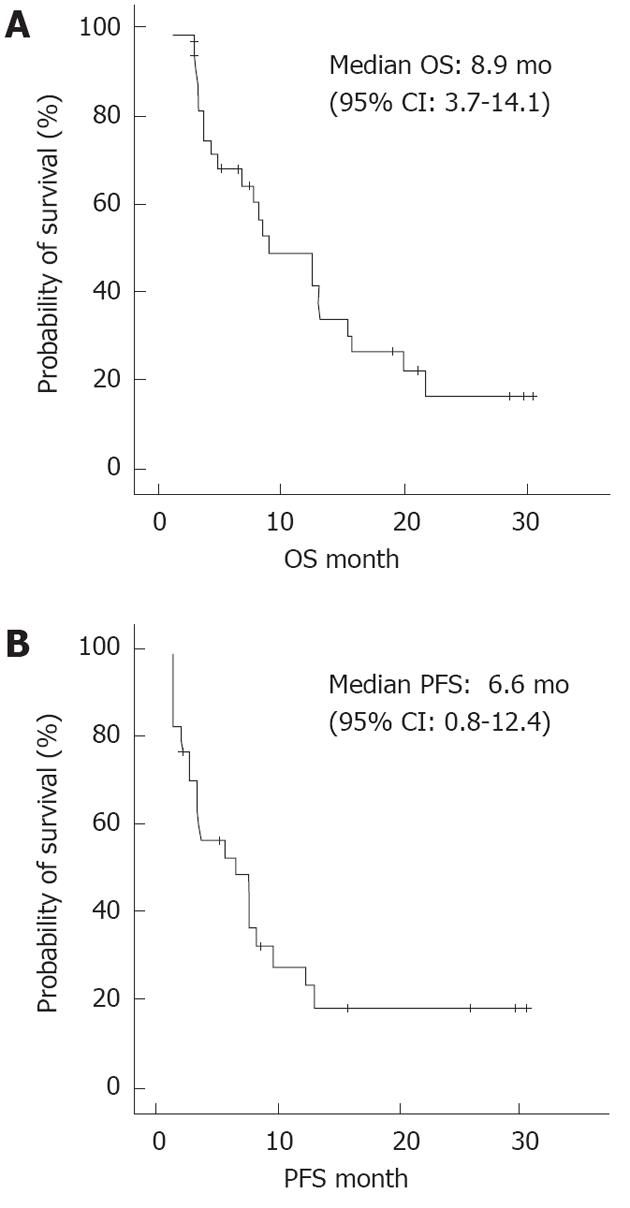
The cumulative survival of the 34 patients is presented in Figure 2A and B. The median OS was 8.9 mo, and the median PFS was 6.6 mo.
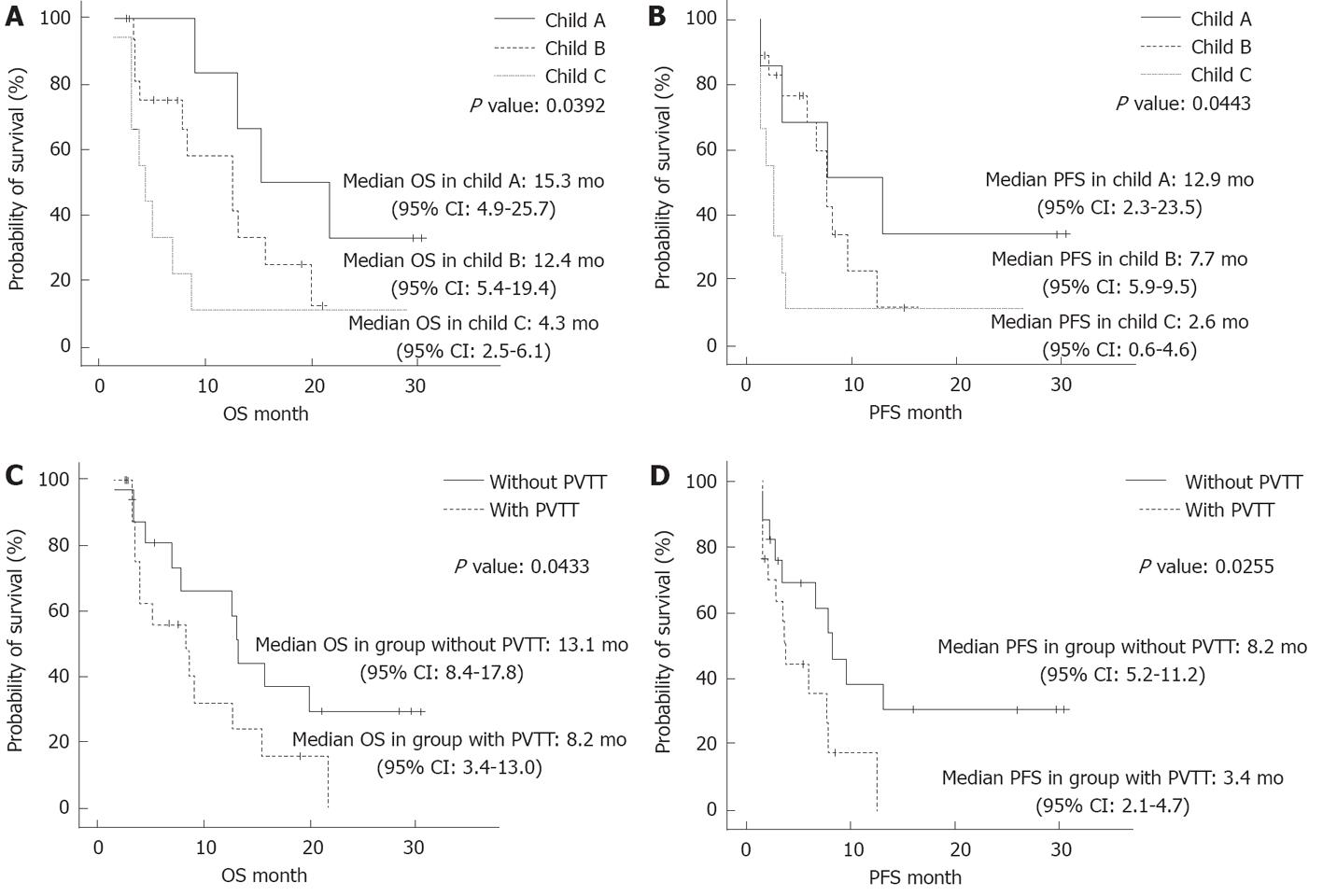
We assessed the cumulative survival according to the Child-Pugh classification and the presence of PVTT (Figure 3). The median OS times were 15.3 mo, 12.4 mo and 4.3 mo in the patients who were classified as Child class A, Child class B and Child class C, respectively, and there were significant differences between the groups (P = 0.0392). Similarly, there were significant differences in the median PFS times, which were 12.9 mo, 7.7 mo and 2.6 mo in the patients who were classified as Child class A, Child class B and Child class C, respectively (P = 0.0443) (Figure 3A and B). These findings showed that the patients who were classified as Child class C had significantly shorter survival times compared with the patients who were classified as Child class A or Child class B.
Further differences were found in the OS and PFS according to the presence of PVTT, which indicated a negative impact of PVTT on survival time. The median OS and PFS of the patients without PVTT were 13.1 mo and 8.2 mo, respectively, compared with 8.2 mo and 3.4 mo, respectively, for the patients with PVTT (Figure 3C and D).
We conducted univariate and multivariate analyses of baseline characteristics, such as age, sex, Child-Pugh classification, PVTT, extent of HCC, bilirubin, albumin and PT international normalized ratio (INR), using a Cox proportional hazards model to evaluate the prognostic factors for survival (Table 3). The multivariate analysis showed that 4 variables (i.e., child classification, PVTT, HCC type and PT INR) were independent predictors of survival.
