Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3288
Revised: January 12, 2012
Accepted: April 10, 2012
Published online: July 7, 2012
AIM: To evaluate the effectiveness of omega-3 polyunsaturated fatty acid (ω-3 PUFA) administration on liver regeneration after 90% partial hepatectomy (PH) in rats.
METHODS: ω-3 PUFAs were intravenously injected in the ω-3 PUFA group before PH surgery. PH, sparing only the caudate lobe, was performed in both the control and the ω-3 PUFA group. Survival rates, liver weight/body weight ratios, liver weights, HE staining, transmission electron microscope imaging, nuclear-associated antigen Ki-67, enzyme-linked immunosorbent assay and signal transduction were evaluated to analyze liver regeneration.
RESULTS: All rats in the control group died within 30 h after hepatectomy. Survival rates in the ω-3 PUFA group were 20/20 at 30 h and 4/20 1 wk after PH. Liver weight/body weight ratios and liver weights increased significantly in the ω-3 PUFA group. The structure of sinusoidal endothelial cells and space of Disse was greatly restored in the ω-3 PUFA group compared to the control group after PH. In the ω-3 PUFA group, interleukin (IL)-4 and IL-10 levels were significantly increased whereas IL-6 and tumor necrosis factor-α levels were dramatically decreased. In addition, activation of protein kinase B (Akt) and of signal transducer and activator of transcription 3 signaling pathway were identified at an earlier time after PH in the ω-3 PUFA group.
CONCLUSION: Omega-3 polyunsaturated fatty acids may prevent acute liver failure and promote liver regeneration after 90% hepatectomy in rats.
- Citation: Qiu YD, Wang S, Yang Y, Yan XP. Omega-3 polyunsaturated fatty acids promote liver regeneration after 90% hepatectomy in rats. World J Gastroenterol 2012; 18(25): 3288-3295
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3288.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3288
The liver is a unique organ with ability to regenerate and recover its original function after extended resection or injury. This occurs due to the hyperplasia of the residual lobes and mitosis of the hepatocytes which are quiescent under normal conditions[1,2]. The development of two-thirds partial hepatectomy (PH) in the rat liver by Higgins and Anderson represented a milestone in the exploration of liver regeneration[3]. In this study, we found that 90% PH was lethal for rats: the cause of death may be associated with acute liver failure induced by small residual liver. Hepatocyte growth factor, platelets and 5-hydroxytryptamine were reported to promote liver regeneration and ameliorate acute liver failure after 90% hepatectomy in previous research[4-6].
Fatty acids, as essential nutrients, have a wide range of biological functions[7] and omega-3 polyunsaturated fatty acid (ω-3 PUFA) supplementation is also reported to be involved in modifying the organic biochemical environment[8]. Recently, ω-3 PUFAs were found to play significantly protective roles in the liver, cardiovascular system and kidney[9-11] and they have been widely used in clinical perioperative total parenteral nutrition[12]. In this study, we demonstrate that ω-3 PUFA plays an important role in stimulating liver regeneration after 90% hepatectomy in rats.
Sprague-Dawley male rats with weights ranging from 180 to 220 g were purchased from Nanjing University. Rats were maintained in a temperature-controlled room on a 12 h light-dark cycle, with free access to water and standard chow. Animals were divided into three groups (n = 6 in each group): the sham group; the control group (rats without ω-3 PUFA treatment); and the ω-3 PUFA group (rats with ω-3 PUFA treatment). In the ω-3 PUFA group, rats were injected intravenously (via rat tail vein) with ω-3 PUFA (Fresenius Kabi Corp., Germany) at a dose of 2 mL/kg body weight once 1 d for 2 d before PH surgery. Animal experiments were approved by the Institutional Animal Experiment Committee of Affiliated Drum Tower Hospital, Medical College of Nanjing University (China), in accordance with the Regulations for Animal Experiments at Nanjing University and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology.
Ninety percent hepatectomy was performed in the control and the ω-3 PUFA groups. The procedure is modified from the Higgins-Anderson operation by removing the left lateral, left median and right median lobes with a single ligature (70% PH), and subsequently resecting the right lateral lobe (20%) and leaving only the caudate lobe. Hepatectomy was carried out under ether anesthesia.
