Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3145
Revised: March 31, 2012
Accepted: April 22, 2012
Published online: June 28, 2012
AIM: To investigate the mechanism of gastric mucosal demage induced by water immersion restraint stress (WRS) and its prevention by growth hormone releasing peptide-6 (GHRP-6).
METHODS: Male Wistar rats were subjected to conscious or unconscious (anesthetized) WRS, simple restraint (SR), free swimming (FS), non-water fluid immersion, immersion without water contact, or rats were placed in a cage surrounded by sand. To explore the sensitivity structures that influence the stress reaction besides skin stimuli, a group the rats had their eyes occluded. Cervical bilateral trunk vagotomy or atropine injection was performed in some rats to assess the parasympathetic role in mucosal damage. Gastric mucosal lesions, acid output and heart rate variability were measured. Plasma renin, endothelin-1 and thromboxane B2 and gastric heat shock protein 70 were also assayed. GHRP-6 was injected [intraperitoneal (IP) or intracerebroventricular (ICV)] 2 h before the onset of stress to observe its potential prevention of the mucosal lesion.
RESULTS: WRS for 6 h induced serious gastric mucosal lesion [lesion area, WRS 81.8 ± 6.4 mm2vs normal control 0.0 ± 0.0 mm2, P < 0.01], decreased the heart rate, and increased the heart rate variability and gastric acid secretion, suggesting an increase in vagal nerve-carrying stimuli. The mucosal injury was inversely correlated with water temperature (lesion area, WRS at 35 °C 56.4 ± 5.2 mm2vs WRS at 23 °C 81.8 ± 6.4 mm2, P < 0.01) and was consciousness-dependent. The injury could not be prevented by eye occlusion, but could be prevented by avoiding contact of the rat body with the water by dressing it in an impermeable plastic suit. When water was replaced by vegetable oil or liquid paraffin, there were gastric lesions in the same grade of water immersion. When rat were placed in a cage surrounded by sand, there were no gastric lesions. All these data point to a remarkable importance of cutenuous information transmitted to the high neural center that by vagal nerves reaching the gastric mucosa. FS alone also induced serious gastric injury, but SR could not induce gastric injury. Bilateral vagotomy or atropine prevented the WRS-induced mucosal lesion, indicating that increased outflow from the vagal center is a decisive factor in WRS-induced gastric injury. The mucosal lesions were prevented by prior injection of GHRP-6 via IP did, but not via ICV, suggesting that the protection is peripheral, although a sudden injection is not equivalent to a physiological release and uptake, which eventually may affect the vagal center.
CONCLUSION: From the central nervous system, vagal nerves carry the cutaneous stimuli brought about by the immersion restraint, an experimental model for inducing acute gastric erosions. GHRP-6 prevents the occurrence of these lesions.
- Citation: Guo S, Gao Q, Jiao Q, Hao W, Gao X, Cao JM. Gastric mucosal damage in water immersion stress: Mechanism and prevention with GHRP-6. World J Gastroenterol 2012; 18(24): 3145-3155
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3145
Stress is an adaptive physiological response to disruption of homeostasis. Serious stress can induce organ injury or contribute to diseases, such as gastric ulcers, hypertension, diabetes, and cancer. The stomach is one of the main targets of stress. Stress-induced gastric ulceration is a typical example of stress-associated organ injuries[1]. Water immersion restraint stress (WRS) mimics the clinical acute gastric ulcerations caused by trauma, surgery, or sepsis[2] and has been widely accepted for studying stress ulceration[3]. It is theoretically and clinically significant to demonstrate the mechanism of stress-induced gastric injury and develop respective therapeutic drugs.
Both psychological and physiological responses occur during stress and these are involved in the pathogenesis of gastric ulceration. The psychological responses include anxiety, depression, feeling of helplessness, fear, threat of drowning, etc. The physiological responses include neurohormonal and immunological activations, including the involvement of corticotropin-releasing factor. These two systems may interact during stressful challenges[4,5], known as psychosomatic reactions. Nonetheless, the mechanisms of gastric stress ulceration remain unclear.
Developing protective drugs against gastric stress ulceration is an important clinical issue. Based on previous studies, agonizing the growth hormone secretagog receptor (GHSR) might be a strategy. Growth hormone releasing peptides (GHRP) are peptidyl growth hormone secretagogs (GHS) and are the synthetic ligands for the GHSR. The family members of GHRP include GHRP-1, GHRP-2, GHRP-6 and hexarelin[6]. GHSR, and its natural ligand ghrelin, are widespread in many tissues, including the gastrointestinal tract[7] and cardiovascular system[8]. Although the GH-releasing actions of both the natural and synthetic GHS have been demonstrated in different species, the function of GHS on alternative physiological systems has not been clearly elucidated. Studies over the past two decades have demonstrated that GHS exerts its physiological or pharmacological actions via GH-independent pathways, except for its GH-dependent action[9]. In the cardiovascular system, GHRP and ghrelin exert protective effects, especially on myocardial infarction[10] and heart failure[8,11,12]. Ghrelin and GHSR are expressed in the rat and human stomach and may have significant physiological/pharmacological effects on gastric function and diseases[13,14]. Ghrelin exerts a potent protective action on the stomach of rats exposed to WRS[15]. However, whether or not GHRP also protects against stress-induced gastric injury is unknown. GHRP are much smaller in molecular weight, effective when administered orally, more stable and economically cheaper than ghrelin, and with minimal toxicity, they are better prospects for developing drugs for gastric protection. The purpose of the study was to further investigate the mechanism of gastric stress ulceration using the WRS rat as a model and observe the potential protective effect of GHRP-6 on this gastric injury.
