INTRODUCTION
A desmoid tumor, also known as aggressive fibromatosis, is a rare benign neoplasm that arises from fascial or musculoaponeurotic tissues. It is usually associated with preneoplastic conditions, such as familial adenomatous polyposis and Gardner syndrome. The sporadic form is uncommon, presenting with a mass, sometimes associated with pain and weight loss. It can occur in any anatomical location, but most commonly in the abdominal wall, shoulder girdle and retroperitoneum. Histological analysis of this type of tumor reveals mesenchymal characteristics, a lack of mitotic activity, and cellular pleomorphism[1-4]. Although desmoid tumors are considered benign, without the ability to metastasize, they do have significant potential for local invasiveness and recurrence[5,6].
We present a case of local recurrence after aggressive surgical resection in a 25-year-old man.
CASE REPORT
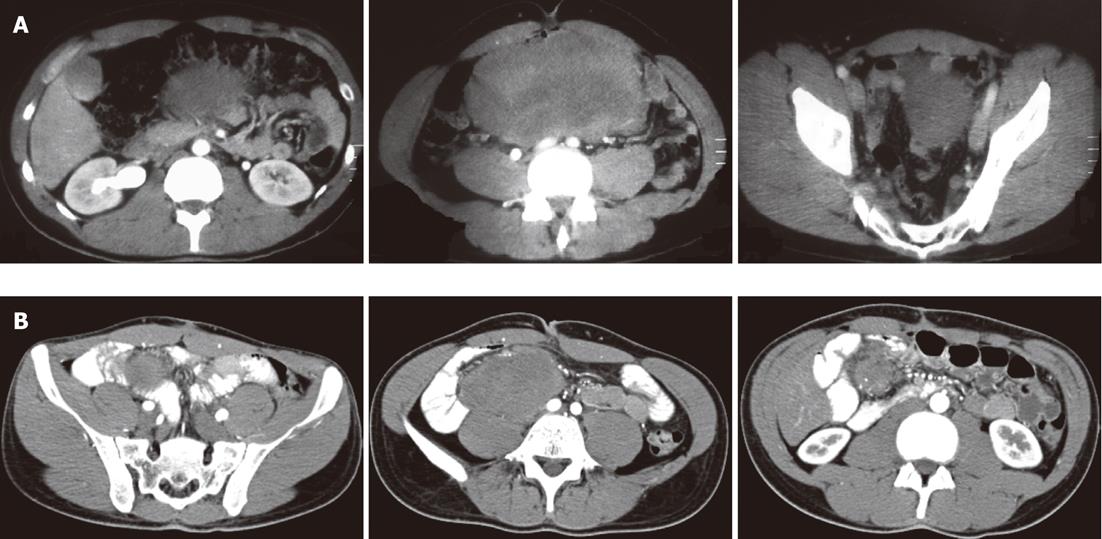
A 25-year-old Caucasian man presented to our department with a histologically confirmed diagnosis of local recurrence of sporadic desmoid tumor. The patient’s disease dated back 2 years, when the patient was admitted to another hospital because of pain in the right anterolateral abdomen. Abdominal computed tomography (CT) revealed a large solid mass (22 cm × 10 cm × 15 cm) with mild enhancement and density equal to that of soft tissue. The mass appeared to arise from the retroperitoneum and caused superior displacement of the stomach and adjacent bowel loops. There was no pathological lymphadenopathy (Figure 1A).
Figure 1 Abdominal computed tomography of desmoid tumour.
A: A solid large mass (22 cm × 10 cm × 15 cm) with density equal to that of soft tissue; B: A perianastomotic solid mass (13 cm × 11 cm × 10 cm) arising from the mesentery.
There was no known history of familial polyposis and no medical history of any abdominal trauma or surgery. His blood parameters were normal, and laboratory studies showed normal renal function and electrolytes.
Radical surgical resection was performed, including 30 cm of small intestine and right hemicolectomy, which was necessary to obtain a wide margin. The histological diagnosis was desmoid tumor. There were no complications in the postoperative phase.