Six rats from each group were sacrificed and liver tissues were collected to investigate the effect of ω-3 PUFA administration on liver regeneration. At 12 h, 18 h and 24 h after PH, rats were sacrificed, regenerated liver samples were collected and wet remnant liver weights were measured. In addition, liver weight/body weight ratios were measured (%) for each rat. Mean value was calculated for each group at each time point. Tissues were divided into three specimens with one immediately frozen in liquid nitrogen and the second immersed into OCT compound and quickly frozen in the liquid nitrogen. The third specimen was fixed in 10% buffered formalin.
Blood was collected from the peripheral vessels in the quantity of 1-1.5 mL. Blood was centrifuged for 10 min at 4 °C at 3500 rpm. Supernatants were collected and stored at -80 °C until tested by a serum multiple biochemical analyzer to measure alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, total protein, serum albumin, total bilirubin and total bile acids.
Liver tissues, fixed in 10% buffered formalin, were used for histological and immunohistological analyses. Samples were stained in hematoxylin-eosin (HE). Liver sections were also incubated with Ki-67 antibody. OCT compound-immersed tissue samples were used for detection of liver sinusoidal endothelial cells (SEC) in the residual liver 24 h after PH.
Livers were removed 5 min after hepatectomy. The liver was cut into small pieces (approximately 1 mm) and the specimens were fixed in 2% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.4, and post-fixed in 1% OsO4 in 0.1 mol/L phosphate buffer. The specimens were dehydrated through a graded series of ethanol, passed through propylene oxide and embedded in EPON 812. Ultra-thin sections mounted on copper grids were stained with uranyl acetate and lead citrate, and then were observed under a Hitachi H-7000 transmission electron microscope.
Serum samples were collected and stored at -80 °C and serum tumor necrosis factor-α (TNF-α), interleukin (IL)-4, IL-6 and IL-10 were quantified using commercially-available enzyme-linked immunosorbent assay kits (Becton, Dickinson and Company, United States). Serum TNF-α, IL-4, IL-6 and IL-10 levels were measured in the control group and the ω-3 PUFA group.
Liver tissue extracts were prepared from the liquid nitrogen frozen specimens as previously described. Western blotting was developed using polyclonal antibodies: phosphoserine Akt, total Akt, phosphotyrosine signal transducer and activator of transcription 3 (STAT3), total STAT3, phosphotyrosine glycogen synthase kinase (GSK) and total GSK.
All data are expressed as the mean ± SD of samples. Statistical analyses were carried out with the T-test for independent samples. In all cases, a P value < 0.05 was considered significant.
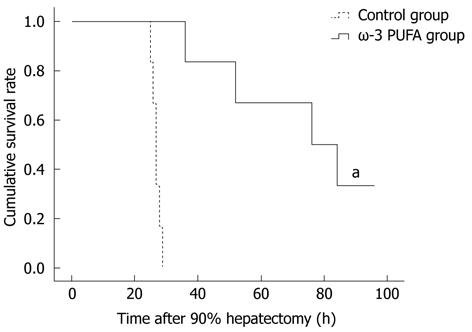
Twenty rats were subjected to PH surgery to examine the survival rates. All rats from the control group died within 30 h after PH. Four rats from the ω-3 PUFA group survived 1 wk after PH. According to the obtained results, the survival rates in the ω-3 PUFA group after 90% PH were 100% at 30 h after PH and 20% (P < 0.05) 1 wk after PH, respectively (Figure 1).
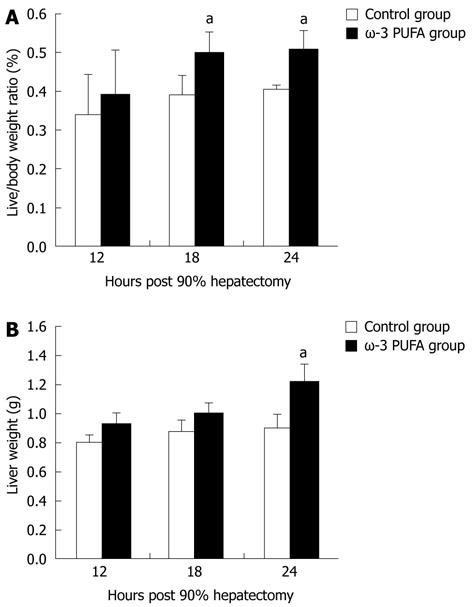
The ratios of LW/BW and liver weights were significantly increased at 24 h after PH in the ω-3 PUFA group in comparison with the control group (P < 0.05) (Figure 2).