| Multivariate | Hazard ratio | 95% CI | P value |
| Age (≥ 60 yr, < 60 yr) | 1.381 | 0.403-4.734 | 0.608 |
| Sex (male, female) | 1.462 | 0.433-4.937 | 0.541 |
| Child-Pugh classification | 3.710 | 1.490-9.238 | 0.005 |
| Portal vein thrombosis (without, with) | 0.086 | 0.019-0.387 | 0.001 |
| Extent of HCC | 0.185 | 0.051-0.679 | 0.011 |
| (multinodular, massive > 50% of liver) | |||
| Bilirubin (≥ 3, < 3) | 0.837 | 0.319-2.195 | 0.718 |
| Albumin (< 3, ≥ 3) | 1.059 | 0.430-2.603 | 0.901 |
| PT INR (< 2.3, ≥ 2.3) | 0.218 | 0.066-0.715 | 0.012 |
The treatment-related adverse reactions and complications that were observed in the 34 patients who were treated with HAIC (Table 4). Severe hematologic toxicity during HAIC was not noted in any of the patients. The most common grade 3-4 adverse reaction was gastric or duodenal ulcer (11.8%). Most of the adverse reactions were improved by medical treatment and/or delaying HAIC. One patient with hepatitis C experienced progressive hepatic failure during the third cycle of chemotherapy and eventually died. In addition, a major complication related to the indwelling catheter occurred in 1 patient. Indeed, an infection occurred around the catheter, but it was controlled by antibiotics and removal of the port. This patient continued HAIC after insertion of another catheter in the opposite site. Another major complication that has commonly been reported to be associated with FUDR treatment is biliary sclerosis; however, the patients in the present study received prophylactic dexamethasone on a regular schedule, and none of the present patients experienced biliary sclerosis.
