Published online Jul 14, 2012. doi: 10.3748/wjg.v18.i26.3400
Revised: March 23, 2012
Accepted: April 2, 2012
Published online: July 14, 2012
AIM: To determine which patients might benefit most from retrograde viewing during colonoscopy through subset analysis of randomized, controlled trial data.
METHODS: The Third Eye® Retroscope® Randomized Clinical Evaluation (TERRACE) was a randomized, controlled, multicenter trial designed to evaluate the efficacy of a retrograde-viewing auxiliary imaging device that is used during colonoscopy to provide a second video image which allows viewing of areas on the proximal aspect of haustral folds and flexures that are difficult to see with the colonoscope’s forward view. We performed a post-hoc analysis of the TERRACE data to determine whether certain subsets of the patient population would gain more benefit than others from use of the device. Subjects were patients scheduled for colonoscopy for screening, surveillance or diagnostic workup, and each underwent same-day tandem examinations with standard colonoscopy (SC) and Third Eye colonoscopy (TEC), randomized to SC followed by TEC or vice versa.
RESULTS: Indication for colonoscopy was screening in 176/345 subjects (51.0%), surveillance after previous polypectomy in 87 (25.2%) and diagnostic workup in 82 (23.8%). In 4 subjects no indication was specified. Previously reported overall results had shown a net additional adenoma detection rate (ADR) with TEC of 23.2% compared to SC. Relative risk (RR) of missing adenomas with SC vs TEC as the initial procedure was 1.92 (P = 0.029). Post-hoc subset analysis shows additional ADRs for TEC compared to SC were 4.4% for screening, 35.7% for surveillance, 55.4% for diagnostic and 40.7% for surveillance and diagnostic combined. The RR of missing adenomas with SC vs TEC was 1.11 (P = 0.815) for screening, 3.15 (P = 0.014) for surveillance, 8.64 (P = 0.039) for diagnostic and 3.34 (P = 0.003) for surveillance and diagnostic combined. Although a multivariate Poisson regression suggested gender as a possibly significant factor, subset analysis showed that the difference between genders was not statistically significant. Age, bowel prep quality and withdrawal time did not significantly affect the RR of missing adenomas with SC vs TEC. Mean sizes of adenomas detected with TEC and SC were similar at 0.59 cm and 0.56 cm, respectively (P = NS).
CONCLUSION: TEC allows detection of significantly more adenomas compared to SC in patients undergoing surveillance or diagnostic workup, but not in screening patients (ClinicalTrials.gov Identifier: NCT01044732).
- Citation: Siersema PD, Rastogi A, Leufkens AM, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI, Dewit O, Ignjatovic A, Odstrcil E, East J, Deprez PH, Saunders BP, Kalloo AN, Creel B, Singh V, Lennon AM, DeMarco DC. Retrograde-viewing device improves adenoma detection rate in colonoscopies for surveillance and diagnostic workup. World J Gastroenterol 2012; 18(26): 3400-3408
- URL: https://www.wjgnet.com/1007-9327/full/v18/i26/3400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i26.3400
Colonoscopy is generally regarded to be the “gold standard” for the detection of colorectal neoplasia, and is the only method that allows detection and removal during a single procedure[1,2]. If the colon were a straight pipe, the standard colonoscope would be the perfect instrument for examining its lining, but lesions can be missed during colonoscopy, especially when located on the proximal aspect of haustral folds or on the inner curve of flexures[3-8].
The Third Eye Retroscope (TER) (Avantis Medical Systems, Sunnyvale, California) provides an additional, retrograde view that helps to visualize areas behind folds and flexures[9-11]. We recently showed in the Third Eye Retroscope Randomized Clinical Evaluation (TERRACE) study that Third Eye colonoscopy (TEC) detected 23.2% additional adenomas compared to standard colonoscopy (SC)[12].
As concerns about medical costs increase, there is growing interest in finding ways to target interventions and new technologies to specific patient sub-populations that will experience the greatest benefit[13,14]. In this follow-up of our initial report of TERRACE results, we performed a post-hoc subset analysis of the data to determine whether specific indications for colonoscopy (screening, surveillance or diagnostic workup) or other patient characteristics were associated with higher additional adenoma detection rates (ADR) with TEC. Such information may be useful for targeting those segments of the colonoscopy population that would benefit most from use of this new technology. We also wished to evaluate the clinical significance of the additional adenomas detected through TEC and determine how finding those additional adenomas would affect recommendations for follow-up intervals.