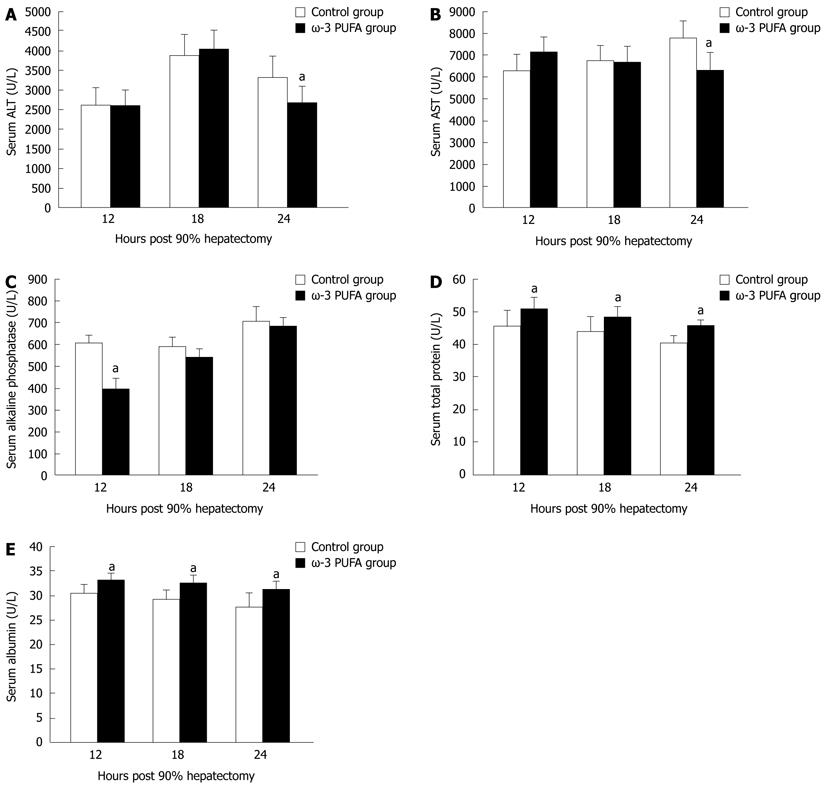
Serum ALT and AST levels were increased in both control and the ω-3 PUFA groups at 24 h after PH. There was a significant difference, with higher levels in the control group (P < 0.05) (Figure 3A and B). Serum alkaline phosphatase levels were lower in the ω-3 PUFA group with significant differences at 12 h after PH (P < 0.05) in comparison with the control group (Figure 3C). Serum albumin and total protein levels were attenuated in both groups after PH with more rapidly decrease in the control group (P < 0.05) (Figure 3D and E). No significant differences were identified between the control and the ω-3 PUFA groups in the levels of total bilirubin, direct bilirubin and total bile acids (P > 0.05) (data not shown).