A 78 4-mo old male Wistar rats of 310 ± 10 g, were involved in the study. Before the experiment, each animal was housed in a single cage that had wire-net bottoms to avoid coprophagy and had free access to tap water and regular chow for at least 7 d. All animals were starved for 24 h before the onset of stress, but had free access to tap water. Animals were conscious during the stress procedures except those in the “WRS + anesthesia” group (described below), in which rats were anesthetized with 50 mg/kg of sodium pentothal intraperitoneal (IP) during the whole 6-h stress procedures. The water temperature was set to 23 ± 0.5 °C, except in the WRS group, in which three water temperatures were tested (see below).
The animals were randomly divided into 11 groups (n = 6 in each group/treatment): (1) WRS: rats were lightly anesthetized by ether inhalation and four limbs of each rat were restrained on a wooden plate (25 cm × 19 cm), with the upper limbs anchored at a horizontal position and the lower limbs extended downward. After awakening (usually 10-15 min after ether anesthesia), rats anchored on the wooden plates were immersed vertically (head up) in water to the level of xiphoid process in a water bath thermostatically controlled at 23 ± 0.5 °C, 19 ± 0.5 °C or 35 ± 0.5 °C, with or without constant pentothal anesthesia, respectively (each n = 6). Anesthesia was achieved with 50 mg/kg of sodium pentothal IP over the whole 6-h stress procedure; (2) simple restraint (SR): the procedure was the same as in the WRS group except that the water bath was empty; (3) free swimming (FS): rats were put into water (water depth 7 cm to avoid drowning) and allowed free movement in the water for 6 h; (4) shallow water touch: rats were put into a water both (water depth 1 cm) and kept for free moving in the water for 6 h; (5) WRS + eye occlusion: animals were eye-occluded with adhesive plasters and then underwent the WRS procedures, in an attempt to determine whether vision plays a role in the development of stress ulcers; (6) immersion without water contact (NWCI): water immersion with the rat body into a plastic bag to avoid water contact but the rat could see the surrounding water; (7) non-water fluid immersion: the procedure was the same as WRS except that water was replaced by salad oil or liquid paraffin (each n = 6), in an attempt to elucidate if skin sensation can differentiate different liquids and induce different gastric responses; (8) “burial” in sand: the restrained rat was placed in a box, the space between the box wall and the rat body was filled with fine sand, the level of filling sand was also to the xiphoid process. To avoid compression of the body, pieces of spongeous material were introduced into the sand; (9) WRS + vagotomy and WRS + atropine: rats underwent bilateral vagal nerve trunk cutting and then underwent WRS. Additional 6 WRS rats (without vagotomy) received atropine (1 mg/kg) IP injection 10 min before the onset of WRS; (10) WRS + GHRP-6: the rat received GHRP-6 (100 μg/kg) (ProSpec-Tany, Israel) IP or intracerebroventricular (ICV) injection 2 h before the WRS procedure. For IP injection, GHRP-6 was dissolved in saline, with a total volume of 0.25 mL per injection; for ICV injection, GHRP was diluted in artificial cerebrospinal fluid, the volume and dosage of GHRP per injection were 5 μL and 20 μg/kg; and (11) normal control: rats were not submitted to any stress procedure. Animals without GHRP-6 IP injection received same volume (0.25 mL) of saline injection (placebo).
The animal use protocol was approved by the Life Ethics Committee of Peking Union Medical College and was conducted in compliance with the United States National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication 85-23).
After the stress procedures, animals were released from the plate and were decapitated under pentobarbital anesthesia. The stomachs were then harvested and opened along the lesser curvature. The severity of mucosal lesions was grossly inspected and digitally photographed. Gastric tissues were then fixed in 10% formalin, dehydrated and imbedded in paraffin wax. Paraffin sections of 5 μm were cut and stained with hematoxylin and eosin. Histological changes were checked under a microscope. The length and width of each lesion, including epithelial cell damage, glandular disruption, vasocongestion, hemorrhage and deep necrosis, were measured by stereoscopy and the total area of the lesions in one stomach was assessed by planimetry[16]. The measurement of ulcer index was determined by protocol-blinded researcher. The number of animals showing these histopathological lesions in each group was compared with that of other groups.