The TERRACE study was a prospective, randomized, controlled trial that used a “tandem” design involving two same-day, back-to-back procedures to provide a head-to-head comparison between TEC and SC.
Between March 2009 and February 2010, 15 experienced endoscopists at 4 European and 5 United States sites enrolled 448 patients who were scheduled to undergo colonoscopy for routine screening, surveillance after previous polypectomy, or diagnostic workup for symptoms possibly related to the lower gastrointestinal tract. Subjects were excluded for history of inflammatory bowel disease, colonic resection, polyposis syndrome or radiation therapy to abdomen or pelvis, or for active diverticulitis, suspicion of colonic stricture or concurrent enrollment in another study.
Upon arrival in the endoscopy suite, subjects were randomized to one of two groups by a research assistant utilizing a Web-based randomization module stratified both by center and by individual endoscopist. Each subject then underwent two complete examinations by the same endoscopist, who was informed of the order of exams immediately before beginning intubation.
Group A underwent SC first, followed by TEC, using the same colonoscope for both procedures. Group B underwent TEC first, then SC.
For TEC, following intubation of the cecum with a standard colonoscope, the TER was inserted through the biopsy channel of the colonoscope. When the TER emerges, its distal tip automatically turns 180 degrees so its miniature video camera and light are oriented backwards. As the colonoscope and TER are withdrawn together, the device provides a continuous retrograde view that complements the forward view of the colonoscope. The two video images are displayed side-by-side on a monitor.
Tandem miss rate studies have demonstrated a “second-pass effect” - i.e., looking a second time generally yields additional lesions[3-5]. In Group B the detection rate for SC as the second procedure served as a proxy for second-pass effect. Subtracting that from the additional detection rate with TEC in Group A yielded the net additional detection rate attributed to TEC.
Polyps were removed when detected and sent for histological evaluation. Advanced adenomas were defined as adenomas that measured at least 1 cm in diameter or those with villous or tubulovillous components, high-grade dysplasia or adenocarcinoma. An assistant recorded withdrawal time (from cecum to anal verge, subtracting pauses for polypectomy or extensive irrigation/suctioning) and total procedure time (from initial insertion of the colonoscope to withdrawal through the anal verge, not subtracting pauses). Bowel preparation was graded with the Ottawa Bowel Preparation Quality Scale Score[15]. Telephone interviews were performed 24-72 h after procedures to assess each subject for adverse events.
Subjects were withdrawn from the study if they withdrew consent, had inadequate bowel preparation or developed complications, if the endoscopist was unable to intubate the cecum or identified a condition in which back-to-back examinations might create risk for the subject, or if there were device malfunctions, technical errors or deviations from the protocol.
The primary outcome measure was per-polyp detection rates for adenomas and for all polyps with SC and TEC. Secondary outcome measures were polyp size, polyp histology, withdrawal time and total procedure time.
Patients presenting for routine screening were regarded as having a low-to-average risk of developing colorectal cancer (CRC), while the non-screening patients - those presenting for surveillance colonoscopy as a follow-up after previous removal of adenomas or for diagnostic workup of symptoms - were considered to have an above-average risk for CRC.
The protocol was approved by the Institutional Review Board of each institution, and all patients signed an informed consent. The study was registered with ClinicalTrials.gov (Identifier Number NCT01044732) and was reported in compliance with the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement.
Statistical analyses were performed on the per-protocol population using the SAS statistical package, version 9.1 (SAS Institute, Cary, NC). Because non-adenomatous polyps are generally regarded as having limited clinical significance, subset analysis was applied only to adenomas. Sample size determination was discussed in our earlier report of TERRACE results[12].
Because it is not possible to directly determine P values for the net additional ADR, we evaluated the statistical significance of the detection rates indirectly by calculating the relative risk (RR) of missing an adenoma during first exams with SC vs first exams with TEC. RR for missing adenomas during the first exam was estimated by Poisson regression including only study group (TEC first vs SC first) as an explanatory variable. P values < 0.05 were considered statistically significant.