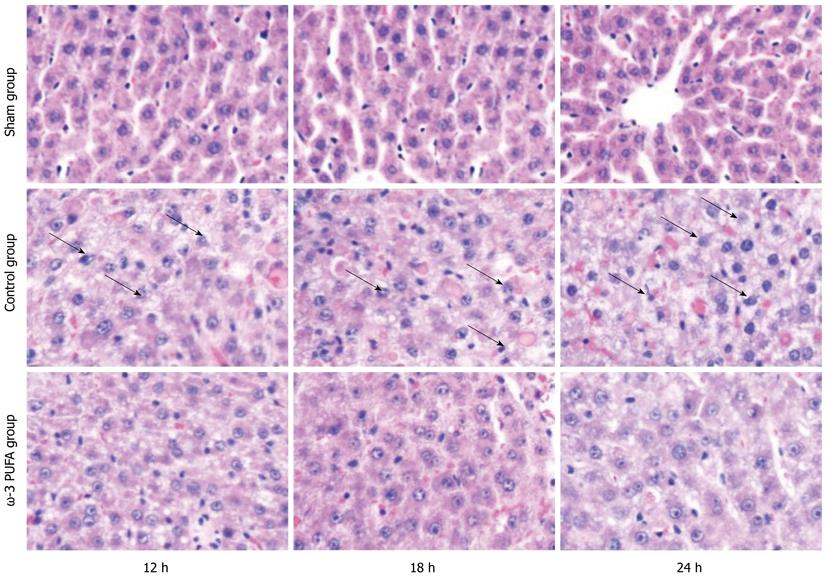
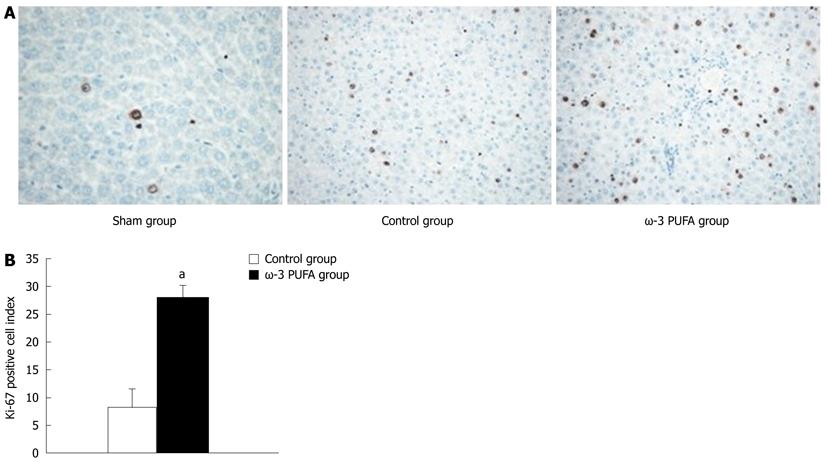
HE staining showed that the structure of liver lobules was severely damaged; hepatocyte swelling and necrosis of hepatocytes were observed after PH at every time point in the control group. Nevertheless, hepatocytes and the structure of liver lobules were greatly restored at 24 h after PH in the ω-3 PUFA group (Figure 4). The number of Ki-67 positive cells in the liver tissue 24 h after 90% PH was much higher in the ω-3 PUFA group (P < 0.05) (Figure 5).
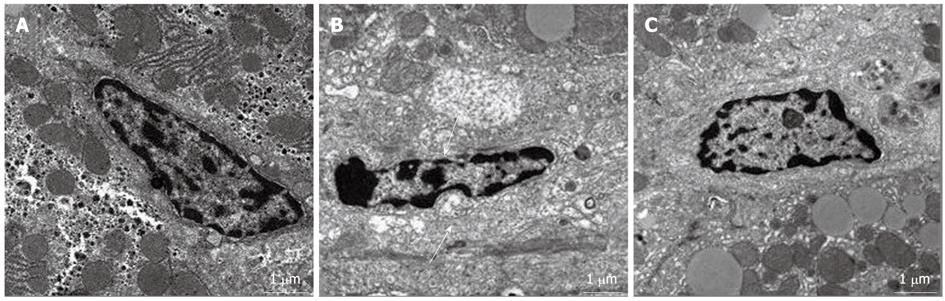
In the control group, the space of Disse was enlarged and the structure of sinusoidal endothelial cells (SECs) was severely damaged at 24 h after PH. However, the SEC structure and space of Disse were greatly restored at 24 h after PH in the ω-3 PUFA group (Figure 6).
