Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3317
Revised: May 17, 2011
Accepted: May 12, 2012
Published online: July 7, 2012
There has been an increasing prevalence of lymphogranuloma venereum (LGV) or Chlamydia trachomatis (C. trachomatis) cases among the men who have sex with men (MSM) population, particularly in Europe and North America. These cases may present with an incomplete or undisclosed history and proctosigmoiditis without characteristic adenopathy syndrome. During the initial evaluation and colonoscopy, there is a strong clinical and endoscopic suspicion of inflammatory bowel disease (IBD) by virtue of presentation and endoscopic and histological findings. The diagnosis of IBD is subsequently modified to LGV proctosigmoiditis when one or more of the following transpire: (1) there is failure of response to IBD therapy; (2) additional components of history (MSM/travel) may be identified; (3) return of initially performed Chlamydia antibody test is positive; and (4) response to antibiotics effective against Chlamydia. We describe three such cases initially suspected to be an inflammatory bowel disease and subsequently identified as C. trachomatis proctosigmoiditis.
- Citation: Gallegos M, Bradly D, Jakate S, Keshavarzian A. Lymphogranuloma venereum proctosigmoiditis is a mimicker of inflammatory bowel disease. World J Gastroenterol 2012; 18(25): 3317-3321
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3317
Lymphogranuloma venereum (LGV), an ulcerative sexually transmitted infection caused by Chlamydia trachomatis (C. trachomatis), has gained recent attention as a cause of hemorrhagic proctitis among men who have sex with men (MSM) in North America, Australia, United Kingdom, and the rest of Europe[1,2]. This infection is found most frequently in tropical and subtropical areas in the world but it is rarely reported in developed countries. However, since 2003, outbreaks have been increasingly reported in Europe and the United States in MSM[3-5]. Human immunodeficiency virus (HIV)-1 infection is the strongest risk factor for LGV infection and it presents in more than 70% of the subjects[6]. The patients usually have high rates of promiscuity and high risk sexual behavior, often involving international sexual networks, which correlates with the high rate of concomitant sexually transmitted diseases (STDs) observed[2,5]. The atypical clinical presentation, the unawareness of physicians and patients in regards to the disease, and the lack of a routine diagnostic tests for LGV serovars of C. trachomatis have contributed to a delay in diagnosis and have caused misdiagnosis[5]. We report three cases of LGV proctitis in MSM patients, initially misdiagnosed or suspected to be inflammatory bowel disease (IBD).
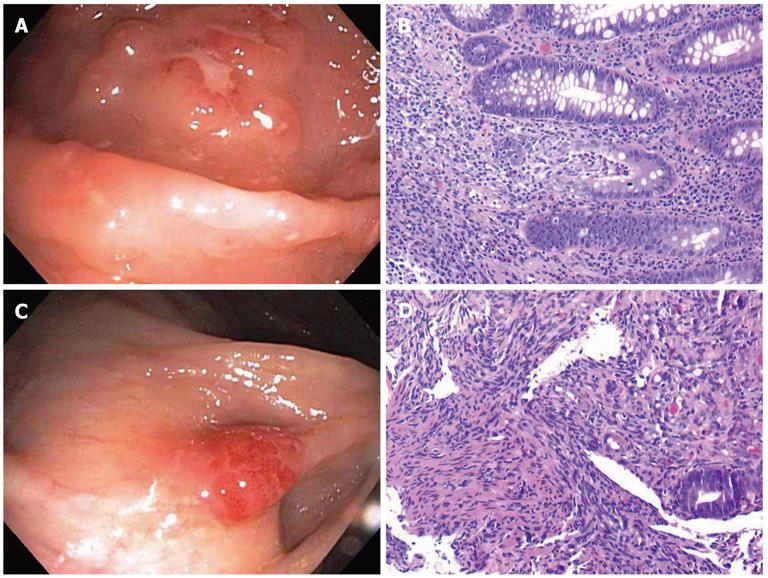
A 30-year-old male presented with a three week history of passing bloody mucus per rectum several times a day. A stool exam was negative for ova and parasites. A limited sigmoidoscopy showed a cobble-stoned and granular mucosa with several ulcers in the distal 5-7 cm of the rectum (Figure 1A). Rectal biopsies showed patchy severely active colitis with distorted and mucodepleted crypts and lymphohistiocytic cryptitis reminiscent of Crohn’s disease (Figure 1B). Immunostains for cytomegalovirus (CMV) and herpes simplex virus and special stains for fungi and acid-fast bacilli, performed retrospectively, were negative. The patient was given mesalamine 2.4 g/d which