To further assess the effect of the order of procedures on adenoma miss rates during the first exam, an exploratory analysis was performed to examine the influence of the independent variables including study group, age, gender, bowel prep quality (Ottawa Score), withdrawal time and indication for procedure (screening, surveillance, or diagnostic workup). Interactions between study groups (i.e., order of exams) and each of the explanatory variables were also examined. A multivariate Poisson regression with backward selection was performed to determine whether the above variables predicted differences in the number of adenomas missed during first exams with SC vs TEC. Independent variables with P values < 0.10 were included in the model for the multivariate analysis. Independent covariates included in the final model from the multivariate Poisson regression were further evaluated through subset analysis, where P values < 0.05 were considered statistically significant.
For the subsets of subjects by indication for procedure, Fisher’s exact test was performed to compare results for second exams in terms of additional subjects found to have at least one adenoma and additional subjects found to meet criteria for shortened surveillance intervals as a result of findings during the second exam.
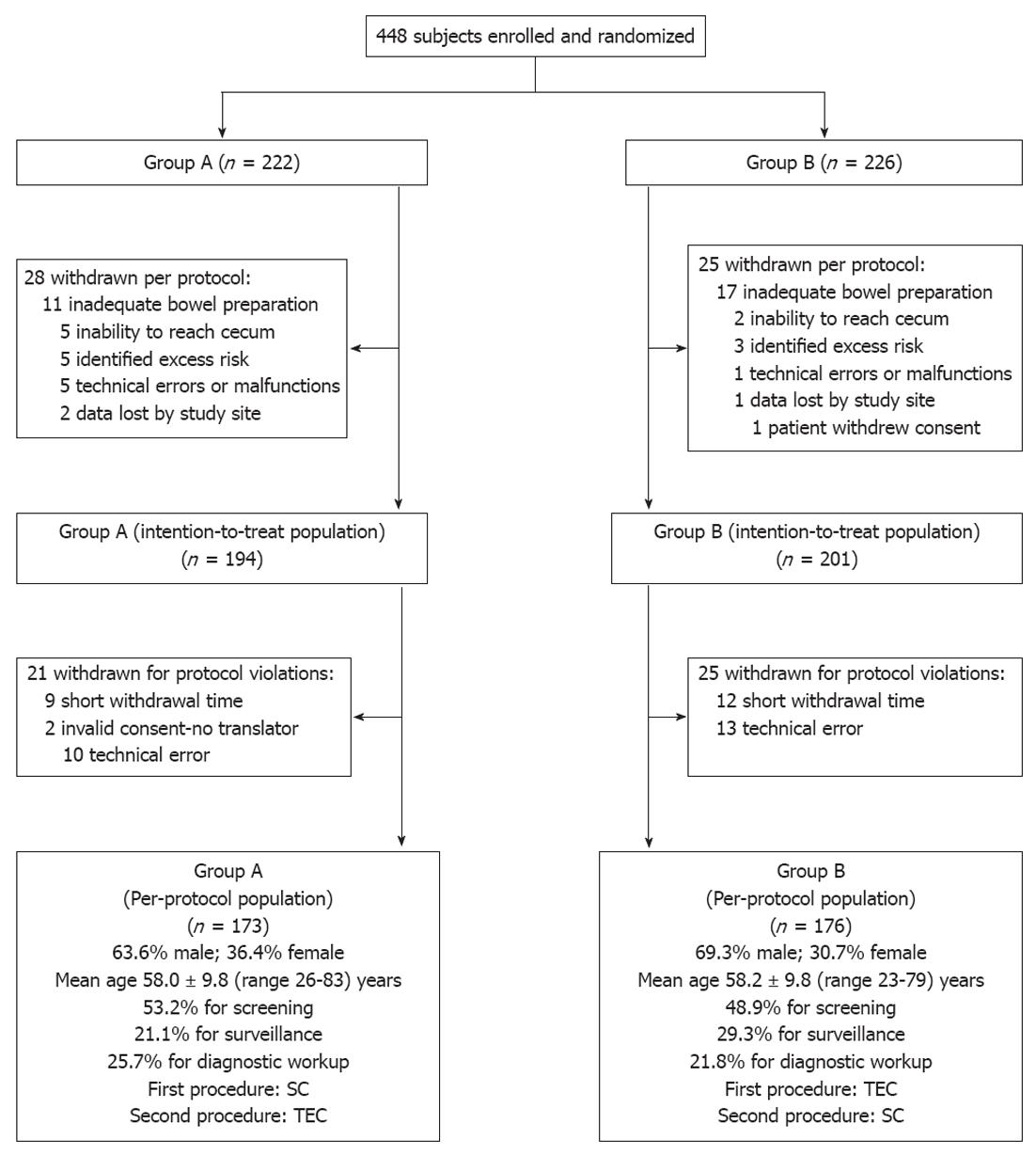
Patient demographics and a study flow diagram describing withdrawal of subjects to yield a per-protocol population of 349 subjects are shown in Figure 1.