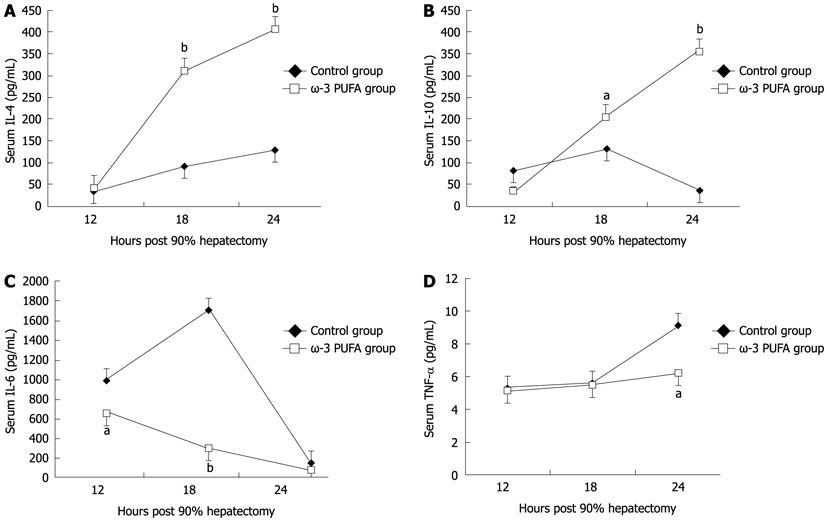
Compared with the control group, the level of pro-inflammatory cytokines IL-6 and TNF-α significantly decreased at 18 h and 24 h after PH (P < 0.05). The levels of anti-inflammatory cytokines IL-4 and IL-10 significantly increased in the ω-3 PUFA group at 24 h after PH (P < 0.05) (Figure 7).
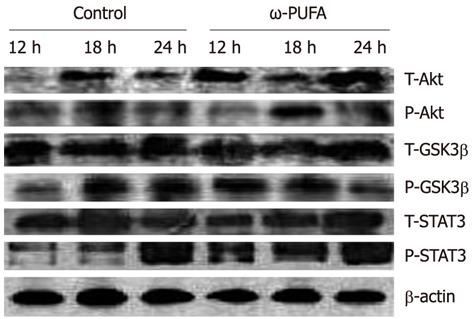
In the ω-3 PUFA group, Akt was phosphorylated in the samples at 12 h after PH and phosphorylated Akt was increased in the samples at 18 h after PH. In contrast, phosphorylated Akt expression was much lower at 18 h after PH in the control group. In addition, GSK3β was expressed at 12 h after PH in the ω-3 PUFA group, which was much earlier than in the normal group. Moreover, the expression of phosphorylated STAT3 was much higher and was sustained longer in the ω-3 PUFA group at 12 h, 18 h and 24 h after PH (Figure 8).
PH is the accepted gold standard of treatment for benign and malignant hepatic space-occupying lesions. The precise liver resection, which is based on preoperative evaluation, meticulous surgical procedure and postoperative management, has been well developed. However, acute liver failure after extended hepatectomy has been shown to be a major contributor to post-operative mortality and morbidity[13,14]. Thus, how to control post-operative liver failure remains an urgent problem to be solved. It has been shown that ω-3 PUFA plays an important role in gastrointestinal surgery; it can protect hepatocytes through inhibiting liver cell peroxidation via anti-oxidation and anti-inflammatory mechanisms of action after PH[15,16]. Nevertheless, no report has shown the effects of ω-3 PUFA on liver regeneration and acute liver failure after extended hepatectomy. Our study is the first to demonstrate that ω-3 PUFA plays a beneficial role on liver regeneration after 90% hepatectomy, which may offer hope for protecting from post-operative liver failure after clinical extended hepatectomy.
In general, serum parameters are a significant index to evaluate liver function. Serum AST and ALT concentrations are inversely related to the severity of liver damage. Albumin is a marker of synthetic function of the liver[17,18]. In this research, both ALT and AST levels were significantly lower in the ω-3 PUFA group at 24 h after PH. In addition, total protein and albumin levels were higher in the ω-3 PUFA at 12 h, 18 h and 24 h after PH. These results strongly indicate that ω-3 PUFA plays a protective role in liver function after 90% hepatectomy.
SECs are a type of special endothelium, which not only take part in the formation of blood vessels[19], but also play a key role in hemodynamic changes of the liver, even physiological and metabolic functions[20-22]. The structural feature of the SEC is a spindle type of cell with plenty of clusters of cribriform fenestrae[23], which can accelerate the exchange of substances between hepatocytes and blood plasma[24]. ω-3 PUFA has been reported to modulate both nitric oxide synthase (NOS) activity and cyclooxygenase expression as antioxidants[25,26]. Oxidative stress and the inactivation of NO by superoxide anions play an important role in disease states. NO is synthesized by three distinct NOS isoforms (neuronal, inducible, and endothelial NOS)[27]. The endothelial NOS III plays physiologically important roles in vascular homeostasis, such as keeping the vasculature dilated, protecting the intima from platelet aggregates and leukocyte adhesion, and combining superoxide[28]. This procedure can generate non-toxic metabolite NO3-, which could circumvent oxygen-free radical damage to the integrity of the structure and function of SECs[29]. In our study, the space of Disse was enlarged and the structure of SECs was severely damaged in the control group, in contrast to the favorable integrity of the SEC structure in the ω-3 PUFA group. These results suggest that ω-3 PUFA promotes liver regeneration through protecting the structure of the SEC in the acute phase after hepatectomy.
ω-3 PUFA is known to play a crucial role in the function of the immune system and its receptors such as Toll-like receptors (TLRs). Recently, TLRs were observed to influence the development of adaptive immune responses in T-helper-1 (Th1) and T-helper-2 (Th2) cells which are characterized by their distinct cytokine patterns including IL-4, IL-6, IL-10 and TNF-α. Jerin et al[30] suggested that the intensity of inflammation could be assessed by the ratio of IL-6/IL-10. In the present study, we showed that pro-inflammatory cytokines IL-6 and TNF-α significantly decreased, and anti-inflammatory cytokines IL-4 and IL-10 significantly increased, in the ω-3 PUFA group in comparison with the control group. TNF-α is produced mainly by resident hepatic macrophages (Kupffer cells)[31], and the expression of IL-6 has been shown to be stimulated in hepatocytes as well as in Kupffer cells[32]. Activation of nuclear factor-κB induced by TNF-α is a key step in liver regeneration[33]. IL-6 is also predominantly produced in Kupffer cells. The role of IL-6 in liver regeneration has not been clearly defined[34]. However, it was found to be one of the strongest inducers of STAT3 in hepatocytes. After IL-6 binds to its receptor on the hepatocyte surface, signal transduction occurs by phosphorylation of cytoplasmic STAT protein 3[35]. STAT3 is an important signal pathway in regulating cell proliferation, transformation and apoptosis of hepatocytes[36]. A previous study has shown that a significantly higher mortality rate occurred in the early phase after 70% PH in STAT3-knockout mice[37]. The Akt pathway is downstream of growth factor receptors[38], and promotes cell proliferation through activation of GSK3β to accelerate nuclear accumulation of cyclin D1[39]. Cyclin D1 can strongly stimulate cell division and thus enhance liver regeneration. In the present study, STAT3 was strongly phosphorylated at 12 h and 18 h after PH in the ω-3 PUFA group. Phosphorylated Akt was also detected at 18 h after PH which was much earlier and stronger than that in the control group. Our results demonstrate that ω-3 PUFA is responsible for earlier and stronger phosphorylation of the STAT3 and Akt in the signaling pathway. However, IL-6 and TNF-α, key cytokines for liver regeneration, were found to decrease in the ω-3 PUFA group. The mechanism of these two opposite effects on liver regeneration is not clear in the present study and needs to be clarified in the future.
Taken together, our results provide strong evidence that intravenous injection of ω-3 PUFA could slow the progress of acute liver failure through an anti-inflammatory action and significantly promote liver regeneration after 90% hepatectomy.
Liver regeneration after the loss of hepatic tissue is a fundamental parameter of liver response to injury. Omega-3 polyunsaturated fatty acid (ω-3) PUFA could promote liver regeneration as an anti-inflammatory agent.
Although a previous study has demonstrated that intravenous administration of ω-3 PUFA is beneficial in the treatment of fatty liver in rats, the effect of liver regeneration was not detected.
This study demonstrated that ω-3 PUFA plays a beneficial role in liver regeneration. ω-3 PUFA decreased the expression of pro-inflammatory cytokines and increased the expression of anti-inflammatory cytokines. ω-3 PUFA could slow the progress of acute liver failure through its anti-inflammation effect and promote liver regeneration.