failed to produce any response after 1 wk. Due to the lack of response to IBD therapy and the clinical need to assess the remainder of the colon, a repeat full colonoscopy was performed. Colonoscopy showed similar findings in the rectum as seen previously, but the cecum showed a bright red nodule (Figure 1C). Biopsies from the cecal nodule showed classic histological features of Kaposi’s sarcoma with spindled cells, vascular proliferation and extravasated erythrocytes (Figure 1D). Immunohistochemically, the cells were positive for CD31 and HHV-8. Random colonic biopsies away from the rectum and cecum were also performed which showed incidental intestinal spirochetosis. Additional clinical history revealed that the patient had unprotected anal intercourse while traveling through Europe three months prior. Microbiological studies were then performed which confirmed the presence of C. trachomatis by DNA probe and serologic testing. The specimen was sent the Center for Disease Control which detected C. trachomatis LGV DNA by sequencing of the outer membrane protein (omp A). The patient was subsequently found to be HIV positive (HIV RNA: 24 177 cop/mL) and was started on antiretroviral therapy. Doxycycline 100 mg twice a day for 21 d was given for his LGV proctitis and he had resolution of symptoms after 4 d.
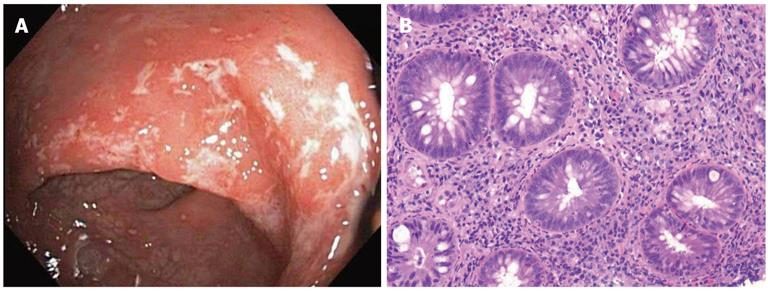
A 28-year-old HIV positive male presented with rectal bleeding and dyschezia. An anoscopy revealed an ulcer at the anal verge. Biopsies showed fragments of anal skin with ulceration, lymphoid tissue, and inflammatory granulation tissue histologically suspicious for an inflammatory bowel disease. Sigmoidoscopy was recommended to assess for Crohn’s disease and it showed an erythematous mucosa in the distal rectum with small areas of white exudates and heaped folds (Figure 2A). These biopsies showed severe lymphohistiocytic proctitis and cryptitis similar to Case 1 and again were suspicious for Crohn’s disease (Figure 2B). Given the history of HIV positivity, LGV was also a clinical consideration and DNA probes and serologic testing were subsequently performed and were positive for C. trachomatis. Retrospectively, the proctitis and anal ulceration was deemed to be LGV-related and not Crohn’s disease.
A 42-year-old male with HIV and prior anal condyloma acuminatum presented with a 3 wk history of bright red blood per rectum. Colonoscopy was performed and showed moderate colitis in the rectum with friable and erythematous mucosa (Figure 3A). The biopsies showed moderately active proctitis with focal crypt distortion and cryptitis reminiscent of Crohn’s-type IBD (Figure 3B). However, the clinical profile and the short duration of symptoms raised the possibility of LGV proctitis. Serologically, IgG antibodies against C. trachomatis were positive and the patient responded to antibiotic therapy for LGV.
Rectal bleeding or diarrhea may be caused by a variety of etiological factors including infections, diverticular disease, inflammatory bowel disease, ischemia, and neoplasia. Acute symptoms in young patients are generally linked to infectious colitis and often associated with travel, food, antibiotic intake, or sexual transmission. Common infectious agents include bacteria such as Escherichia coli, Clostridium difficile, Salmonella, Shigella and Campylobacter, viruses and parasites. When an infectious agent cannot be identified and/or symptoms persist, IBD is a clinical consideration.