Indication for colonoscopy was screening in 176/345 subjects (51.0%), surveillance after previous polypectomy in 87 (25.2%) and diagnostic workup in 82 (23.8%). In 4 subjects no indication was specified.
Previously reported overall TERRACE results had shown a net additional ADR with TEC of 23.2% compared to SC (Table 1). The RR of missing adenomas with SC vs TEC was 1.92 (P = 0.029) (Table 2).
| Indication | Group A (SC, then TEC) | Group B (TEC, then SC) | Net additional detection with TEC (%) | ||||
| SC 1st | TEC 2nd | Additional in 2nd exam (%) | TEC 1st | SC 2nd | Additional in 2nd exam (%) | ||
| All indications | 107 | 49 | 45.8 | 115 | 26 | 22.6 | 23.2 |
| Screening | 54 | 19 | 35.2 | 52 | 16 | 30.8 | 4.4 |
| Surveillance | 37 | 20 | 54.1 | 49 | 9 | 18.4 | 35.7 |
| Diagnostic | 16 | 10 | 62.5 | 14 | 1 | 7.1 | 55.4 |
| Surveillance + diagnostic | 53 | 30 | 56.6 | 63 | 10 | 15.9 | 40.7 |
| Indication | Group A standard colonoscopy first | Group B Third Eye colonoscopy first | Relative risk2 (95% CI) | P |
| All Indications3 | 49/173 = 0.283 | 26/176 = 0.148 | 1.92 (1.25-2.94) | 0.029 |
| Screening | 19/91 = 0.209 | 16/85 = 0.188 | 1.11 (0.62-2.04) | 0.815 |
| Surveillance | 20/36 = 0.556 | 9/51 = 0.176 | 3.15 (1.63-6.10) | 0.014 |
| Diagnostic | 10/44 = 0.227 | 1/38 = 0.026 | 8.64 (1.16-64.41) | 0.039 |
| Surveillance + diagnostic | 30/80 = 0.375 | 10/89 = 0.112 | 3.34 (1.74-6.39) | 0.003 |
Using a multivariate Poisson regression, we determined the risk of missing adenomas during the first exam. Study group (TEC first vs SC first) and gender were selected as possibly significant factors, and the interaction between indication for procedures and study group was found to be predictive. Age, bowel prep quality and withdrawal time did not significantly affect the risk.
Subset analysis segmenting the population by indication showed that additional ADRs for TEC compared to SC were 4.4% in the screening group, 35.7% in the surveillance group, 55.4% in the diagnostic group and 40.7% in the non-screening patients (surveillance and diagnostic groups combined) (Table 1). The RR of missing adenomas with SC vs TEC was 1.11 (P = 0.815) for screening subjects, 3.15 (P = 0.014) for surveillance subjects, 8.64 (P = 0.039) for diagnostic subjects and 3.34 (P = 0.003) for the surveillance and diagnostic subjects combined (Table 2).
In the diagnostic group, due to detection of lesions with TEC that had been missed with SC (after correction for second-pass effect), 75.0% additional subjects were found to have at least 1 adenoma. In the surveillance group, 42.9% additional subjects were found to have at least 1 advanced adenoma or at least 3 small adenomas criteria for 3-year follow-up according to joint guidelines published by the United States Multi-Society Task Force on CRC, American College of Radiology and American Cancer Society[16]. However, the numbers of subjects with these findings were small, and the differences did not reach statistical significance.