The results of this study suggest ω-3 PUFA can promote liver regeneration in rats. Further studies are required before applying this treatment to patients with partial hepatectomy.
The ω-3 PUFA enter human bodies mainly through consumption of cold-water fish products, and they have been widely clinically used in total parenteral nutrition treatment.
It is of interest and importance that ω-3 PUFA may contribute to the recovery of severely damaged livers.
Peer reviewer: Toshihiro Mitaka, MD, PhD, Professor, Department of Pathophysiology, Cancer Research Institute, Sapporo Medical University School of Medicine, South-1, West-17, Chuo-ku, Sapporo 060-8556, Japan
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
| 3. | Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, Tanaka K, Shimizu T, Matsuo K, Nagashima Y. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477-4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 616] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438-463. [PubMed] |
| 8. | Hwang D. Fatty acids and immune responses--a new perspective in searching for clues to mechanism. Annu Rev Nutr. 2000;20:431-456. [PubMed] |
| 9. | Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care. 2010;13:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569-2578. [PubMed] |
| 11. | Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010;56:728-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Koch T, Heller AR. Benefits of ω-3 fatty acids in parenteral nutrition. Clin Nutr Suppl. 2005;1:17-24. |
| 13. | Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862-1872. [PubMed] |
| 14. | O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273-275. [PubMed] |
| 15. | Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 17. | Yap FH, Joynt GM, Buckley TA, Wong EL. Association of serum albumin concentration and mortality risk in critically ill patients. Anaesth Intensive Care. 2002;30:202-207. [PubMed] |
| 18. | Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693-703. [PubMed] |
| 19. | Gervaz P, Scholl B, Mainguene C, Poitry S, Gillet M, Wexner S. Angiogenesis of liver metastases: role of sinusoidal endothelial cells. Dis Colon Rectum. 2000;43:980-986. [PubMed] |
| 20. | Wisse E, Braet F, Luo D, De Zanger R, Jans D, Crabbé E, Vermoesen A. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol. 1996;24:100-111. [PubMed] |
| 21. | Limmer A, Knolle PA. Liver sinusoidal endothelial cells: a new type of organ-resident antigen-presenting cell. Arch Immunol Ther Exp (Warsz). 2001;49 Suppl 1:S7-11. [PubMed] |
| 22. | Weik C, Warskulat U, Bode J, Peters-Regehr T, Häussinger D. Compatible organic osmolytes in rat liver sinusoidal endothelial cells. Hepatology. 1998;27:569-575. [PubMed] |
| 23. | Braet F, De Zanger R, Baekeland M, Crabbé E, Van Der Smissen P, Wisse E. Structure and dynamics of the fenestrae-associated cytoskeleton of rat liver sinusoidal endothelial cells. Hepatology. 1995;21:180-189. [PubMed] |
| 24. | Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. [PubMed] |
| 25. | Radosinska J, Bacova B, Bernatova I, Navarova J, Zhukovska A, Shysh A, Okruhlicova L, Tribulova N. Myocardial NOS activity and connexin-43 expression in untreated and omega-3 fatty acids-treated spontaneously hypertensive and hereditary hypertriglyceridemic rats. Mol Cell Biochem. 2011;347:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 474] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Berdeaux A. Nitric oxide: an ubiquitous messenger. Fundam Clin Pharmacol. 1993;7:401-411. [PubMed] |
| 28. | Förstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 1998;12:773-790. [PubMed] |
| 29. | Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Simpson KJ, Lukacs NW, Colletti L, Strieter RM, Kunkel SL. Cytokines and the liver. J Hepatol. 1997;27:1120-1132. [PubMed] |
| 33. | Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160-171. [PubMed] |
| 34. | Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403-411. [PubMed] |
| 35. | Rakemann T, Niehof M, Kubicka S, Fischer M, Manns MP, Rose-John S, Trautwein C. The designer cytokine hyper-interleukin-6 is a potent activator of STAT3-dependent gene transcription in vivo and in vitro. J Biol Chem. 1999;274:1257-1266. [PubMed] |
| 36. | Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411-28417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J Hepatol. 2005;43:799-807. [PubMed] |
| 38. | Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, Evers BM. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401-G1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun. 2005;330:722-730. [PubMed] |