Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3282
Revised: October 19, 2011
Accepted: January 18, 2012
Published online: July 7, 2012
AIM: To introduce a bioimpedance gastric motility measurement method based on an electrical-mechanical composite concept and a preliminary clinical application.
METHODS: A noninvasive gastric motility measurement method combining electrogastrogram (EGG) and impedance gastric motility (IGM) test was used. Preliminary clinical application studies of patients with functional dyspepsia (FD) and gastritis, as well as healthy controls, were carried out. Twenty-eight FD patients (mean age 40.9 ± 9.7 years) and 40 healthy volunteers (mean age 30.9 ± 7.9 years) were involved. IGM spectrum was measured for both the healthy subjects and FD patients, and outcomes were compared in the FD patients before treatment and 1 wk and 3 wk after treatment. IGM parameters were obtained from 30 erosive gastritis patients (mean age 50.5 ± 13.0 years) and 40 healthy adults, and IGM and EGG results were compared in the gastritis patients before treatment and 1 wk after treatment.
RESULTS: There were significant differences in the IGM parameters between the FD patients and healthy subjects, and FD patients had a poorer gastric motility [percentage of normal frequency (PNF) 70.8 ± 25.5 in healthy subjects and 28.3 ± 16.9 in FD patients, P < 0.01]. After 1 wk administration of domperidone 10 mg, tid, the gastric motility of FD patients was not improved, although the EGG of the patients had returned to normal. After 3 wk of treatment, the IGM rhythm of the FD patients became normal. There was a significant difference in IGM parameters between the two groups (PNF 70.4 ± 25.5 for healthy subjects and 36.1 ± 21.8 for gastritis patients, P < 0.05). The EGG rhythm of the gastritis patients returned to normal (frequency instability coefficient 2.22 ± 0.43 before treatment and 1.77 ± 0.19 one wk after treatment, P < 0.05) after 1 wk of treatment with sodium rabeprazole tablets, 10 mg, qd, po, qm, while some IGM parameters showed a tendency toward improvement but had not reached statistical significance.
CONCLUSION: The electrical-mechanical composite measurement method showed an attractive clinical application prospect in gastric motility research and evaluation.
- Citation: Zhao S, Sha H, Li ZY, Ren CS. Electrical bioimpedance gastric motility measurement based on an electrical-mechanical composite mechanism. World J Gastroenterol 2012; 18(25): 3282-3287
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3282
An electrical bioimpedance technique is a method used to extract physiological and pathological information from the human body according to the electrical property of the tissue and organs. This method has the advantage of being noninvasive and convenient and can provide functional information. It is a powerful tool in clinical diagnosis and medical research[1]. There are many applications that use bioimpedance signals for different pathological conditions, but their use in gastric motility assessment needs further exploration[2].
Gastrointestinal motility as an interdisciplinary subject has developed rapidly in recent years. Gastric motility is one of the most critical physiological functions of the human body. Coordinated gastric motility is necessary for digestion and the absorption of dietary nutrients. Impairment of gastric motility results in delayed gastric emptying and symptoms such as nausea, vomiting, abdominal pain and discomfort[3]. The research on gastric motility function has fallen behind as compared with the studies on gastric endocrine and exocrine secretion functions and gastric morphology. One of the important reasons for this is the absence of convenient and effective measurement methods for gastric motility function[4].
Stomach volume distends gradually after the ingestion of food. In the period of digestion, such as contraction and peristalsis after a meal, the stomach content changes greatly and so does the impedance of the stomach. When measuring the impedance of the stomach during digestion, information reflecting the stomach volume (gastric emptying) and gastric motility can be extracted. Sutton et al[5] in 1985 reported results on extracting the signal of gastric movement by an electrical impedance method. The curve reflecting gastric emptying was obtained and, from the curve, the gastric peristaltic information was extracted showing a rhythm of 2-4 cycles per minute (cpm), which is in accordance with gastric contraction. In 1987, Familoni et al[6] presented a technique to monitor gastric electrical activity and mechanical activity as an aid in assessing gastric motor function. In 1992, Kothapalli et al[7] established a three-dimensional abdomen model to study the origin of changes in the epigastric signal, and analyzed the relationship between the gastric impedance signal and food capacity, resistivity of a test meal, and gastric contraction when the exciting current electrodes and the measuring voltage electrodes were located at different positions.