Surface electrocardiograms (ECG) were recorded with a computer assisted BL-420S system (Chengdu Technology and Market Co. Ltd., Chengdu, China) with a sample rate of 1000 HZ. To avoid the influence of circadian variation of gastric susceptibility, we restricted the ECG recording time to 09:00-15:00 for all subjected animals. R waves were detected off-line with wavelet transform algorithm and then by manual artifact removal. Linear parameters of heart rate variability (HRV), including mean R-R intervals, standard deviation of the normal-to-normal R-R intervals (SDNN) and root mean square of successive difference (RMSSD) of R-R intervals, and non-linear parameter (Poincaré plot) were analyzed as we previously described[17].
Under light ether inhalation anesthesia, bilateral cervical vagal nerve trunks were exposed and cut off. After closure of the incision, rats were allowed 3 h to recover from the surgery and anesthesia before the WRS procedure.
To avoid interrupting the development and observation of gastric erosion, an additional 24 rats were used to measure gastric acid secretion during the stress. These rats were randomly assigned to four groups: WRS, WRS + GHRP-6, RS and Normal + GHRP-6 groups, respectively (each n = 6). Gastric acid outputs were measured according to the reported protocols[18,19] with minor modifications. After a 24-h fast, animals were anesthetized by light ether inhalation. For each animal, a transverse incision was made in the abdomen. Both cardia and pylorus were intubated via incisions with open polyethylene cannulae and then ligated. The incisions were closed with thread adhesive to avoid water invasion, and ether was discontinued. To remove any solid contents, the stomach was gently rinsed with 2 mL of saline at 37 °C three times before the drainage of the gastric juice. Two milliliters of saline warmed to 37 °C were then injected into the stomach, left for 30 min and then aspirated and replaced by a fresh saline solution. The process was repeated twice to obtain acid secretion before stress and once every 30 min after the beginning of stress, for 3 h. The aspirated fluids were titrated to pH 7.0 with 0.01 mol/L (normality) NaOH using a pH meter, and acid output was calculated as μEq/30 min.
At least one week before the ICV injection, a brain cannula made of polyethylene tubing (PE-10; Clay Adams, Parsippany, NJ) was inserted into the left lateral cerebral ventricle (A-P, 1.5 mm caudal to the bregma; L, 2.0 mm lateral to the midline; V, 3.0 mm below the skull surface) under pentobarbital anesthesia (35 mg/kg, IP), as recently reported[20]. The cannula implanted into the brain was securely fixed by dental cement and synthetic resin. When injections were given to the rats, a microsyringe for injection was directly connected to the cannula. ICV injections were performed only in conscious rats.
Blood was sampled from the ventroartery and prepared for the measurements of stress-related vasoconstrictive factors. Kits for assaying the factors were purchased from the People’s Liberation Army General Hospital, Beijing, China. Plasma renin activity (PRA) was indicated by the production of angiotensin I (Ang I) in a reaction system including rat plasma (containing renin and angiotensinogen), rabbit anti-human Ang I antiserum, Ang I standards, and 125I-Ang I. Ang I was measured by the respective radioimmunoassay (RIA) kit. The direct reaction between sample plasma and Ang I antiserum served as a control. The radiation intensity (counts/min) in each tube was converted to nanograms per milliliter (ng/mL), with reference to the Ang I standard curve. PRA was calculated by the equation: PRA (ng/mL per hour) = (Ang I concentration in test tube-Ang I concentration in control tube)/incubation time (h). All assays were performed in duplicate.
Plasma endothelin-1 (ET-1) was measured with a RIA kit according to the manufacturer’s protocols. The primary antibodies were rabbit anti-human ET-1 which showed interactions with rat ET-1. We also used standards of these hormones and blank controls to guarantee the quality of the measurement. The measuring sensitivities were < 5 pg/mL for ET-1. The intra- and interassay variabilities were < 10% and < 15% for ET-1.
Plasma thromboxane B2 (TXB2) was measured with an RIA kit (People’s Liberation Army General Hospital, Beijing), according to the manufacturer’s protocols. Thromboxane A2 (TXA2) is unstable (half-life 30 min) and is rapidly metabolized to the relatively stable TXB2; therefore, we used TXB2 as an indicator of TXA2 level. One milliliter of blood was drawn from the abdominal aorta into a test tube containing 0.06 mL of indomethacin-EDTA solution and then mixed. The blood was centrifuged at 3500 r/min for 15 min and the plasma was separated and stored at -20 °C. At the beginning of the measurement, the plasma was defrosted and centrifuged again at 3500 r/min for 10 min. The supernatant was used to measure the TXA2 level using the RIA kit.
The gastric mucosal tissues were harvested immediately after decapitation, and 100 mg of mucosal tissues for each animal were used for the following procedures. Total protein extracts were prepared by homogenizing mucosal tissues in lysis buffer. Protein (80 μg per sample) electrophoresis were subjected to sodium dodecylsulfate-polyacrylamide gel and then transferred onto a nitrocellulose membrane. Primary antibodies [goat anti-rat heat shock proteins 70 (HSP70), polyclonal] (Santa Cruz Biotechnology, Inc., dilution 1:500) were added onto the membrane to react overnight at 4 °C and then incubated with rabbit anti-goat horseradish peroxidase-conjugated secondary antibody (Santa Cruz, Inc., dilution 1:2500) for 1 h. The immunoreactive bands were visualized using Western blotting luminal reagents and were scanned with Image Analysis software (Alpha Innotech, United States).