Patients with LGV proctitis typically present with a rectal syndrome (> 90%) with moderate to severe ulcerative proctitis or proctocolitis with mucopurulent discharge, constipation, and tenesmus. Systemic symptoms like fever, malaise, and weight loss are also relatively common[5]. LGV is typically a disease of lymphatic tissue. When the primary inoculation is in the penis or vagina, inguinal or femoral lymphadenitis develops, with a severe inflammatory reaction that suppurates and extends to adjacent structures. However, when the rectum is the site of primary inoculation, proctitis is the most common reaction and enlarged inguinal lymphadenopathies are absent[6]. Simultaneous penile lesions are rarely seen. The ulcerative nature of LGV proctitis could enhance the transmission and acquisition of HIV and other STDs, as well as other blood-borne diseases like Hepatitis C[5].
LGV proctitis must be routinely ruled out in MSM with unspecific anorectal complaints. A high index of suspicion is the mainstay to its early diagnosis in order to prevent avoidable complications. If left untreated, the disease evolves to a late stage, which is marked by fibrosis and strictures in the anogenital tract. Late complications include chronic genital ulcerations, anal fistulae, infertility, and disfiguring conditions such as genital elephantiasis and esthiomene[4,6].
The endoscopic findings include mucopurulent exudates with a hyperemic and friable mucosa, multiple ulcers and erosions, and granulation tissue in the rectum. However, in some reports, up to 40% of men with LGV proctitis showed no anoscopic abnormalities[5]. Also, in rare cases there were normal endoscopic findings[7]. Hence, the endoscopic findings in LGV are nonspecific and can be easily misdiagnosed[5].
Microscopically, the main changes are inflammatory changes, which can be easily misinterpreted even by experienced pathologists. Findings include lymphohistiocytic colitis, lymphoplasmacytic infiltrates, cryptitis, crypt abscesses, and crypt distortion.
These changes are often interpreted as IBD-type (particularly Crohn’s-like) or described as non-specific inflammatory changes[7,8].
LGV proctitis is caused by serovars L1, L2, and L3 of the obligate intracellular bacterium C. trachomatis, which is classified into 15 serovars based on immunogenic epitope analysis of the major outer membrane protein. Serovars A, B, Ba, and C are associated with ocular trachoma and strains D-K with genital tract disease and with inclusion conjunctivitis. Only serovars L1, L2, and L3 cause LGV, probably due to their tropism for the lymphatic system, in contrast to serovars A-K, which affect mucocutaneous tissue. The L2 serovar can be further classified into L2, L’, L2a, and L2b, and the recently described L2c-L2g according to amino acid differences. The most common serovar causing LGV proctitis to date is L2b[4,5].
A wide variation in the diagnosis and management of chlamydial infection in MSM currently exists which needs to be standardized[9]. Historically, the laboratory diagnosis of LGV was based on culture, but this method is very tedious, has a low sensitivity (50%-80%), and cannot differentiate between different strains, therefore other detection methods have been applied. Serologic tests (complement fixation or immunofluorescence) have been used; however, these tests lack standardization, do not distinguish the different serotypes, nor do they differentiate past from present infections. Nucleic acid testing of clinical specimens has emerged as a promising technique that is both sensitive and convenient. Specialized laboratories such as the Center for Disease Control can further amplify sequences in the major outer-membrane protein and reliably distinguish among lymphogranuloma venereum serotypes[5,6,10].
The diagnosis of LGV is challenging to clinicians because the clinical presentation may be nonspecific and specific laboratory procedures for its diagnosis require a high index of suspicion. The main pathological differential diagnosis of LGV proctitis includes other forms of infectious colitis and inflammatory bowel disease[6].
Herpes, CMV, syphilis, and Neisseria gonorrhea infections can also cause proctocolitis. Patients generally present with similar general symptoms such as anal discharge, pain, diarrhea, constipation, bloody stool, and tenesmus. Proctoscopic findings are again nonspecific and range from normal to erythema, mucosal friability, and surface erosions[11]. Virally infected cells with herpes are histologically identified with molding and multinucleation of cells and the peripheralization of the chromatin. CMV infections have large cells with identifiable nuclear and cytoplasmic inclusions. These two viral infections are seen in a background of ulcerations with associated acute and chronic inflammation and granulation tissue and immunohistochemical markers are also helpful with identification. Syphilis is associated with a chronic inflammatory infiltrate rich in plasma cells and may also show granulomatous inflammation. With Neiserria gonorrhea, as with Chlamydia, one may see evidence of cryptitis, crypt abscesses, and reactive epithelial changes[12]. Since there are overlapping histological features with these two infectious processes, microbiologic studies become necessary.
There is considerable mimicry between IBD and LGV histologically[8]. In IBD, crypt architectural distortion, the presence of a prominent increase in the cellularity of the lamina propria, and the development of well-formed granulomas can be seen. However, some of these pathologic features are often also seen in LGV proctitis. Furthermore, with more advanced LGV infection, transmural inflammation can ensue, further resembling Crohn’s disease[12]. There are no pathognomonic features of LGV, and this is why clinical suspicion along with microbiological identification in a patient with risk factors is crucial.
According to the current European and United States STD guidelines, the recommended treatment for LGV is doxycycline (100 mg orally twice a day for 21 d or as long as anorectal symptoms persist) in contrast to infection with other Chlamydia serovars, where only 1 wk (or a single azithromycin dose) is required. This cures the infection and prevents further tissue damage. An alternative regimen is erythromycin (500 mg orally four times daily for 21 d). However, this recommendation is not supported by randomized evidence in clinical trials, but by empirical data. Patients should be followed up until symptoms have disappeared and clinically reviewed at 3-6 wk. Likewise, sexual partners who have had unprotected contact with the patient within the previous 60 d of onset of clinical symptoms should be screened or empirically treated with an LGV regimen[5].
In the absence of laboratory confirmation of L serovars, physicians are advised to treat possible cases presumptively for LGV and provide medical management of sexual partners[1]. Patients should receive risk education counseling and be routinely screened for other STDs, especially HIV and hepatitis B and C[5].
In conclusion, patients with C. trachomatis who present without classical history or symptoms and have isolated proctosigmoiditis may be confused clinically, endoscopically and histologically with inflammatory bowel disease. When there is lack of response to IBD therapy and/or the clinical profile raises the potential for LGV, serological and DNA probe studies for Chlamydia are recommended. Awareness of its mimicry to IBD and prompt identification of C. trachomatis are crucial for treatment and prevention of further complications from this disease.
Peer reviewers: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan; Dr. Giovanni Latella, MD, Professor, Department of Internal Medicine, GI Unit, University of L’Aquila, 67100 L’Aquila, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zhang DN
| 1. | Pathela P, Blank S, Schillinger JA. Lymphogranuloma venereum: old pathogen, new story. Curr Infect Dis Rep. 2007;9:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | de Vries HJ, van der Bij AK, Fennema JS, Smit C, de Wolf F, Prins M, Coutinho RA, Morré SA. Lymphogranuloma venereum proctitis in men who have sex with men is associated with anal enema use and high-risk behavior. Sex Transm Dis. 2008;35:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Vall-Mayans M, Caballero E, Sanz B. The emergence of lymphogranuloma venereum in Europe. Lancet. 2009;374:356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Stary G, Stary A. Lymphogranuloma venereum outbreak in Europe. J Dtsch Dermatol Ges. 2008;6:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Martin-Iguacel R, Llibre JM, Nielsen H, Heras E, Matas L, Lugo R, Clotet B, Sirera G. Lymphogranuloma venereum proctocolitis: a silent endemic disease in men who have sex with men in industrialised countries. Eur J Clin Microbiol Infect Dis. 2010;29:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Heras E, Llibre JM, Sirera G, Mate JL, Boix V, Rey-Joly C, Clotet B. Lymphogranuloma venerium proctitis in the setting of HIV: a case report and differential diagnosis. AIDS Patient Care STDS. 2009;23:493-494. [PubMed] |
| 7. | Soni S, Srirajaskanthan R, Lucas SB, Alexander S, Wong T, White JA. Lymphogranuloma venereum proctitis masquerading as inflammatory bowel disease in 12 homosexual men. Aliment Pharmacol Ther. 2010;32:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Høie S, Knudsen LS, Gerstoft J. Lymphogranuloma venereum proctitis: a differential diagnose to inflammatory bowel disease. Scand J Gastroenterol. 2011;46:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | McMillan A, Kell P, Ward H. Diagnosing chlamydia and managing proctitis in men who have sex with men: current UK practice. Sex Transm Infect. 2008;84:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Lamps L. Infectious disorders of the GI Tract. Surgical Pathology of the GI tract, liver, biliary tract, and pancreas. 2nd ed. Philadelphia, PA: Saunders Elsevier 2009; 65-66. |
| 12. | Iacobuzio-donahue CA. In: Odze RD, Goldblum JR. Surgical Pathology of the GI tract, liver, biliary tract, and pancreas. 2nd ed. Philadelphia, PA: Saunders Elsevier 2009; 740-741. |