Early research using an impedance method to measure the digestion course mainly focused on gastric emptying measurement[8-10]. There have been few researches on the extraction of gastric motility information[11-13]. One of the primary reasons is that the rhythm of gastric motility is much lower; i.e., about 3 cpm. It is difficult to extract the gastric motility signal and eliminate respiration interference. In 1991, Chen et al[14] reported their work on obtaining an electric impedance signal, which reflected gastric contraction, and measurement devices were subsequently developed[15]. However, the impedance signals obtained by the devices were all sine waveforms that were similar for both healthy and diseased subjects because of an incorrect filter processing. It was difficult to differentiate normal or abnormal conditions from the signals. In 2007, we proposed a noninvasive electrical impedance method for gastric motility measurement and evaluation[16]. Multi-resolution analysis of the wavelet was adopted to separate the gastric motility signal from the mixed impedance signal obtained on the body surface[17].
In this paper, a gastric motility measurement method based on an electrical-mechanical composite concept was introduced and results from some preliminary clinical application studies of gastric motility measurement for patients with functional dyspepsia (FD) and gastritis, as well as normal controls, were presented.
Gastric contraction is a mechanical behavior that follows electrical activity occurring on the cell membrane surface of smooth muscles. It begins with electrical activity of the smooth muscle, followed by evoked contraction of the gastric corpus and antrum, and is then transmitted to the distal pylorus. It is a composite course from electrical activity to mechanical contraction, which then leads to gastric peristalsis and transmission. Gastric contraction complies with the rhythm of electrical activity and is affected by the amplitude, time limitation, transmission direction and distance of the transmission contraction. Gastric motility is a complex electrical-mechanical composite course. It is very important to measure and evaluate gastric motility according to an electrical-mechanical composite mechanism[18].
There are two kinds of gastric myoelectric activities: slow wave and spike potential. The gastric antrum contracts only when the slow wave occurs accompanied by the spike potential. The spike potential appears during the slow wave phase, and the rhythm of gastric contraction may be determined by the slow wave. Electrogastrogram (EGG) signals recorded from the body surface reflect the myoelectric activity of different regions of the stomach. It corresponds to the gastric slow wave accurately and, therefore, can be used to investigate the rhythm of gastric contraction. EGGs reflect myoelectric activity of the stomach and the rhythm of gastric contraction. The information from the impedance measurement of the stomach directly corresponds to gastric motility. A combined measurement of EGG and impedance gastric motility (IGM) is a new approach for evaluating gastric motility based on an electrical-mechanical composite mechanism.
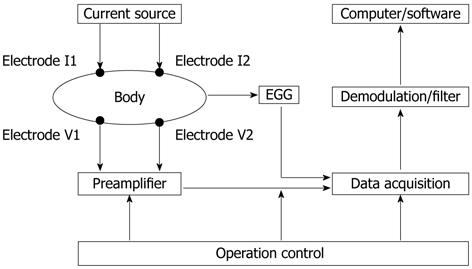
Based on an electrical-mechanical composite mechanism and combined IGM and synchronous EGG measurements, a gastric motility measurement method has been developed, which was reported in our previous paper[19]. The block diagram of the measurement system is shown in Figure 1.
The measurement system consists of a current source, electrodes, preamplifier, demodulation/filter circuit, data acquisition, operation control and an upper computer. Four electrodes (I1, I2, V1 and V2) placed on the epigastric surface of the body are used to inject measurement current and measure impedance signals. An electric current of 50 kHz and 1 mA provided by the current source injects into the epigastric area of the subject via excitation electrodes I1 and I2. The IGM signal picked up from the measurement electrodes V1 and V2 is put into the preamplifier and the demodulation/filter, and then goes into the data acquisition system for analog/digital (A/D) conversion and numeralization processing of the signal. The digitized data are transmitted to the computer where proprietary software performs the IGM and EGG information extraction, analysis and calculation of the gastric motility parameters.