Segmenting the population by gender, additional ADRs for TEC compared to SC were 25.9% for males and 8.5% for females (P = NS). Segmenting by age, additional ADRs for TEC compared to SC were 22.0% for subjects less than 65 years of age and 25.8% for those at least 65 years (P = NS).
Per-polyp miss rates in the surveillance and diagnostic groups were lower for TEC than for SC, and no large adenoma (at least 10 mm) was missed with TEC in any sub-group (Table 3).
| Adenomas missed with standard colonoscopy during 1st exams in group A (%) | Adenomas missed with Third Eye colonoscopy during 1st exams in group B (%) | |||||
| < 10 mm | ≥10 mm | All sizes | < 10 mm | ≥10 mm | All sizes | |
| All indications | 33.8 | 11.8 | 31.4 | 20.8 | 0 | 18.4 |
| Screening | 27.7 | 12.5 | 26.0 | 28.1 | 0 | 23.5 |
| Surveillance | 37.7 | 0 | 35.1 | 16.7 | 0 | 15.5 |
| Diagnostic | 42.9 | 20.0 | 38.5 | 7.1 | 0 | 6.7 |
| Surveillance + diagnostic | 39.2 | 11.1 | 36.1 | 14.7 | 0 | 13.7 |
Mean sizes of adenomas detected with TEC and SC were similar at 0.59 cm and 0.56 cm, respectively (P = NS). Mean procedure times are shown in Table 4.
| Indication | All exams with SC | All exams with TEC | ||
| Withdrawal time | Total procedure time | Withdrawal time | Total procedure time | |
| All indications | 7.6 | 17.0 | 9.5 | 20.9 |
| Screening | 7.5 | 16.0 | 9.6 | 19.4 |
| Surveillance | 7.9 | 16.4 | 9.5 | 21.8 |
| Diagnostic | 7.5 | 19.8 | 9.6 | 23.2 |
| Surveillance + diagnostic | 7.7 | 18.1 | 9.5 | 22.5 |
No adverse events were reported.
We report a subset analysis of results from the TERRACE study, which was the first “head-to-head” comparison of TEC with SC and was designed to provide correction for the “second-pass effect” that invariably occurs in tandem studies[12]. Reversing the order of procedures in the control group (TEC first, followed by SC) provided a proxy for the second-pass effect, which was then subtracted from the additional detection rate in the study group (SC first, followed by TEC) to yield the net additional ADR for TEC compared to SC.
This subset analysis provides additional insight regarding which segments of the population are likely to benefit most from TEC and the clinical significance of the adenomas that were detected with TEC after having been missed during SC.
As shown in Table 1, overall results demonstrated a 23.2% net additional ADR for TEC compared to SC. This was consistent with the outcome of a previous study by DeMarco et al[11], in which investigators using a different methodology found a 25% additional ADR with the TER after each endoscopist had gained experience through performing 15 procedures.
Subset analysis segmenting the population by indication for procedure showed that in subjects undergoing colonoscopy for surveillance or diagnostic workup, TEC demonstrated additional ADRs of 35.7% and 55.4%, respectively, compared to SC. Pooling the results for those two groups of patients, who can be considered to have above-average risk for CRC, TEC showed an additional ADR of 40.7% compared to SC. In subjects undergoing screening colonoscopy, who can be regarded as having a low-to-average risk for CRC, TEC showed an additional ADR of 4.4% after correction for second-pass effect (Table 1).
In order to determine the statistical significance of these differences, we calculated the RR of missing an adenoma during first exams with SC vs first exams with TEC. The advantage with TEC proved to be statistically significant for the surveillance and diagnostic groups, both separately and combined. However, the difference for the screening population was not significant (Table 2).
It is not surprising to find proportionally more lesions behind folds in surveillance patients because, by definition, they have had at least one previous colonoscopy during which polyps that were seen with the colonoscope were removed, while lesions that were hidden behind folds may have been left behind. In contrast, screening patients have not had adenomas removed, so whatever polyps they have are likely to be more evenly distributed. However, the reason for the difference in results between diagnostic patients and screening patients is not clear.