After 10 mo, the patient came to our hospital because of persistent diarrhea. A CT scan was performed, which demonstrated a perianastomotic solid mass (13 cm × 11 cm × 10 cm) arising from the mesentery. The mass caused displacement of the common iliac artery and spread downward to the psoas muscle, without infiltrating it (Figure 1B). A second surgical resection was performed, without complications, including anastomosis and part of the intestinum tenue. The histological diagnosis was aggressive fibromatosis, with uninvolved surgical margins. The patient tolerated the surgical excision of the mass, but he had a nutritional examination because of short bowel syndrome acquired due to the surgery (leaving a residual 75 cm of small intestine). The patient received an optimized dietary and medication plan.
One month later, the patient underwent an abdominal CT scan that revealed an oval mass with a diameter of 18 mm in the mesenteric root, close to the metallic clips of the second surgical excision. This mass was open to a different interpretation. Therefore, the patient underwent an oncological examination, and as we suspected, it was found to be a recurrent lesion. We also recommended a biopsy. The test reported a histological diagnosis of desmoid tumor, and the patient was treated with curative radiotherapy. He received 3D-conformal external beam irradiation using megavoltage photon and conventional fractionation: 2 Gy daily for 5 d/wk, for a total dose of 50 Gy. The planned target volume included the previous tumor bed and actual relapse. Acute toxicity was observed, primarily as a function of the volume of the radiation field. After 2 wk of radiotherapy, the treatment was interrupted for 13 d because of grade 3 gastrointestinal toxicity, according to the Common Terminology Criteria for Adverse Events v3.0. The patient had progressive weight loss, fatigue and severe radiation enterocolitis, resulting in urgency, increased frequency (> 7/d) and decreased consistency of bowel movements. The radiotherapy was temporarily interrupted, and the patient received parenteral nutrition for 1 wk.
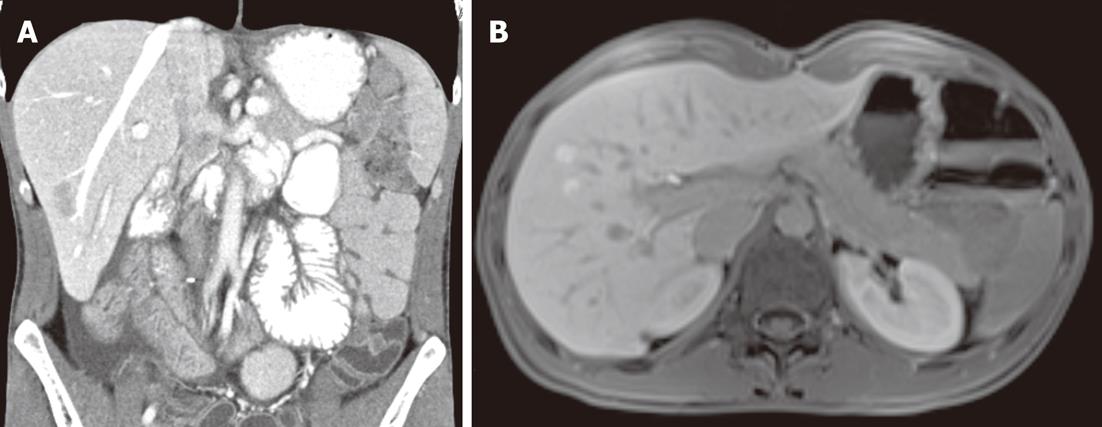
We radiologically assessed the response to radiotherapy after 4, 8 and 12 mo, and the outcome was complete remission. It is important to note that abdominal CT carried out 8 mo after the end of radiotherapy revealed liver alterations with no distinguishing marks (Figure 2). Such alteration had not been observed in previous examinations; therefore, the patient underwent magnetic resonance imaging and then liver biopsies, because the first was not sufficient to clarify the situation (Figure 2). The histological diagnosis was macrovesicular and microvesicular steatosis. Radiotherapy increases the risk of hepatobiliary complications in short bowel syndrome, therefore, a wait-and-see approach, consisting of radiological and clinical evaluations performed every 3 mo, was chosen. The patient achieved complete tumor regression at 4 mo after radiotherapy, and he is clinically free of disease at 12 mo after the end of treatment, with an acceptable quality of life.
Figure 2 Fatty liver.
Abdominal computed tomography (A) and magnetic resonance imaging (B) demonstrated liver alterations with no distinguishing marks.