Data are presented as mean ± SD. Student’s t-test was used for two group comparison and analysis of variance followed by Newman-Keuls multiple comparisons were used in case for multiple comparisons. Differences with P value < 0.05 were considered significant.
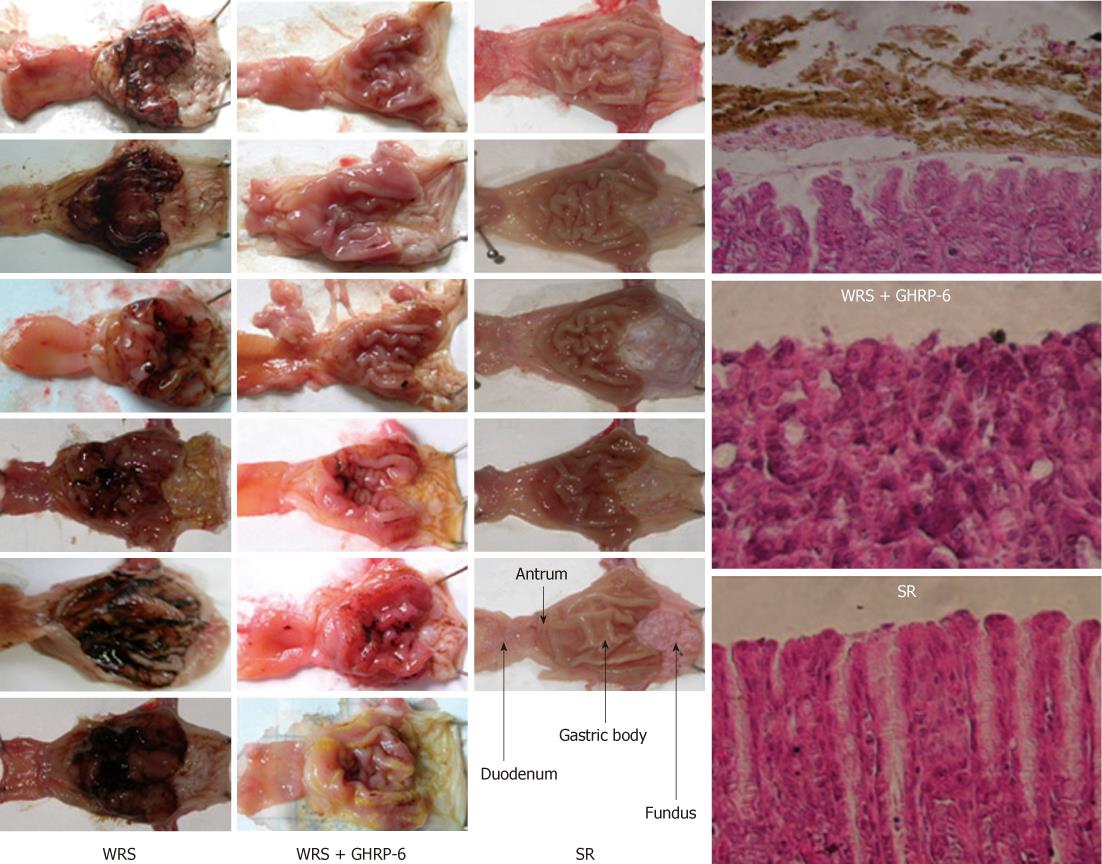
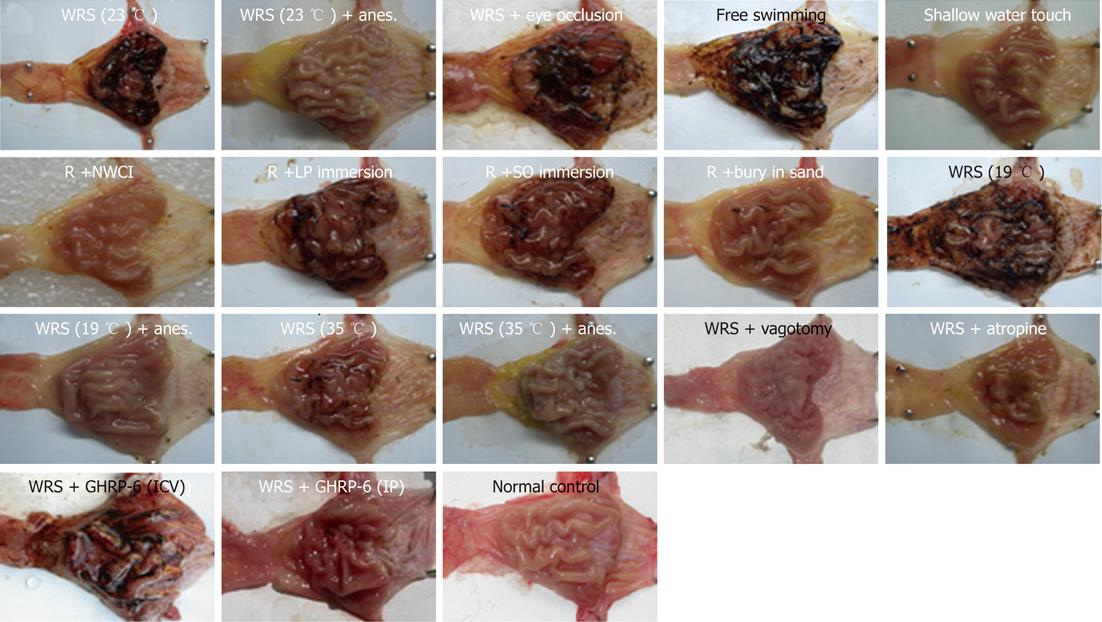
Visual inspection showed that WRS for 6 h (water temperature 23 °C) induced serious gastric bleeding erosions, as indicated by the mucosal hemorrhage and mucosal erosive lesion (Figure 1) and the calculated area of the lesions (Table 1). The hemorrhage was observed mainly in the gastric body and antrum, but not in the fundus and duodenum (Figure 1). Under the microscope, the mucosa in the WRS rat was disrupted and covered with coagulated blood and inflammatory cell infiltration (Figure 1). Rats with SR did not show gastric mucosal lesions (Figure 1, Table 1). Compared with the serious gastric mucosal damage in all the WRS rats (Figures 1 and 2), eye occlusion of WRS rats did not provide any protection from the mucosal lesions (Figure 2, Table 1), suggesting that vision does not play an important role in the pathogenesis of gastric damage. Rats with NWCI showed an intact mucosa (Figure 2, Table 1), again supporting that the view that vision is not important in inducing gastric mucosal lesion. FS for 6 h also induced serious mucosal bleeding erosions (Figure 2, Table 1), indicating that water immersion without restraint is also sufficient for inducing gastric mucosal lesions.
| Group | Lesion area (mm2) |
| WRS (23 °C) | 81.8 ± 6.4b |
| WRS (19 °C) | 97.5 ± 8.7b |
| WRS (35 °C) | 56.4 ± 5.2d |
| WRS + GHRP-6 | 12.0 ± 2.8d |
| SR | 0.0 ± 0.0d |