| Treatment group (toxicity) | NCI-CTC grade | |
| 1-2 | 3-4 | |
| Fever | 2 (5.9) | 0 (0) |
| Nausea/vomiting | 0 (0) | 0 (0) |
| Gastric or duodenal ulcer | 3 (8.8) | 4 (11.8) |
| Mucositis | 4 (11.8) | 3 (8.8) |
| Diarrhea | 4 (11.8) | 3 (8.8) |
| Leukopenia | 2 (5.9) | 1 (2.9) |
| Thrombocytopenia | 2 (5.9) | 1 (2.9) |
| Bilirubin elevation | 4 (11.8) | 1 (2.9) |
| AST/ALT elevation | 5 (14.7) | 1 (2.9) |
| BUN/Cr elevation | 0 (0) | 1 (2.9) |
| Catheter infection | 1 (2.9) | |
| Total | 19 | 9 |
During treatment and follow-up, 9 patients died from various causes, including tumor progression, hepatic failure, gastrointestinal bleeding and sepsis. Among the patients who died, 4 (44.4%) died of hepatic failure related to an advanced cirrhotic condition. Only 1 of the 4 patients had hepatic failure in relation to therapy, and the remaining patients died after loss to follow-up or the incidence of another illness. Tumor progression and sepsis were the causes of death in 2 patients each (22.2%). One patient died of upper varix bleeding.
Many therapeutic modalities are available for the treatment of HCC, such as hepatic resection, percutaneous ethanol injection, RFA, TACE, radiotherapy and liver transplantation[15]. Among these therapies, TACE has been the main treatment modality for the management of unresectable or recurrent HCC. A randomized controlled trial revealed that the median survival for patients undergoing TACE was approximately 14 mo[8]. However, this treatment has not been useful in patients with PVTT or large infiltrative HCC because of the potential risk of hepatic failure resulting from ischemia[16,17].
HAIC can be safely used in patients with impaired liver function due to advanced HCC or underlying liver cirrhosis because hepatic arterial blocking agents, such as lipiodol or gelatin sponges, are not used in HAIC[18]. Thus, HAIC is not limited by tumor size, number, and/or proximity to major vasculature, all of which are common contraindications to resection and/or ablation. In addition, HAIC has several other advantages. For example, in most cases, there is no need for the administration of antiemetics or exogenous hydration, which can cause ascites or peripheral edema. Moreover, the higher first-pass hepatic extraction of infused drugs produces elevated local concentrations with lower systemic exposure, which results in fewer side effects than systemic chemotherapy[10].
HAIC using 5-FU with cisplatin has been extensively studied as a treatment for advanced HCC[9,19,20]. However, the pharmacokinetics of 5-FU are not linear over a hepatic extraction gradient of 19%-90%, and there are decreases in both systemic clearance and hepatic extraction at higher doses, which reduce the selective regional advantage[21].
Intra-arterial FUDR, which is a metabolite of fluorouracil, is preferable because it is associated with an increased response rate due to its higher hepatic extraction (> 95%) and an intrahepatic concentration that is more than tenfold greater than that of 5-FU, cisplatin, mitomycin or doxorubicin[11,12]. Therefore, FUDR is associated with decreased toxicity and improved survival through maximal tumor cell death. Despite these advantages, regional chemotherapy using FUDR via an implantable pump has rarely been studied in HCC, although it has been studied extensively in patients with liver metastasis from colorectal cancers[14,22-24]. Therefore, we hypothesized that the study of HAIC using FUDR in patients with advanced HCC could yield significant results.