A multivariate analysis indicated that more adenomas were found during second exams with TEC than with SC, and more were found during second exams in males than in females. However, subset analysis showed that while the net additional ADR for TEC compared to SC trended lower for females than for males, the total number of adenomas detected in females during all exams was relatively small, and the difference between genders was not statistically significant.
The multivariate Poisson regression showed no significant influence of age, bowel preparation quality or withdrawal time on adenoma miss rates. These findings were confirmed by subset analysis in which patient age and bowel prep quality had no significant impact.
Mean withdrawal times for TEC were essentially identical for the groups segmented by indication for procedure, suggesting that the amount of time spent examining the mucosa could not explain the differences among those groups. However, total procedure times for TEC were somewhat longer for the surveillance and diagnostic populations, most likely because many more polypectomies were performed in those subjects compared to the screening group.
In this study, overall adenoma miss rates for SC were 31.4% for all-size and 11.8% for large adenomas (at least 10 mm). These miss rates are higher than suggested by previous studies involving two same-day colonoscopies. One such study found miss rates of 24% for all-size and 6% for large adenomas[3]. Another found miss rates of 21% for all-size and 11% for advanced adenomas (but did not specify how many of those advanced adenomas were at least 10 mm)[5]. These discrepancies are not surprising, since many authors have acknowledged the limitation inherent in comparing two examinations with identical technology: lesions that are hidden from the colonoscope during one exam are likely to be hidden during the second exam as well, causing them to be missed on the second exam and thus underestimating the true miss rate[3-5,17].
Our 11.8% miss rate for large adenomas with SC is similar to the finding of a 12% miss rate for large adenomas in one study comparing colonoscopy with CT colonography (CTC)[8]. Another comparison with CTC showed a 17% miss rate with SC for all polyps at least 10 mm, but did not indicate a miss rate specifically for large adenomas[17]. Due to the limited sensitivity of CTC for smaller lesions, neither study could reliably estimate the all-size adenoma miss rate for SC.
In Tables 1 and 2, we show that, except in the screening group, TEC provided a significant improvement in ADR on a per-polyp basis (i.e., number of adenomas per patient). As we currently think that each adenoma has the potential to develop into malignancy, it is desirable to detect and remove as many adenomas as possible except in the relatively uncommon circumstance in which the patient has a high risk for complications from a polypectomy of small lesions.
One might argue that it is important to determine ADR on a per-patient basis (i.e., the number of patients in whom at least one adenoma is found), because the detection of even one adenoma identifies the patient as a “polyp-former” who might be followed more carefully than a “non-polyp-former.” However, for example, if a patient had one diminutive tubular adenoma (5 mm or smaller) and two large advanced adenomas, this measure would attribute the same quality to an exam whether the endoscopist detected all 3 lesions or found only the small adenoma and missed the 2 more significant lesions.
Furthermore, the finding of a single small adenoma does not necessarily dictate close follow-up according to joint guidelines published by the United States Multi-Society Task Force on CRC, American College of Radiology and American Cancer Society[16], which recommend follow-up at 3 years only for those patients who are at a high risk, defined as those with at least 1 advanced adenoma or at least 3 small adenomas. For patients with only 1-2 small tubular adenomas, who are at a low risk, the guidelines recommend repeat colonoscopy in 5-10 years.
Therefore, if we wished to consider a quality metric other than the per-polyp ADR, it might be more useful to calculate the number of subjects who are determined to be at high risk, i.e., those with at least 1 advanced adenoma or at least 3 small adenomas. In the surveillance group, due to detection of lesions with TEC that had been missed with SC, 42.9% additional subjects would be advised to return for 3-year follow-up per guidelines. However, the numbers were small and were not statistically significant.
Our results suggest that TEC offers an improvement in adenoma detection that can currently not be matched by other techniques or technologies. There is growing interest in improving quality in colonoscopy, and substantial evidence indicates that optimizing bowel preparation[18-20], spending adequate time examining the mucosa[21-24] and practicing high-quality withdrawal technique[21,25,26] are associated with improved detection rates. However, none of these measures fully addresses the difficulty of detecting neoplasia located behind folds and flexures. According to one study comparing SC with CTC, two-thirds of adenomas missed with the colonoscope were located behind folds[8], where they would likely be missed again during repeated exams.