| FS (23 °C) | 99.5 ± 6.9b |
| Shallow water touch | 0.0 ± 0.0d |
| WRS + eye occlusion | 91.2 ± 8.4b |
| R + NWCI | 0.0 ± 0.0b |
| R + SO immersion | 80.6 ± 6.9b |
| R + LP immersion | 82.3 ± 7.1b |
| R + sand immersion | 0.0 ± 0.0d |
| WRS + anesthesia | 0.0 ± 0.0d |
| WRS + vagotomy | 0.0 ± 0.0d |
| WRS + atropine | 0.0 ± 0.0d |
| Normal control | 0.0 ± 0.0 |
We also observed the influence of water temperature on WRS-induced gastric mucosal lesions. WRS with cool water (23 °C) (Figure 1) or cold water (19 °C) (Figure 2) both induced serious mucosal lesions, but the extent of the lesions was smaller when warm water (35 °C) was used (Figure 2, Table 1).
Water immersion (WI) to the level of the xiphoid induced serious gastric mucosal lesions (Figure 1), but partial WI (shallow water tough) did not induce mucosal lesions (Figure 2, Table 1), suggesting that the depth of the immersion determines the occurrence of mucosal lesions. In an attempt to determine if different liquids would lead to different response patterns in the gastric mucosa, we observed the effects of immersion with two other liquids (salad oil and liquid paraffin, which are not obviously skin-hazardous) on gastric mucosa. Immersion to the level of xiphoid process with either of the two liquids induced similar gastric mucosal lesion (Figure 2, Table 1) as WRS did (Figure 2). “Burying” the body into sand (with the head exposed) did not induce mucosal lesions (Figure 2, Table 1). These results suggest that it is the liquid, but not the chemical nature of the liquid, that determines whether the mucosal lesions would occur, and “burying” the body in solid materials does not induce gastric mucosal lesions.