IGM and EGG signals are classified according to their rhythm. The rhythm of 2-4 cpm is considered a normal rhythm, while that below 2 cpm is bradygastria, and above 4 cpm is tachygastria. Based on this classification, frequency spectra, energy spectra, dynamic spectra, running spectra, percentage of normal frequency (PNF), percentage of normal power (PNP), the frequency instability coefficient (FIC) and the power instability coefficient (PIC), for both IGM and EGG, were analyzed as temporal features for the subjects. FIC and PIC represent the stability of the frequency (e.g., the rhythm of the gastric motility signals) and power of the rhythm for both IGM and EGG, respectively. The higher FIC or PIC means that the stability of the rhythm and the power of gastric motility signals are worse.
The subjects included FD patients, erosive gastritis patients and healthy volunteers. All patients with FD and erosive gastritis came from the First Affiliated Hospital of Chongqing University of Medical Sciences. Twenty-eight FD patients (mean age 40.9 ± 9.7 years) who self-reported the symptoms of abdominal bloating, belching and gastric acid reflux and were diagnosed according to the Rome III criteria of FD. All selected FD patients were taking domperidone(10 mg, tid)for treatment. The 30 erosive gastritis patients (mean age 50.5 ± 13.0 years) were diagnosed through gastroscopy and were taking sodium rabeprazole tablets (10 mg, qd, po, qm) for treatment. The 40 healthy volunteers were university students and teachers (mean age 30.9 ± 7.9 years) who served as a control group.
In order to validate the feasibility and effects of the proposed method in this study, a preliminary clinical application study of gastric motility measurement for healthy volunteers and patients with FD and gastritis was conducted. The IGM measurement is a noninvasive method. The study was approved by the ethical committee of the institute and all subjects in the study signed a consent form. The statistical software SPSS 13.0 was used to analyze the data. The data were expressed as mean ± SD. Variance analysis between the study and control groups was undertaken and a significant difference was accepted at P < 0.05.
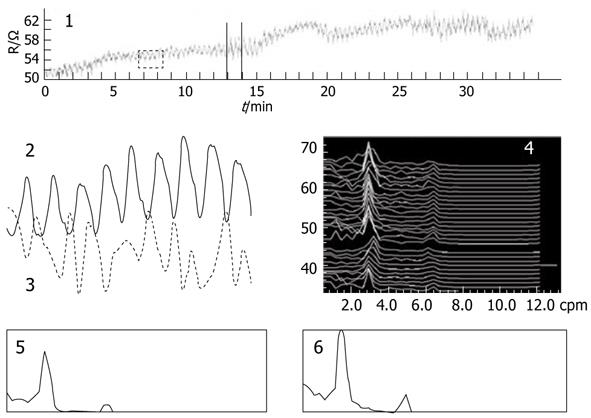
IGM measurement results for healthy volunteers are illustrated in Figure 2. Part 1 represents the original mixed impedance signal picked up from the abdominal surface, part 2 is the IGM signal extracted from the mixed signal, part 3 is the synchronous EGG, part 4 is the dynamic spectrum of IGM, and parts 5 and 6 are the power spectrums before and after a test meal. The dominant frequency of the IGM signal was 2.8 cpm in the dynamic spectrum of part 4.
The IGM parameters for 40 healthy volunteers and 28 FD patients are shown in Table 1. It can be seen that PNF in the FD group was obviously lower than that in the control group (P < 0.01), and FIC was higher than that in the control group (P < 0.01). The PNP and PIC showed similar differences between the two groups (P < 0.01). These results suggest that FD patients have a poorer rhythm in gastric motility and energy deficiency. This result is consistent with digestion pathology and physiology in FD.
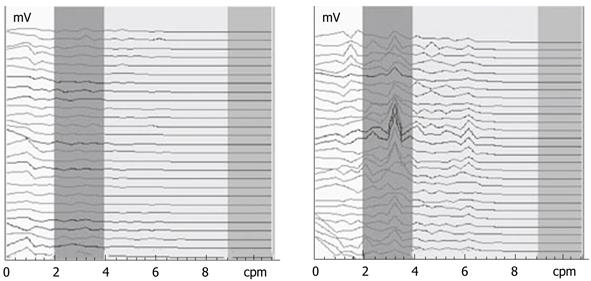
Figure 3 shows the EGG dynamic spectrum of FD patients before and 1 wk after administration of dompendone tablets, 10 mg, tid, po, taken half an hour before meals. The interval for each spectrum line was 1 min in duration. In Figure 3, the EGGs of FD patients were weak and the rhythms were disordered before treatment (left). After 1 wk of treatment, the EGG improved and the rhythm returned to 2-4 cpm (right). This suggested that the GEA of FD patients tended towards normal after 1 wk of treatment.