CTC can reveal lesions on both sides of folds, but lacks sensitivity for small and flat lesions, and requires referral for colonoscopy when abnormalities are identified[27,28]. Cap-assisted colonoscopy does not require a second procedure, but studies have shown mixed results[29-32] and even the most encouraging study demonstrated an advantage only for the detection of diminutive adenomas[32]. Retroflexion of a colonoscope in the proximal colon has been found to be feasible by highly-experienced endoscopists, but results of studies so far have been mixed[33-35]. Moreover, the safety, practicality and time requirement for routine retroflexion proximal to the rectum still need to be established.
Reducing the prevalence of CRC in a cost-effective manner requires use of the proper examination for each patient, so it is important to identify which segments of the population are most likely to benefit from various technologies. The findings in this subset analysis may have implications for evidence-based patient selection for TEC. In our study, TEC demonstrated a clinically and statistically significant benefit for the subsets of patients who presented for surveillance in follow-up of previous polypectomy and for diagnostic workup of symptoms. However, our results did not provide evidence for efficacy in patients who presented for routine screening.
Cost-effectiveness also requires appropriate timing of examinations. There is evidence that many endoscopists perform surveillance colonoscopy at shorter intervals than those recommended by guidelines[16,36,37]. Their decisions regarding surveillance intervals may be influenced by the growing recognition that limitations in technology result in substantial miss rates during SC and by associated medico-legal concerns[36,37]. Improving the quality of colonoscopy through use of a retrograde-viewing device could instill confidence in physicians and patients that the likelihood of missing significant neoplasia is very low and thus foster greater compliance with guidelines for surveillance intervals.
The TERRACE study was not powered for analysis of subsets, and some numerical trends revealed by this post-hoc analysis, such as difference in additional detection rates between genders, did not reach statistical significance.
Only 33.5% of the subjects were female. Some of this discrepancy was due to one of the sites being a Veteran’s Administration Medical Center, where 55 of the 57 subjects were male. Another, probably more significant, factor was that the TERRACE study was performed with the first-generation Retroscope, which was too large to fit through the working channel of “pediatric” size colonoscopes. With the growing trend toward using these smaller colonoscopes when examining female patients, it is likely that some investigators may have chosen not to enroll some of their female patients in the study on the basis that use of an “adult” colonoscope might have made the examination more difficult and/or uncomfortable (The second-generation device that is currently in use differs in that it has a higher-resolution camera and a smaller diameter that allows its use with all colonoscopes).
In conclusion, the results of this subset analysis demonstrated that adenoma miss rates for SC are even higher than those previously estimated through performance of back-to-back colonoscopy exams.
Use of a retrograde-viewing device significantly improves the quality of colonoscopy by increasing the detection of adenomas of all sizes. Use of the retrograde-viewing device along with a colonoscope appears to have the greatest value for patients undergoing colonoscopy for surveillance or for diagnostic workup of symptoms, and the study results do not provide evidence for efficacy of the device in routine screening colonoscopy. These findings could provide endoscopists with an evidence-driven basis for selection of patients most likely to benefit from this new technology.
This article expands on a previously-published report of the Third Eye Retroscope Randomized Clinical Evaluation (TERRACE), which was a large randomized, controlled trial. TERRACE evaluated the effectiveness of a device that provides an additional, retrograde (backward) view to allow examination of areas located behind folds in the colon wall that hide them from the forward-viewing camera during standard colonoscopy (SC). The previously-published overall TERRACE results showed that Third Eye colonoscopy (TEC) detected 23.2% additional pre-cancerous adenomas not found with SC.
This new report focuses on post-hoc analyses of the TERRACE data to determine whether some subsets of patients might benefit more than others from TEC, to evaluate the clinical significance of the additional adenomas detected through TEC and to determine how finding those additional adenomas would affect recommendations for follow-up intervals.
These new analyses of the TERRACE data showed that, of all the variables that might influence the effectiveness of TEC, only the indication - i.e., the reason for performing the colonoscopy procedure - was significant. Patients who were having “surveillance” colonoscopy because they were found to have adenomas during previous exams, or “diagnostic” colonoscopy to look for a cause for symptoms, are considered to have above-average risk for colorectal cancer (CRC), and pooled results for those groups showed a 40.7% net additional adenoma detection rate (ADR) for TEC compared to SC. In contrast, patients who were having routine “screening” colonoscopy - who are considered to have low-to-average risk - had a 4.4% net additional ADR with TEC. In all of the groups combined, SC missed 11.8% of large adenomas, while none were missed during TEC.