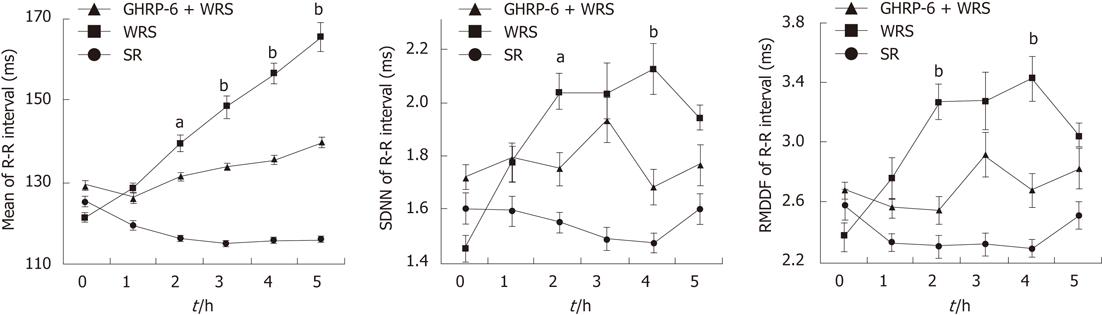
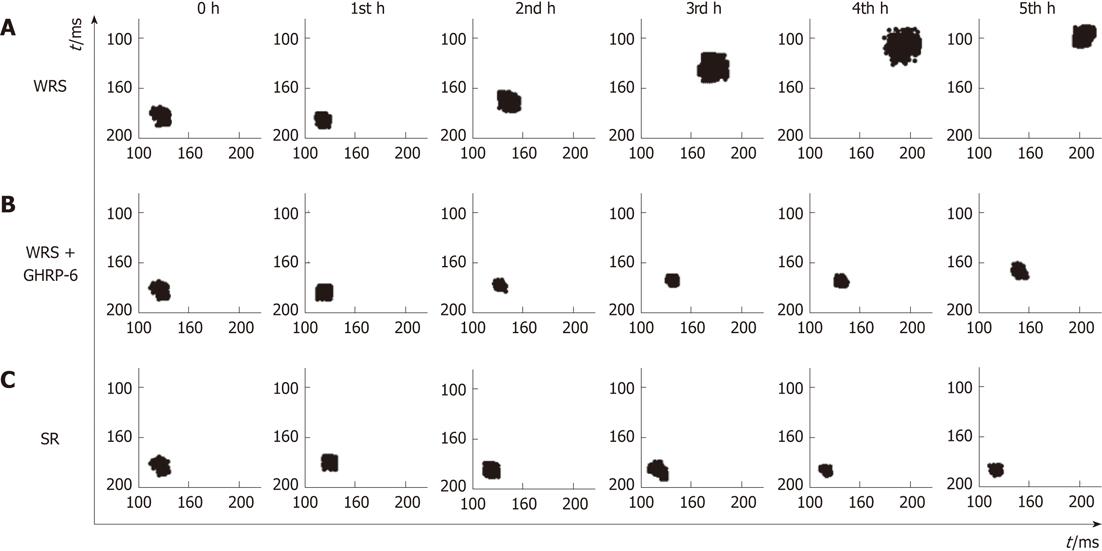
WRS without anesthesia (i.e., conscious rats) induced serious gastric mucosal lesions (Figure 1, Table 1), but WRS with anesthesia (unconscious rats) did not, no matter what a water temperature was used (Figure 2, Table 1). The HRV analyses (Figures 3 and 4) showed that the R-R intervals of the ECG in WRS rats gradually became longer, in other words, the HR gradually decreased; the SDNN and RMSSD of the R-R intervals increased, suggesting an increase of HRV, underlying an increase of the vagal outflow. Simple restraint induced a gradual shorting in R-R intervals and decreases in SDNN and RMSSD (Figure 3), suggesting an increase in sympathetic outflow to the heart. The Poincaré plot of R-R intervals (Figure 4) also supported the above observations. Previous injection of atropine also abolished the WRS-induced gastric mucosal lesion (Figure 2), further supporting the vagal hypothesis of this injury.
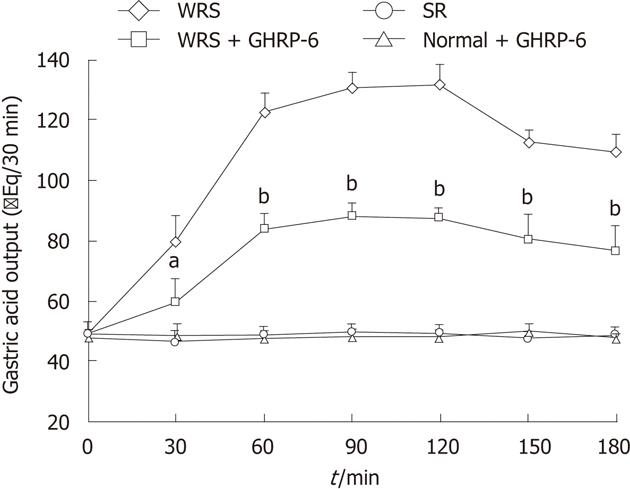
WRS stimulated gastric acid secretion (Figure 5), also indicative of increased vagal efferent activity. Simple restraint did not affect gastric acid output (Figure 5), indicating that restraint alone did not stimulate parasympathetic activity. Bilateral vagotomy totally prevented the development of WRS-induced mucosal lesions (Figure 2), also supporting the hypothesis that increased vagal outflow to the stomach plays a leading role in the development of WRS-induced mucosal lesions.