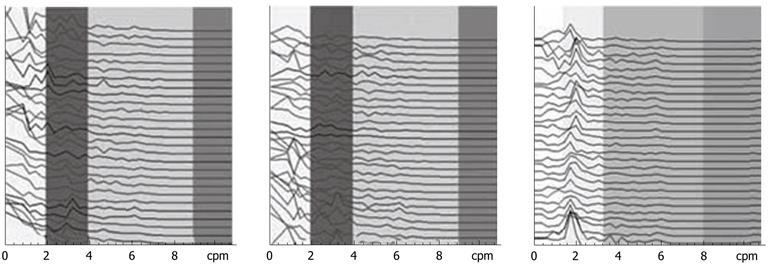
The IGM signal spectrums of one FD patient before treatment and 1 wk and 3 wk after treatment are shown in Figure 4. Compared with the EGG dynamic spectrum in Figure 3, the IGM spectrum after 1 wk of treatment in Figure 4 showed little change and the rhythm was also disordered. After 3 wk of treatment, the IGM rhythm returned to 2-4 cpm.
The parameters for IGM and EGG in the gastritis patients before and 1 wk after administration of sodium rabeprazole tablets, 10 mg, qd, po, qm are shown in Tables 1 and 2.
| Treatment | 0-2 cpm PNP | 2-4 cpm PNP | > 4 cpm PNP | FIC | PIC |
| EGG parameters | |||||
| Before | 24.0 ± 5.6 | 51.5 ± 11.1 | 24.4 ± 5.5 | 2.22 ± 0.43 | 0.34 ± 0.03 |
| After 1 wk | 22.7 ± 3.4 | 54.3 ± 6.7 | 23.1 ± 3.3 | 1.77 ± 0.19a | 0.23 ± 0.02a |
| IGM parameters | |||||
| Before | 27.5 ± 2.4 | 44.6 ± 4.8 | 27.9 ± 2.4 | 2.23 ± 0.55 | 0.24 ± 0.05 |
| After 1 wk | 27.4 ± 2.2 | 44.9 ± 4.4 | 27.8 ± 2.2 | 1.91 ± 0.65 | 0.21 ± 0.06 |
It can be seen from Table 1 that there was a significant difference in IGM parameters between the gastritis patients and healthy controls. The PNF and PNP of the gastritis patients were significantly lower than that of the healthy controls (P < 0.05). The FIC and PIC of the gastritis patients were evidently higher than the healthy controls (P < 0.05). This suggests a weakening gastric motility function and disorder of stomach peristalsis in gastritis patients. Table 2 indicates that the EGG power of the normal rhythm (2-4 cpm) for the patients was raised and the power of the abnormal rhythm (0-2 cpm and > 4 cpm) declined after 1 wk of treatment, although this was not statistically significant (P > 0.05). It should be noted that the FIC and PIC of the patients decreased significantly (P < 0.05). It suggests that the EGG of gastritis patients tended to be normal and stable after 1 wk of treatment, and the rhythm of the EGG improved.
IGM parameters in Table 2 show that the power ratios of IGM signals for the patients in all frequency bands did not change much before and after treatment (P > 0.05), although the FIC and PIC showed a decreasing trend (P > 0.05).
The motility function of the stomach is regulated by nerve and body fluid and is accomplished by the coordinated movement of smooth muscle[4,20]. It can be seen from Figures 3 and 4 that for FD patients the IGM was not improved with 1 wk of treatment, while the EGG returned to normal. After 3 wk of treatment, the regular IGM rhythm of the FD subjects became normal and the contraction function of the stomach was recovered. It is understandable that although the EGG returned to normal by nerve regulation with 1 wk of treatment, improvement of the electric activity had not coupled with or transferred to the mechanical activity of the stomach. After 3 wk of treatment, when the electrical activity had already coupled with or transferred to the mechanical activity of the stomach via the regulation mechanism for nerve and body fluid, the normal IGM rhythm could be seen in the spectrum, which suggests the recovery of the contraction function of the stomach.
There were significant differences in IGM parameters between the gastritis patients and healthy controls. After 1 wk of treatment, the EGG rhythm of the gastritis patients returned to normal while the IGM parameters only showed a tendency to improve, which had no statistical significance. This suggests that the influence of the GEA may not have coupled with the mechanical contraction of the stomach after only 1 wk of treatment. On the other hand, although the patients felt some alleviation after 1 wk of treatment, the cardinal symptoms of gastritis were not completely relieved. This fact coincided with the results of Table 2, and therefore, the patients should be advised to continue the treatment.
The mixed signal acquired from the abdominal surface contains not only IGM information but also the components of impedance blood flow, breath and some other disturbances. The normal rhythm of IGM is about 3 cpm. Within the mixed signal, the rhythm of the breath impedance signal is about 12 cpm, which is close to the rhythm of the IGM. Signals of the IGM and breath both are ultra-low frequency signals and the amplitude of the breath signal is usually much higher than that of the IGM signal. It is a challenge to extract the IGM signal from the mixed signals effectively. A low-pass filter may be good enough to reduce the effect of high-frequency noise and heart activity, however, it is difficult to eliminate respiration influence and separate the IGM signal from the mixed signal. Therefore, a narrow bandpass filter and a high-order active low-pass filter are required. In the measurement system for gastric motility described in this paper, a wavelet transform was introduced and the IGM signal was separated successfully from impedance signals, including breath and blood flow.
In this study, we focused on the stomach contraction rhythm. Some events without a rhythm, such as gastric acid secretion, did not affect the measurement results.
The EGG reflects GEA of the stomach and is sensitive to regulation mechanisms from nerve and electrical activity. The improvement of the EGG after treatment is only the beginning of improvement in gastric motility function and does not indicate a cure of gastric disorder or the recovery of gastric motility. The IGM is a veritable measure of gastric contraction and peristalsis, and reflects the gastric motility function. Gastric motility measurement is based on an electrical-mechanical composite mechanism. The combined measurement of IGM and EGG is a noninvasive, convenient and effective method that extracts information that directly reflects the gastric motility state. It can be used to measure gastric contraction and peristalsis during digestion and evaluate gastric motility function in different physiological and pathological conditions.
Gastric motility is one of the most critical physiological functions of the human body. The research on gastric motility function has fallen behind that on gastric endocrine and exocrine secretion functions and gastric morphology. One of the important reasons for this is the absence of a convenient and effective measurement method for gastric motility function. There are many applications using bioimpedance signals for different pathological conditions, but their use in gastric motility assessment needs to be explored in detail and there are few researches on the extraction of gastric motility information.
In the period of digestion, the stomach content changes greatly and so does the impedance of the stomach. When measuring the impedance of the stomach during digestion, the information reflecting the stomach volume (gastric emptying) and gastric motility can be extracted. The method combining electrical bioimpedance measurement with an electrogastrogram (EGG) is one of the research frontiers for gastric motility measurement.
Gastric motility is a complex composite course from electrical gastric activity to mechanical gastric activity. The EGG reflects the myoelectric activity of the stomach and the rhythm of gastric contraction. Information from impedance measurement on the stomach directly corresponds to gastric motility. A combination of EGG and impedance gastric motility (IGM) measurements is a new approach for measuring and evaluating gastric motility based on an electrical-mechanical composite mechanism.
A combination of IGM and EGG measurement is a noninvasive, convenient and effective method that can be used to measure gastric contraction and peristalsis during digestion and evaluate gastric motility function in different physiological and pathological conditions. This method has shown an attractive application prospect in gastric motility research and evaluation.
An electrical bioimpedance technique is a method to extract physiological and pathological information from the human body according to the electrical property of the tissue and organs by means of injecting a small current and measuring the electrical potentials from electrodes on the body surface. The method has the advantages of being noninvasive, convenient, and providing considerable functional information.
This is a good article illustrating how technology can be used for clinical measurement. The authors have put a lot of efforts to develop and present their work.