DISCUSSION
The definition of a rare tumor is based on an incidence of ≤ 3/100 000/year[7]. Desmoid tumors fall under this category because they have an annual incidence of 0.2-0.4/100 000 population. For that reason, desmoids are not extensively discussed in the literature: most reports are case reports[1,3,8-11], and some are retrospective analyses[5,6,12,13], but randomized series do not exist.
The etiopathogenesis is poorly defined; trauma, endocrine factors and genetic predisposition all seem to play important roles in the development of the disease. It is significantly associated with familial adenomatous polyposis and has a more pronounced association with the subgroup of Gardner syndrome. Desmoid tumors are a fibroblastic proliferations that arise mainly from fascial or musculoaponeurotic structures. The typical clinical presentation is a painless mass with a slow and progressive invasion of contiguous structures, and it is associated with a high local recurrence rate after resection. Factors predictive of relapse include an age between 18 and 30 years, a tumor size > 8 cm, incomplete resection, or close positive margins, and no adjuvant radiotherapy for gross residual disease[5,6,12].
The rarity of these tumors, coupled with the variability in their clinical course, has prevented the demonstration of the efficacy of any specific intervention[13]. Many issues regarding the optimal treatment of desmoid tumors remain controversial. However, true R0 margins are difficult to achieve because of the indistinct borders in this type of tumor[14,15]. Aggressive surgical resection with a wide margin (2-3 cm) remains the gold standard treatment with regard to the preservation of quality of life[16].
Radiotherapy alone has been shown to be effective for the control of unresectable or recurrent lesions. In 1928, Ewing et al proposed radiotherapy as a treatment modality for the management of these lesions and reported that desmoid tumors respond slowly but satisfactorily to radiation[16]. In subsequent years, several studies[17-20] have confirmed that radiotherapy can result in lasting local control in inoperable or incompletely resected tumors. A comparative review of 22 articles[12] has shown that radiotherapy, delivered as a single modality or as an adjuvant treatment, resulted in significantly better local control than surgery alone (78%, 75% and 61%, respectively). However, the role of radiotherapy in the management of desmoid tumors is still not defined.
Strong indications for radiotherapy include unresectable lesions, macroscopic or microscopic residual disease after resection and recurrent tumors.
The radiation dose appropriate for treating desmoid tumors remains controversial. Ballo et al[14] have demonstrated that doses of 56 Gy at 2 Gy per fraction (equivalent to approximately 60 Gy at 1.8 Gy per fraction) produce significantly higher control rates than doses ≤ 50 Gy. In other published studies[17,19-22], patients were treated with doses of 50-60 Gy. To date, no improvement of results with total doses higher than 60 Gy has been observed, while an increased incidence of radiation-related toxicity has been documented[12-15,17,20]. As desmoid tumors tend to be locally infiltrative, the fields must be generous to prevent marginal recurrence. Of course, normal tissue toxicity and potential late radiation effects are important to consider in the treatment. Radiotherapy for gross disease is associated with a relatively high rate of complications, which are dose-dependent. Complications have included edema, fibrosis, ulcers, paraesthesia and secondary malignancy[8]. Radiation enteritis and radiation-induced liver disease are also potential complications of radiotherapy. In the study of Boland et al[23], a total of 48 patients treated with radiotherapy for intra-abdominal malignancy were evaluated: 16 (33%) patients developed short bowel syndrome within 12 mo of treatment. Thompson et al[24] found that patients with short bowel syndrome and a history of radiotherapy were more likely to develop cirrhosis and portal hypertension than short bowel syndrome patients not subject to radiotherapy. Although diabetes mellitus and obesity are the conditions most frequently associated with nonalcoholic fatty liver disease, which is histologically defined by the presence of macrovesicular steatosis, intestinal resection has also been recorded as one of the predisposing factors[25]. Our patient, who did not conform to the usual profile of an obese and diabetic man, developed short bowel syndrome as a complication of the second surgical resection. Consequently, radiotherapy might have worsened an already present malabsorption and so led to steatohepatitis. Immediate adjuvant radiotherapy after the first radical surgical approach would likely have produced a better result in terms of toxicity and quality of life, reducing the likelihood of malabsorption due to repeated surgical resections.
Peer reviewer: Sanaa Ahmed Ali, Professor, Department of Theraputic Chemistry, National Research Centre, El Behooth St. Dokki Giza, Cairo 12622, Egypt
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L