GHRP-6 pre-injection via IP 2 h before the start of WRS dramatically prevented the WRS-induced mucosal bleeding erosion; only very slight or no hemorrhaging was observed in the WRS + GHRP-6 group (Figure 1). The HE stains of the gastric tissues (Figure 1) also confirmed that the mucosal injury/hemorrhage was minimal or not observed in WRS rats pretreated with GHRP-6. Planimetry analyses (Table 1) showed that the lesion area was large in the WRS group; but was minimal in the WRS + GHRP-6 group; lesion area was zero in the SR group. GHRP-6 did not have a protective effect on the mucosa of WRS rats if administrated centrally via ICV (Figure 2), suggesting that the protective effect of GHRP-6 is mainly peripheral.
GHRP-6 alleviated the changes of HRV parameters induced by WRS (Figures 3 and 4), and decreased the gastric acid output during WRS (Figure 5), suggesting that GHRP-6 protects the mucosa, at least in part, by suppressing the vagal efferent effect on the stomach.
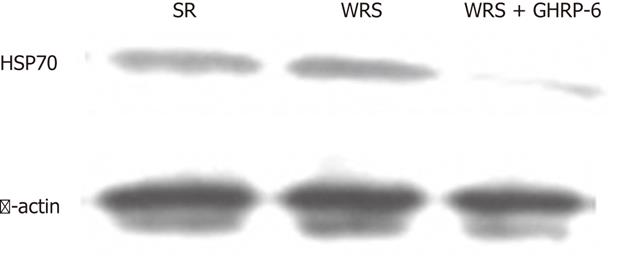
GHRP-6 could alleviate the intensity of gastric stress response, which is reflected by the level of expression of HSP70 in the mucosa. Western blotting results showed that both the WRS and SR induced a high expression of HSP70 in the gastric mucosal tissues (Figure 6), indicating a nonspecific response of HSP70 expression to stress. GHRP-6 pretreatment significantly decreased the protein level of HSP70 in the WRS rats (Figure 6), suggesting a decrease in the stress intensity.
WRS significantly increased the plasma levels of ET-1, renin activity and TXB2 compared with that of the SR group, while GHRP-6 pretreatment significantly attenuated the increases in these vasoconstrictive hormones (Table 2).
The mechanism of WRS-induced gastric mucosal lesion is complicated and not yet fully understood. The pathogenesis of the injury may be recognized at different levels, for example, at psychological, physiological, psychosomatic[21], integrative, organic, cellular and molecular levels. This study focuses on the psychosomatic mechanism of WRS-induced gastric mucosal lesions in vivo.
We first differentiated the relative importance of the pathways by which the stress stimulus signals were sensed and transferred to the central nervous system (CNS). When a conscious rat was immersed in water, it saw (intact vision) that its body was almost drowning, which may have induced fear. At the same time, the rat’s skin also sensed liquid immersion and generated a physiological response and subsequent psychological stress response. By eye occlusion or NWCI manipulations, we determined that vision alone is not sufficient to induce gastric mucosal lesion, while WI alone is sufficient for the induction of the lesion, because free swimming rats showed serious mucosal injury. These results also showed that immersion depth significantly affects the severity of mucosal lesions, as partial immersion in shallow water could not induce mucosal lesions. These results indicate that skin sensation is the leading input pathway for WRS-induced gastric bleeding erosion. The cutaneous stimuli may reach the integrative structures of the upper central nervous system and, by the vagal pathway, produce gastric mucosa lesions. Rat is an animal of nocturnal habit; therefore vision is not a fundamental sense for its defense. In all environments, rat performs a cognitive map to run away from an eventual predator. In an unstable environment, such as fluid, they may aware of the difficulty of running away.
Skin receptors can sense temperature, touch and noxious stimulation. Whether or not skin sensors can also distinguish different liquids is unknown. To determine this point, we examined gastric mucosal responses to immersion in different liquids or solid materials. Immersion in either salad oil or liquid paraffin induced serious mucosal hemorrhage similarly to WI, indicating that liquid immersion-induced gastric mucosal injury does not depend on the chemical nature of a liquid, but depends on liquid itself. “Burial” in sand did not induce gastric lesions, even when combined with restraint, suggesting that skin sensors can differentiate whether a material is fluid or dry matter, and immersion in a liquid or “burial” in dry material would lead to different gastric mucosal responses. Another possibility is that liquid immersion leads to lower body temperature compared with surrounding the body with solid materials.
The present data also indicated that the functional integrity of neural regulation is essential for the induction of gastric mucosal injury by WRS. In conscious animals, WRS induced injury that was inversely related to water temperature. However, in unconscious rats exposed to even the most severe condition (19 °C), no ulceration occurred, which agreed with the result of Murison et al[21]. Pentobarbital does not block vagal output to the stomach[22], but even enhances vagal output[21] ; therefore, the lack of gastric erosion by WRS in unconscious rats may be caused by certain selective interruptions of CNS-stomach communications by the anesthesia, potentially including sensation of body temperature and mobilization of vasoconstrictive hormones, such as renin-angiotensin system[23], ET-1[24] and TXB2[25]. These vasoconstrictive factors may reduce gastric blood flow and lead to changes in the ratio of gastric blood flow/acid output, which favors the formation of gastric ulcers[26].