Peer reviewer: Justin C Wu, Professor, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, 9/F, Clinical Science Building, Prince of Wales Hospital, Shatin, Hong Kong, China
S- Editor Shi ZF L- Editor Ma JY E- Editor Zheng XM
| 1. | Gajre SS, Anand S, Singh U, Saxena RK. Novel method of using dynamic electrical impedance signals for noninvasive diagnosis of knee osteoarthritis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2207-2210. [PubMed] |
| 2. | Hadi NA, Giouvanoudi A, Morton R, Horton PW, Spyrou NM. Variations in gastric emptying times of three stomach regions for simple and complex meals using scintigraphy. IEEE Trans Nucl Sci. 2002;49:2328-2331 doi: 10.1109/TNS.2002.803785. |
| 3. | Chen JD, Lin XM, Abo M. Multi-channel gastric electrical stimulation for the acceleration of gastric emptying. In: Institution of Engineering and Technology, editors. Advances in Medical Signal and Information Processing. Proceedings of First International Conference on Advances in Medical Signal and Information Processing; 2000; IEE Conference Publication No. 476, 2000: 60-65. |
| 4. | Zhou L, Ke MY. Textbook of neurogastroenterology and motility: basic and clinical aspects. Beijing: Science Press 2005; . |
| 5. | Sutton JA, Thompson S, Sobnack R. Measurement of gastric emptying rates by radioactive isotope scanning and epigastric impedance. Lancet. 1985;1:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Familoni BO, Kingma YJ, Bowes KL. Noninvasive assessment of human gastric motor function. IEEE Trans Biomed Eng. 1987;34:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Kothapalli B. Origin of changes in the epigastric impedance signal as determined by a three-dimensional model. IEEE Trans Biomed Eng. 1992;39:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Huerta-Franco R, Vargas-Luna M, Hernandez E, Capaccione K, Cordova T. Use of short-term bio-impedance for gastric motility assessment. Med Eng Phys. 2009;31:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Chaw CS, Yazaki E, Evans DF. The effect of pH change on the gastric emptying of liquids measured by electrical impedance tomography and pH-sensitive radiotelemetry capsule. Int J Pharm. 2001;227:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Giouvanoudi A, Amaee WB, Sutton JA, Horton P, Morton R, Hall W, Morgan L, Freedman MR, Spyrou NM. Physiological interpretation of electrical impedance epigastrography measurements. Physiol Meas. 2003;24:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Soulsby CT, Khela M, Yazaki E, Evans DF, Hennessy E, Powell-Tuck J. Measurements of gastric emptying during continuous nasogastric infusion of liquid feed: electric impedance tomography versus gamma scintigraphy. Clin Nutr. 2006;25:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Garay L, Ramos EG, Cardiel E, Muñoz R, Hernández PR. In vivo and in situ measurement of electrical impedance for determination of distention in proximal stomach of rats. Med Eng Phys. 2006;28:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Giouvanoudi AC, Spyrou NM. Epigastric electrical impedance for the quantitative determination of the gastric acidity. Physiol Meas. 2008;29:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Chen RX, Wan DR. Further investigation of reliability on impedance gastrography for continuous measurement of human gastric contractile activity. In: Proceedings of the 8th international conference on electrical bio-impedance; 1992; University of Kuopio, 1992: 151-152. |
| 15. | Chen RX, Wan DR. Abnormal impedance gastrogram and upper gastrointestinal symptom. Med Biol Eng comput. 1991;29 Suppl:14-15. |
| 16. | Li ZY, Sha H, Wang Y, Zhao S, Wang W, Ren CS. A new approach of gastric motility measurement and evaluation by bioimpedance. In: Scharfetter H, Merva R, editors. IFMBE Proceedings. ICEBI 2007; Proceedings of the 13th International Conference on Electrical Bio-Impedance & 8th Conference on Electrical Impedance Tomography; 2007 Aug 29-Sep 2; Graz, Austria. Berlin: Springer, 2007, 691-694. |
| 17. | Li Z, Ren C. Gastric motility measurement and evaluation of functional dyspepsia by a bio-impedance method. Physiol Meas. 2008;29:S373-S382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ren CS, Li ZY, Zhao S. Use of electrical bioimpedance for gastric motility measurement and evaluation. Shijie Huaren Xiaohua Zazhi. 2010;18:1-8. |
| 19. | Li ZY, Sha H, Zhao S, Wang Y, Ren CS. [Liquid gastric-emptying measurement using an electrical bio-impedance method]. Zhongguo Yiliao Qixie Zazhi. 2008;32:253-256. [PubMed] |
| 20. | Ma DS, Zhou B, Fu B, Song M. Weichang Yundong Yu Linchuang. Beijing: Military Medicine Science Press 2006; 62-84. |