In general, the prognosis is poor for patients with advanced HCC. Many studies have reported a median survival of 3-6 mo for unresectable and untreated HCC[25]. Recently, a multinational phase III, randomized, double-blind, placebo controlled trial was conducted to assess the efficacy and safety of new therapeutic options in a group of Asian-Pacific patients with advanced HCC [26]. The controlled trial reported a median OS and PFS of 4.2 mo and 1.4 mo, respectively, in the patients without treatment. Interestingly, most of the patients were classified as Child class A (Child class A: 220 and Child class B: 6). Many studies of HAIC have reported good results compared with those of untreated cases[27,28]. In the present study, the response rate and the median survival time of the 34 patients who were treated with intra-arterial FUDR were 41.2% and 8.9 mo, respectively. Despite the inclusion of patients who were classified as Child class C, the present outcomes are similar or better than those observed in other studies.
Studies have clearly shown that survival times are longer in patients with a good functional hepatic reserve. Many studies have shown that a patient’s Child-Pugh status significantly influences survival, which is consistent with the present results[29,30]. The OS times of the patients who were classified as Child class A, B and C were 15.3 mo, 12.4 mo and 4.3 mo, respectively. Similarly, the PFS times of the patients who were classified as Child class A, B and C were 12.9 mo, 7.7 mo and 2.6 mo, respectively. Most other studies excluded patients who were classified as Child class C because early reports demonstrated poor outcomes in these patients and no differences in survival between treated and untreated groups[29]. The present study included patients who were classified as Child class C if they demonstrated a desire to receive therapy and had a relatively good performance status. The nine patients in the present study who were classified as Child class C had similar or slightly better outcomes compared with untreated patients. Interestingly, only 1 of the Child class C patients suffered from a serious adverse event (i.e., hepatic failure). Compared with other studies, the present study also showed relatively good results in the patients who were classified as Child class B.
Like the Child-Pugh classification, PVTT is a major independent factor in the determination of a poor prognosis in patients with advanced HCC[30]. The median survival of untreated HCC with PVTT was reported to be 2.7 mo[31]. One study reported a median survival time of 6 mo in patients with advanced HCC with PVTT (excluding Child class C patients) who received HAIC with 5-FU and cisplatin along with systemic chemotherapy[32]. In the present study, the median OS in the groups with and without PVTT were 8.2 mo and 13.1 mo, respectively, and this difference was significant. In addition, the difference in the median OS times in the groups with and without PVTT also demonstrated the relatively good outcomes that were observed in the present study compared with those of other studies (despite the inclusion of Child class C patients with PVTT). The present study showed that the presence of PVTT, the extent of HCC, and hepatic function (as assessed by the Child classification) were major predictors of survival. Indeed, the present study demonstrated the significance of these factors using multivariate analysis.
Treatment tolerability and patient quality of life are also important when deciding therapies for advanced cancers. Only 1 of the 34 patients in the present study (2.9%) experienced progressive impaired hepatic function that justified the withdrawal of FUDR, and the majority of the patients demonstrated a relatively sustained quality of life during the HAIC.
Several studies have reported the outcomes of HAIC using FUDR in advanced HCC. One study investigated the efficacy of HAIC using FUDR for 5 patients with HCC and reported a response rate of 80% and a 1-year survival rate of 100%[22]. In 2009, Jarnagin et al[33] reported that HAIC with FUDR therapy could be effective and safe in patients with unresectable primary liver cancer. They reported a response rate of 25% and a tumor control rate of 62.5%. These two studies demonstrated positive results of HAIC with FUDR for HCC; however, they only included 5 and 8 patients, respectively. Compared with these two reports, the present study was significant in that it included more patients and demonstrated the efficacy and safety of HAIC with FUDR even in patients with advanced HCC.
Sorafenib was the first systemic agent to be approved for the treatment of advanced HCC. Both the Study of Heart and Renal Protection trial, which was conducted in Europe and North America, and an Asia-Pacific trial showed that sorafenib prolonged the time to progression by 1.4-2.7 mo and prolonged the OS by 2-3 mo[26,34]. In the present study, the outcomes of HAIC with FUDR were superior to treatment with sorafenib. In addition, there are limitations to sorafenib therapy, namely its decreased efficacy over time (i.e., disease-stabilization for only a few months) and potential side effects. Moreover, the safety and efficacy of sorafenib in patients who are classified as having Child class B or C cirrhosis remain unclear. Many factors could eventually limit the potential advantages of anti-angiogenic sorafenib effects. Several mechanisms of resistance to vascular endothelial growth factor signaling-targeted therapy have been proposed, and other pre-existing or distinct oncogenic signaling pathways may begin to drive tumor growth during therapy[35]. Recently, approaches to overcome resistance to anti-angiogenic sorafenib therapy in advanced HCC are being pursued. One of these approaches is the combination of anti-angiogenic therapy and metronomic chemotherapy to induce durable tumor shrinkage or disease stabilization in refractory cancer[36,37]. Therefore, the combination of sorafenib and HAIC with FUDR may be a promising therapeutic approach for advanced HCC.