These results confirm that use of a retrograde-viewing device allows detection of substantial numbers of clinically-significant adenomas that would be missed with the colonoscope alone. The finding that use of the device appears to offer more benefit for patients undergoing surveillance or diagnostic colonoscopy than for screening patients may provide physicians with a basis for selecting patients in whom they will use the device.
Adenomas are polyps that have the potential to transform into CRC, and most cases of CRC arise from adenomas. The term “post-hoc” refers to additional analyses that were not anticipated in the original study protocol.
The results of this post-hoc analysis will be helpful in supporting use of the Third Eye Retroscope (TER) in high-risk patients, which will allow for a more cost-effective approach as endoscopists continue to improve quality in colonoscopy. The authors are congratulated on demonstrating how TEC can detect additional adenomas during colonoscopy. Furthermore, the post-hoc analysis answers the question of who should have a TER used during colonoscopy. Based on the data from this manuscript, it would be those undergoing surveillance and diagnostic exams.
Peer reviewer: Seth Gross, MD, Norwalk Hospital, Maple Street, Norwalk, CT 06856, United States
S- Editor Gou SX L- Editor Logan S E- Editor Zheng XM
| 1. | Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 723] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 2. | Lieberman D. Quality and colonoscopy: a new imperative. Gastrointest Endosc. 2005;61:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1049] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 5. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Postic G, Lewin D, Bickerstaff C, Wallace MB. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol. 2002;97:3182-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, Shike M, Lanza E, Schatzkin A. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352-359. [PubMed] |
| 9. | Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy. 2008;40:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, Li J, Ramrakhiani S, Edmundowicz SA, Early DS. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos). Gastrointest Endosc. 2010;71:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, Gupta K, Wolf L, Dewar T, Deas TM. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Saini SD, Schoenfeld P, Vijan S. Surveillance colonoscopy is cost-effective for patients with adenomas who are at high risk of colorectal cancer. Gastroenterology. 2010;138:2292-229, 2299.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Saini SD, Fendrick AM. Value-based insurance design: implications for gastroenterology. Clin Gastroenterol Hepatol. 2010;8:767-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 16. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1203] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 17. | Van Gelder RE, Nio CY, Florie J, Bartelsman JF, Snel P, De Jager SW, Van Deventer SJ, Laméris JS, Bossuyt PM, Stoker J. Computed tomographic colonography compared with colonoscopy in patients at increased risk for colorectal cancer. Gastroenterology. 2004;127:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 560] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 19. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 701] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 20. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 951] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 24. | Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Rex DK, Hewett DG, Raghavendra M, Chalasani N. The impact of videorecording on the quality of colonoscopy performance: a pilot study. Am J Gastroenterol. 2010;105:2312-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, Bain AS, Mackintosh EE, Paek AM, Crissien AM. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc. 2011;74:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1284] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 28. | Cotton PB, Durkalski VL, Pineau BC, Palesch YY, Mauldin PD, Hoffman B, Vining DJ, Small WC, Affronti J, Rex D. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 29. | Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Tee HP, Corte C, Al-Ghamdi H, Prakoso E, Darke J, Chettiar R, Rahman W, Davison S, Griffin SP, Selby WS. Prospective randomized controlled trial evaluating cap-assisted colonoscopy vs standard colonoscopy. World J Gastroenterol. 2010;16:3905-3910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 32. | Hewett DG, Rex DK. Cap-fitted colonoscopy: a randomized, tandem colonoscopy study of adenoma miss rates. Gastrointest Endosc. 2010;72:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Rex DK. Accessing proximal aspects of folds and flexures during colonoscopy: impact of a pediatric colonoscope with a short bending section. Am J Gastroenterol. 2003;98:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Harrison M, Singh N, Rex DK. Impact of proximal colon retroflexion on adenoma miss rates. Am J Gastroenterol. 2004;99:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246-252. [PubMed] |
| 36. | Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264-271. [PubMed] |
| 37. | Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don't gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol. 2009;43:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |