Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3303
Revised: March 19, 2012
Accepted: April 28, 2012
Published online: July 7, 2012
AIM: To investigate the frequency and clinical significance of the myeloid-derived suppressor cells (MDSC) in human colorectal carcinoma (CRC).
METHODS: Samples of peripheral blood and tumor tissue from 49 CRC patients were analyzed. Mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation and were subjected to a flow cytometry-based immunophenotypic analysis.
RESULTS: A considerable increase in the percentage of CD33+HLA-DR- MDSCs was observed in the peripheral blood (1.89% ± 0.75%) and tumor tissues (2.99% ± 1.29%) of CRC patients as compared with that in the peripheral blood of healthy controls (0.54% ± 0.35%). This expanded CD33+HLA-DR- subset exhibited immature myeloid cell markers, but not lineage markers, and showed up-regulation of CD18/CD11b expression as compared with the MDSCs from healthy donors. Further studies showed that the MDSC proportion in CRC peripheral blood was correlated with nodal metastasis
(P = 0.023), whereas that in tumor tissues was correlated with nodal/distant metastasis (P = 0.016/P = 0.047) and tumor stage (P = 0.028), suggesting the involvement of MDSCs in CRC tumor development.
CONCLUSION: Characterization of MDSCs in CRC suggests the clinical significance of circulating and tumor-infiltrating MDSCs and may provide new insights into the CRC immunotherapy targeting MDSCs.
- Citation: Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, Guo HF, Miao ZN. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol 2012; 18(25): 3303-3309
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3303
Immune escape is not merely a passive process of immune evasion, but is rather an active one in which tumor cells, stroma cells, and immune cells present within the tumor microenvironment actively suppress the antitumor immune response. Thus, although host immune surveillance may prevent tumor outgrowth during the earliest stages of tumor development, locally invasive or metastatic tumors must evade host immunity[1]. In the tumor microenvironment, the “mission” of immune cells is to execute an antitumor function, but some of these cells are converted to act as confederates of the tumor. The inability of immune cells to mount an effective antitumor response, even following vaccination, is an immunological hallmark of cancer and represents a critical problem for the development of effective immunotherapeutic strategies. Myeloid-derived cells and lymphocytes subsets, such as regulatory T cells (Tregs), collaborate with their malignant counterparts to suppress host immunity[2,3].
Myeloid-derived suppressor cells (MDSCs) are a large group of myeloid cells comprising immature macrophages, granulocytes, and dendritic cells (DCs) as well as myeloid cells at earlier stages of differentiation[4-6]. In mice, MDSCs express the myeloid lineage differentiation antigens Gr-1 and CD11b, and undergo dramatic expansion during tumor development. In mouse models, MDSCs are found in tumors and lymph nodes[2,4,5], and the proportion of MDSCs has been shown to exceed 20% in the spleen. There are some controversies about the phenotype of MDSCs in humans, but these cells are now generally defined as CD11b- and CD33-positive, but lacking mature myeloid and lymphoid cell markers and the major histocompatibility complex (MHC) class II molecule, HLA-DR[7-9]. In the appropriate cytokine environment, MDSCs can differentiate into mature myeloid cells, but this differentiation is blocked in the presence of tumor-cell-conditioned medium or in tumor-bearing hosts, indicating that MDSC expansion can be induced in a tumor microenvironment. Recent studies have shown that MDSCs inhibit the proliferation and activation of T cells, and suppress maturation of DCs, which together contribute to the negative regulation of the immune responses and promote immune escape of tumors and pathogens[10,11]. In vivo depletion of MDSCs has been shown to improve T cell-mediated immune responses and suppress tumor growth in murine models[12]. Therefore, depletion of MDSCs in tumor-bearing hosts has been proposed as a new approach for cancer immunotherapy. Colorectal carcinoma (CRC) ranks as the third most common cancer and the fourth leading cause of cancer-related deaths worldwide[13]. In China, the incidence of CRC is increasing due to changes in diets and lifestyle[14]. Numerous pathological factors and transformation of multiple genes are involved in tumor genesis and progression. It has been demonstrated that an immune-escape microenvironment shaped by chronic inflammation or autoimmune diseases is clearly associated with increased risk of CRC[15]. A variety of therapeutic strategies, including conventional surgery, chemotherapy, radiotherapy and immunotherapy, alone or in combination, are currently available for the treatment of CRC patients. However, these therapies lead to different outcomes due to the different physical situation of each patient, which also construct the different tumor microenvironment through immune suppression[16-18]. Therefore, it is critical that clinicians perform further analyses of immune-suppression status and establish individualized therapeutic strategies for CRC patients. Several studies have described the presence of abnormalities in the immune system of patients with CRC, including defective function of natural killer cells, DCs and Tregs[19-21], but little is known about MDSCs in CRC.
In the present study, we investigated the frequency and characterized the phenotype of MDSCs in CRC patients and evaluated the clinical significance of MDSCs in CRC clinical status and outcome. The results might suggest a new strategy for efficient, individualized treatment of CRC.
Peripheral blood and tumor tissue samples were collected from 49 CRC patients who underwent surgery in the Third People’s Hospital of Wuxi, China from January 2010 to January 2011 after the approval by the Ethics Committee of the hospital. All patients were diagnosed with CRC for the first time, and had not been previously treated. Forty age-matched healthy donors were used as controls. Clinical parameters were acquired from the medical records of patients with the permission of the hospital.
Fresh tumor specimens were gently minced over a wire mesh screen to obtain a cell suspension. The cell suspension was layered over Ficoll-Hypaque (Amersham Biosciences, Sweden) and centrifuged at 500 ×g for 25 min. After density gradient centrifugation, mononuclear cells were collected and washed with RPMI 1640 media (Gibco, United States) containing 5% fetal bovine serum (FBS; Hyclone, United States) and 1% penicillin/streptomycin (Sigma-Aldrich, United States). Peripheral blood mononuclear cells (PBMCs) were also isolated by Ficoll-Hypaque density gradient centrifugation. PBMCs were collected, washed, and analyzed immediately. Viable cell counts were obtained using trypan blue dye.
Antibodies against the following proteins, purchased from BD Pharmingen or eBioscience, were used for flow cytometry: CD33, HLA-DR, CD3, CD14, CD19, CD56, CD11b, CD18, and CD1а. PBMCs (1 × 105) were suspended in phosphate-buffered saline (PBS) and incubated with antibodies for 30 min at 4 °C, and then washed twice with cold PBS. Fluorochrome-conjugated antibodies were used as isotype controls. Nonspecific staining was prevented by blocking Fc receptors. A Beckman Coulter flow cytometer equipped with Expo 32 software was used to analyze the stained cells. For MDSC marker analysis, the gate was set on the CD33+HLA-DR- cell subset.
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, United States). Paired or unpaired Student’s t tests, Wilcoxon signed-rank tests, and Pearson χ2 tests were used as appropriate. P value < 0.05 was considered statistically significant.
The percentage of MDSCs was analyzed in 49 CRC patients and 40 healthy donors. Circulating and tumor-infiltrating MDSCs were defined as the CD33+HLA-DR- double-staining cell subset (Figure 1A). As shown in Figure 1B, MDSCs were present in the peripheral blood of both healthy donors and CRC patients, but the percentages were increased in CRC patients (1.89% ± 0.75%) as compared with healthy donors (0.54% ± 0.35%, P < 0.05) Infiltrating MDSCs were also found in CRC tumor tissues, and the percentage of MDSCs among tumor-infiltrating mononuclear cells (2.99% ± 1.29%) was remarkably elevated as compared with that among PBMCs of both healthy donors and CRC patients (P < 0.05).
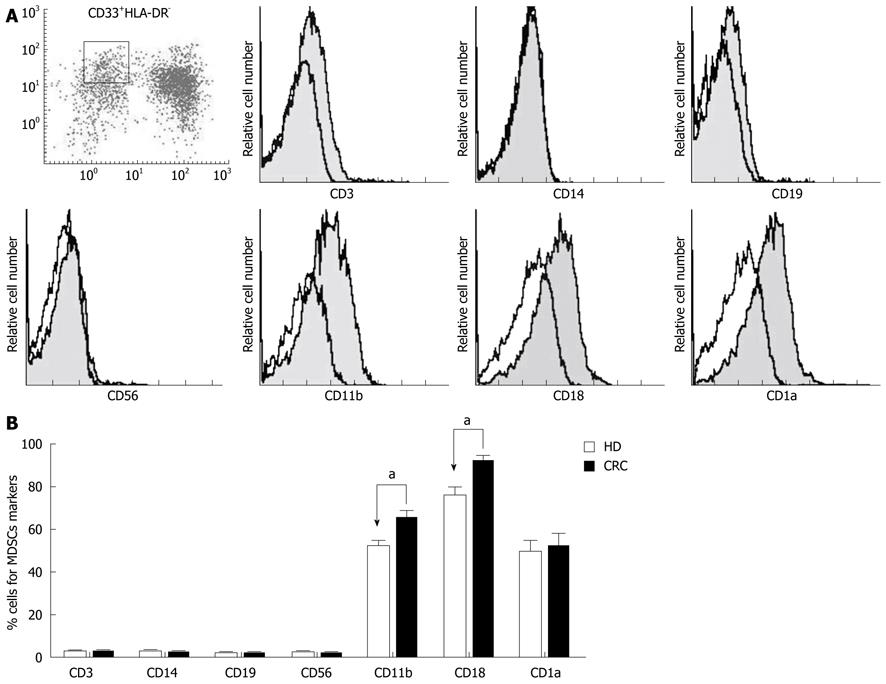
We analyzed the cell surface markers of the CD33+HLA-DR- MDSC subset in each CRC patient by immunostaining and flow cytometry. Cells were gated on CD33+HLA-DR-, and cell surface expression of CD3, CD14, CD19, CD56, CD11b, CD18, and CD1а was analyzed. As shown in Figure 2A, CD33+HLA-DR- cells exhibited high expression of CD11b, CD18 and CD1а, but low expression of CD3, CD14, CD19 and CD56. An analysis of phenotypic differences in peripheral blood MDSCs between CRC patients and healthy donors showed that CD11b and CD18 expressions were increased on MDSCs in CRC patients (Figure 2B).
As shown in Table 1, patients were divided into high and low MDSC groups according to the median MDSC percentages in peripheral blood and tumor tissues. A statistical analysis showed that the proportion of MDSCs in CRC patient peripheral blood was correlated with distant metastasis (P = 0.023): CRC patients with distant metastases showed a higher level of circulating MDSCs than CRC patients without distant metastases. The proportion of MDSCs in tumor tissues was correlated with nodal metastasis, distant metastasis and tumor stages. CRC patients with metastases (nodal/distant) exhibited a higher degree of MDSC infiltration than those without metastases (P = 0.016/P = 0.047). Among stage IV CRC patients, 60% had a high-level infiltration of MDSCs in tumor tissues (P = 0.028), suggesting the involvement of MDSCs in tumor development.
| Clinical parameters | Cases | MDSC proportion in peripheral blood | MDSC proportion in CRC tumor tissues | ||||
| Low | High | P value | Low | High | P value | ||
| Gender | 0.252 | 1.000 | |||||
| Male | 26 | 14 | 12 | 12 | 14 | ||
| Female | 23 | 8 | 15 | 11 | 12 | ||
| Age (yr) | 0.229 | 0.292 | |||||
| < 60 | 16 | 5 | 11 | 8 | 18 | ||
| ≥ 60 | 33 | 17 | 16 | 15 | 18 | ||
| Tumor size (cm) | 1.000 | 0.571 | |||||
| ≤ 5 | 24 | 11 | 13 | 10 | 14 | ||
| > 5 | 25 | 11 | 14 | 13 | 12 | ||
| Tumor (T) status1 | 0.179 | 0.466 | |||||
| pT1 | 4 | 2 | 2 | 1 | 3 | ||
| pT2 | 10 | 6 | 4 | 5 | 5 | ||
| pT3 | 12 | 9 | 5 | 4 | 8 | ||
| pT4 | 23 | 7 | 16 | 13 | 10 | ||
| Nodal (N) status2 | 0.248 | 0.016a | |||||
| N0 | 31 | 16 | 15 | 19 | 12 | ||
| N1 | 18 | 6 | 12 | 4 | 14 | ||
| Distant metastasis (M)3 | 0.023a | 0.047a | |||||
| M0 | 22 | 14 | 8 | 14 | 8 | ||
| M1 | 27 | 8 | 19 | 9 | 18 | ||
| TNM stage | 0.207 | 0.028a | |||||
| I | 6 | 2 | 4 | 2 | 4 | ||
| II | 12 | 7 | 5 | 8 | 4 | ||
| III | 11 | 7 | 4 | 8 | 3 | ||
| IV | 20 | 6 | 14 | 5 | 15 | ||
| Total | 49 | 22 | 27 | 23 | 26 | ||
Many studies have highlighted the role of MDSCs in cancer immune suppression[3,4]. Infiltration of these cells in the tumor host is promoted by the tumor microenvironment, and tumor-associated expansion of MDSCs contributes to tumor escape from the immune system. The decline of immune function in the tumor leads to ineffective tumor treatment outcomes, due to the insufficient activity of antigen-specific antitumor responses and the possible extension of immune tolerance in the tumor host. Many studies have addressed MDSCs in solid tumors, but little is known about MDSCs in CRC. In this study, we investigated circulating and tumor-infiltrating MDSCs in CRC patients, characterizing surface marker expression on MDSCs and demonstrating the clinical significance of MDSCs in CRC.
MDSCs represent 20%-30% of normal bone marrow cells and 1%-4% of all nucleated cells in the spleen[22,23]. Our study showed that the CD33+HLA-DR- subset was present at a very low proportion in the peripheral blood of healthy donors. CD33+HLA-DR- MDSCs represent a homogeneous cell population that is significantly elevated in the peripheral blood of CRC patients. Furthermore, CD33+HLA-DR- MDSCs were found at a relative high density in CRC tumor tissues compared with peripheral proportions in healthy donors or CRC patients. These data show that MDSCs expansion could be involved in CRC development, and suggest that the tumor tissue microenvironment might serve to promote MDSC expansion. Our data confirm previous studies demonstrating a dramatic expansion of MDSCs during tumor progression, infection, and even following immunization.
MDSCs are identified as a population of myeloid cells at earlier stages of differentiation[4-6]. In CRC, these CD33+HLA-DR- MDSCs displayed characteristics of immature myeloid cells, expressing high levels of CD33, CD11b, CD18 and CD1а, but a very low level of HLA-DR. Notably, the lineage markers CD3, CD14, CD19, and CD56 were not expressed in this subset. Thus, the phenotype of MDSCs in CRC is consistent with previous descriptions. Interestingly, however, we found that CD18/CD11b expression was considerably increased in MDSCs from CRC patients compared with those from healthy donors, indicating that the MDSCs in tumors might alter their expression of functional molecules according to the tumor microenvironment. Because CD18/CD11b (also known as Mac-1, CR-3) is critical for cell adhesion and migration[24,25], MDSCs in CRC might contribute to invasion and metastasis.
We further explored the clinical significance of circulating and tumor-infiltrating MDSCs in CRC. We found that the percentage of MDSCs in the peripheral blood of CRC patients was correlated with distant metastasis, whereas the percentage of MDSCs in tumor tissue was correlated with nodal metastasis, distant metastasis, and tumor stage. These data revealed the clinical significance of MDSCs in CRC. Previous studies have indicated that the main action of MDSCs in tumors is suppression of antigen-specific immune responses. Our demonstration that CD18/CD11b is up-regulated on tumor-associated MDSCs is consistent with the correlation between MDSC expansion and metastasis, indicating that MDSCs may play an important role in tumor invasion and metastasis. MDSC infiltration was also found to correlate with CRC tumor stage. Taken together, these observations suggest that MDSCs in CRC may substantially contribute to immune invasion, thereby promoting the tumor development.
In summary, we evaluated the frequency, phenotype and clinical significance of MDSCs in CRC. The increased frequency of MDSCs in CRC likely plays an important role in tumor metastasis and progression. Our study is of considerable significance for developing immunotherapeutic strategies via targeting and eliminating MDSCs in CRC.
We thank Professor Jian-Zhong Zhu for his advice and technical support. We especially thank Dr. Jing Sun for his valuable suggestions and critical review of the manuscript.
In the tumor microenvironment, the principal “mission” of immune cells is to execute an antitumor program, but a portion of such cells become confederates of the tumor. Myeloid-derived suppressor cells (MDSCs) are a large group of myeloid cells comprising immature macrophages, granulocytes, and dendritic cells (DCs), as well as myeloid cells at earlier stages of differentiation. Recent studies have shown that MDSCs can inhibit proliferation and activation of T-cell and suppress maturation of DCs, which together contribute to the negative regulation of immune responses and the promotion of immune escape of tumors and pathogens. MDSCs have been extensively studied in solid tumors, but little is known about MDSCs in colorectal carcinoma (CRC).
In humans, the phenotype of MDSCs has been a matter of some controversy, but it is now generally agreed that MDSCs are defined as CD11b- and CD33-positive cells that lack mature myeloid and lymphoid cell markers and do not express the MHC class II molecule, HLA-DR. In vivo depletion of MDSCs has been shown to improve T cell-mediated immune responses and suppress tumor growth in murine models. Therefore, depletion of MDSCs in tumor-bearing hosts has been proposed as a new approach for cancer immunotherapy. Several studies have described the presence of abnormalities in the immune system of patients with CRC, including defective function of natural killer cells, DCs, and Tregs, but little is known about MDSCs in CRC patients.
In this study, the authors investigated the circulating and tumor-infiltrating MDSCs in CRC patients and examined the expression of molecules characteristic of MDSCs. A considerable increase was found in the percentage of CD33+HLA-DR- MDSCs in the peripheral blood and tumor tissues of CRC patients. The expanded CD33+HLA-DR- subset exhibited immature myeloid cell markers, but not lineage markers, and showed up-regulation of CD18/CD11b expression as compared with MDSCs from healthy donors. These data demonstrated the existence of an MDSC population in CRC patients; further studies showed that the proportion of MDSCs in the peripheral blood and tumor tissues of CRC patients was correlated with nodal/distant metastases and tumor stage, strongly suggesting the involvement of MDSCs in CRC tumor development.
The characterization of the frequency and phenotype of MDSCs in CRC presented in this article suggests the clinical significance of circulating and tumor-infiltrating MDSCs, and it may provide new insights into the CRC immunotherapy targeting MDSCs.
In the present paper, the authors evaluated the frequency and clinical significance of MDSCs in human CRC and they found that this population of cells was considerably increased in CRC as compared with the healthy donors. Moreover, they showed that in CRC peripheral blood, the MDSCs frequency was correlated to distant metastasis whereas, in tumor tissues the percentage was correlated to nodal and distant metastasis and tumor stages. Finally, the authors speculate that their study could have clinical implication contributing to the identification of new target for CRC immunotherapy. A major concern is related to the definition of the MDSC population. The authors have defined MDSCs as a large group of myeloid cells consisting of immature macrophages, granulocytes and dendritic cells as well as myeloid cells at earlier stages of differentiation. The description itself is not a definition but a “Pandora box”. Each cell type has its own identity and specificity. Two or more cell types interact in unpredictable ways, and in the absence of a clear definition, each observation has little value. Given these premises, the attempt to clarify the confused MDSC issue by the authors is respectable, and their results, albeit flebile, are worth publication.
Peer reviewer: Dr. Lucia Ricci Vitiani, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena, 299, 00161 Rome, Italy
S- Editor Shi ZF L- Editor Ma JY E- Editor Zheng XM
| 1. | Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807-839. [PubMed] |
| 2. | Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267-296. [PubMed] |
| 3. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [PubMed] |
| 4. | Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237-245. [PubMed] |
| 5. | Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155-1166. [PubMed] |
| 6. | Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243-5248. [PubMed] |
| 7. | Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678-689. [PubMed] |
| 8. | Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044-3048. [PubMed] |
| 9. | Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49-59. [PubMed] |
| 10. | Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777-2790. [PubMed] |
| 11. | Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest. 2006;116:2587-2590. [PubMed] |
| 12. | Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741-1752. [PubMed] |
| 13. | Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153-165. [PubMed] |
| 14. | Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281-285. [PubMed] |
| 16. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [PubMed] |
| 17. | Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743-751. [PubMed] |
| 18. | Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Präuer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066-1071. [PubMed] |
| 19. | Furue H, Matsuo K, Kumimoto H, Hiraki A, Suzuki T, Yatabe Y, Komori K, Kanemitsu Y, Hirai T, Kato T. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis. 2008;29:316-320. [PubMed] |
| 20. | Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30:5091-5097. [PubMed] |
| 21. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [PubMed] |
| 22. | Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64-72. [PubMed] |
| 23. | Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53-65. [PubMed] |
| 24. | Mobberley-Schuman PS, Weiss AA. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 2005;73:7317-7323. [PubMed] |