The present study has several limitations. For example, high doses of FUDR in HAIC can produce toxicity, which results in a fibrotic narrowing of the bile ducts that is similar to primary biliary sclerosis (up to 30% of patients)[38]. The use of regional dexamethasone with FUDR, however, can reduce hepatic toxicity. In some studies, the biliary sclerosis rate was 3% or lower, and the patient response rate and survival also improved with the addition of dexamethasone[39]. None of the patients in the present study had biliary sclerosis, which was likely due to the inclusion of dexamethasone in our treatment protocol.
Another limitation of the present study was that FUDR had to be administered for 14 d with continuous infusion, which might have been associated with poor patient compliance. Hepatic drug uptake and metabolic capacity can be saturated at high drug delivery rates[40]. Therefore, continuous hepatic arterial infusion is regarded as the most effective means of delivery to maximize the regional advantage. In the present study, all but one patient continued therapy and showed relatively good compliance.
The final limitation of the present study was that we did not use a randomized design (i.e., HAIC was not compared with other therapeutic modalities). However, the present study was the first formal attempt to test HAIC using FUDR in patients with advanced HCC, including patients with advanced cirrhosis. Importantly, the present study demonstrated that survival was better in patients who received HAIC with FUDR compared with patients with unresectable tumors or in whom other therapeutic modalities had been used unsuccessfully.
In conclusion, the present study shows that intra-arterial FUDR chemotherapy is safe and effective for patients with severely advanced HCC confined to the liver for which other therapeutic modalities are ineffective, even in patients with advanced cirrhosis. Based on these results, additional large prospective randomized clinical trials should be performed to prove the efficacy and safety of HAIC using FUDR. Eventually, HAIC using FUDR could be widely applied for the treatment of advanced HCC.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Despite recent advances in therapeutic modalities, the prognosis of advanced HCC remains poor. The current therapies have many limitations, and recurrence or metastasis is relatively frequent. Regional hepatic arterial infusion chemotherapy (HAIC) has been used in patients with advanced HCC. HAIC is believed to provide higher concentrations of chemotherapeutic agents directly to the HCC, which would minimize systemic concentrations of chemotherapeutic agents and potentially minimize systemic toxicity. However, previous studies of HAIC in HCC have shown various results.
Floxuridine (FUDR) is an active metabolite of 5-fluorouracil (5-FU) that has the advantage of being rapidly metabolized (i.e., a 94%-99% extraction rate within the liver). Furthermore, FUDR is maintained at an exceptionally high intrahepatic concentration (i.e., more than tenfold greater than 5-FU, cisplatin, mitomycin or doxorubicin), which permits maximal tumor cell death while preventing systemic toxicity. In the present study, the authors demonstrated the efficacy and safety of HAIC using FUDR in patients with advanced HCC that was confined to the liver.
Most previous studies have reported the efficacy of HAIC using 5-FU and cisplatin in advanced HCC, whereas HAIC using FUDR in advanced HCC has rarely been reported. The present paper describes the results of HAIC using FUDR in 34 patients with advanced HCC.
HAIC with FUDR can be performed in patients with advanced HCC that is unresectable or has not responded to previous therapy.
HAIC provides chemotherapeutic agents directly to the HCC via a pump catheter. The pump catheter is inserted at the proper hepatic artery from the superior mesenteric artery. The distal gastroduodenal artery, right gastric artery and small branches supplying the stomach or duodenum are ligated to prevent gastrointestinal toxicity.
The importance of the present study is that HAIC with FUDR could be a promising therapeutic approach for patients with advanced HCC.
Peer reviewer: Markus Raderer, Professor, Department of Internal Medicine I, Medical University Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2597] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 2. | Lee JS. Advances in the treatment of hepatocellular carcinoma. Korean J Med. 2009;77:290-297. |
| 3. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [PubMed] |
| 4. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 5. | Ohtomo K, Furui S, Kokubo T, Yamauchi T, Itai Y, Yashiro N, Iio M. Transcatheter arterial embolization (TAE) in treatment for hepatoma--analysis of three-year survivors. Radiat Med. 1985;3:176-180. [PubMed] |
| 6. | Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023-1028. [PubMed] |
| 7. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [PubMed] |
| 8. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] |
| 10. | Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Ensminger WD, Rosowsky A, Raso V, Levin DC, Glode M, Come S, Steele G, Frei E. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2'-deoxyuridine and 5-fluorouracil. Cancer Res. 1978;38:3784-3792. [PubMed] |
| 12. | Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176-182. [PubMed] |
| 13. | Itamoto T, Nakahara H, Tashiro H, Haruta N, Asahara T, Naito A, Ito K. Hepatic arterial infusion of 5-fluorouracil and cisplatin for unresectable or recurrent hepatocellular carcinoma with tumor thrombus of the portal vein. J Surg Oncol. 2002;80:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Oberfield RA, Sampson E, Heatley GJ. Hepatic artery infusion chemotherapy for metastatic colorectal cancer to the liver at the lahey clinic: comparison between two methods of treatment, surgical versus percutaneous catheter placement. Am J Clin Oncol. 2004;27:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [PubMed] |
| 17. | Bismuth H, Morino M, Sherlock D, Castaing D, Miglietta C, Cauquil P, Roche A. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387-394. [PubMed] |
| 18. | Reidy DL, Schwartz JD. Therapy for unresectable hepatocellular carcinoma: review of the randomized clinical trials-I: hepatic arterial embolization and embolization-based therapies in unresectable hepatocellular carcinoma. Anticancer Drugs. 2004;15:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW, Chang JC. Hepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-alpha for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol. 2009;44:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 20. | Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | American Society of Clinical Oncology. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998;16:301-308. [PubMed] |
| 22. | Clavien PA, Selzner N, Morse M, Selzner M, Paulson E. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Patt YZ, Charnsangavej C, Yoffe B, Smith R, Lawrence D, Chuang V, Carrasco H, Roh M, Chase J, Fischer H. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma: effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12:1204-1211. [PubMed] |
| 24. | Skitzki JJ, Chang AE. Hepatic artery chemotherapy for colorectal liver metastases: technical considerations and review of clinical trials. Surg Oncol. 2002;11:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4634] [Article Influence: 272.6] [Reference Citation Analysis (0)] |
| 27. | Seno H, Ito K, Kojima K, Nakajima N, Chiba T. Efficacy of an implanted drug delivery system for advanced hepatocellular carcinoma using 5-fluorouracil, epirubicin and mitomycin C. J Gastroenterol Hepatol. 1999;14:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, Kiriyama S, Suga T, Takahashi M. The efficacy of continuous local arterial infusion of 5-fluorouracil and cisplatin through an implanted reservoir for severe advanced hepatocellular carcinoma. Oncology. 1995;52:295-299. [PubMed] |
| 29. | Takizawa D, Kakizaki S, Sohara N, Sato K, Takagi H, Arai H, Katakai K, Kojima A, Matsuzaki Y, Mori M. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. 2007;52:3290-3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Martins A, Cortez-Pinto H, Marques-Vidal P, Mendes N, Silva S, Fatela N, Glória H, Marinho R, Távora I, Ramalho F. Treatment and prognostic factors in patients with hepatocellular carcinoma. Liver Int. 2006;26:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 904] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 32. | Cheong JY, Lee KM, Cho SW, Won JH, Kim JK, Wang HJ, Hahm KB, Kim JH. Intra-arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein thrombosis. Korean J Med. 2004;67:40-48. |
| 33. | Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, D'Angelica M, Fong Y, Dematteo R, Tse A. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] |
| 35. | Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2319] [Cited by in RCA: 2260] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 36. | Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, Cheng AL. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Kemeny N, Seiter K, Niedzwiecki D, Chapman D, Sigurdson E, Cohen A, Botet J, Oderman P, Murray P. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327-334. [PubMed] |
| 39. | Kemeny N, Conti JA, Cohen A, Campana P, Huang Y, Shi WJ, Botet J, Pulliam S, Bertino JR. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 1994;12:2288-2295. [PubMed] |
| 40. | Boublil JL, Milano G, Khater R, Bourry J, Thyss A, Bruneton JN, Renée N, Philip C, Namer M. Continuous 5-day regional chemotherapy by 5-fluorouracil in colon carcinoma: pharmacokinetic evaluation. Br J Cancer. 1985;52:15-20. [PubMed] |