Gastric acid secretion is controlled by sympathetic and parasympathetic nerves, and by certain hormones, such as gastrin. Generally, sympathetic activity inhibits, and parasympathetic activity stimulates, gastric acid secretion. Occasionally, sympathetic stimulation may also increase gastric acid secretion, because adrenalin releases gastrin; and vagal nerves may exert some sympathetic-like effect as they have adrenergic fibers. Our results indicate that increased vagal efferent activity is the leading cause of WRS-induced gastric mucosal injury, because HRV analyses showed heart rate slowing and increasing of some HRV parameters, and furthermore, bilateral vagotomy or atropine totally prevented the injury induced by WRS. This result is consistent with our previous study[17]. Our HRV analyses also showed that restraint alone induced moderate sympathetic hyperactivity, while sympathetic hyperactivity in the stomach prevents WRS-induced gastric injury formation mainly via the inhibition of gastric acid secretion, as observed in stroke prone spontaneously hypertensive rats[27].
Heart rate slowing is a universal response in all air-breathing vertebrates when immersed in water (drowning or diving), this is called diving bradycardia[28]. Diving bradycardia is triggered by apnea and accentuated by immersion of the face or whole body in cold water[28]. The diving response is mainly characterized by bradycardia, decreased cardiac output, peripheral vasoconstriction and increased arterial blood pressure[29,30]. The physiological significance of this response is to conserve oxygen, a mechanism of defense against hypoxic damage[28,31]. Our previous[17] and present observations in the rat model indicate that bradycardia appears even when the immersion is partial and the face is not immersed (for example, immersed to the xiphoid process in the present study), suggesting that heart rate slowing during water immersion does not necessarily depend on face immersion. This reaction pattern may be formed in development, and is a heritable trait[32]. However, when humans are diving or swimming for longer times (for example, 8-h swimming), they usually do not develop gastric mucosal injury, while rats do. One potential mechanism for these differential gastric responses may be the psychological component: a man who is diving or swimming knows that he is just at work or recreation and will not drown; therefore, he has no severe psychological reactions. By contrast, a rat would not think so, it would feel it was about to drown and die, and therefore severe psychological responses would be triggered, which may partially contribute to gastric injury.
Ghrelin, a peptide hormone originally isolated mainly from the stomach, is the endogenous ligand for the GHSR. In the gastrointestinal tract, ghrelin regulates the motility of the stomach and gut[33], gastric acid secretion[34] and gastric mucosal defense[35,36]. Intravenous administration of rat ghrelin dose-dependently increases both gastric acid secretion and gastric motility, actions that are blocked by pretreatment with either atropine or bilateral cervical vagotomy, but not by the histamine H2-receptor antagonist famotidine, suggesting ghrelin might have a physiological role in the vagal control of gastric function in rats[37]. Another study indicated that ghrelin inhibits gastric acid secretion[38]. This controversy deserves further investigation. GHRP, the mimetic of ghrelin, has been shown to have gastric motor effects[39]. However, the effect of GHRP on gastric acid secretion is unknown. We show here that WRS significantly increased the gastric acid output, but restraint did not; GHRP-6 significantly suppressed WRS-stimulated gastric acid secretion, although GHRP-6 did not significantly affect the basal gastric acid output in normal rats. These results, combined with the HRV data, suggest that the protective effect of GHRP-6 on WRS-induced gastric mucosal injury is affected, at least in part, by suppressing vagal efferent effect on the stomach, including gastric acid secretion, as gastric acid play an important role in the development of WRS-induced gastric ulcers[40]. Our results also indicate that the protective effect of GHRP-6 is likely peripheral, potentially by affecting the function of vagal efferent terminals and/or cell protection. However, we cannot exclude the possibility that GHRP-6 might also affect the vago-vagal or vago-sympathetic reflexes. One possibility is that GHRP-6 injected via ICV may not effectively reach its target CNS site (for example, the dorsal vagal complex); the other possibility is that GHRP-6 may also affect the vagal afferent nerves, which in turn affects the neuronal reflex.
The protection of GHRP-6 on WRS-induced gastric injury could also be reflected by the level of expression of HSP70 in the gastric mucosal tissue. HSP are crucial for cell survival during and after various cellular stresses. WRS rapidly induces HSP70 expression and accumulation; the HSP70 level is inversely correlated with the severity of mucosal lesions[41]. GHRP-6 significantly decreased the HSP70 protein level in the gastric mucosa of WRS rats compared with WRS alone, indicating that the stress intensity is low in the GHRP-6 treated animals. This result also suggests that GHRP-6 can exert a cell protective effect.
Interestingly, we found that gastric mucosal injury never occurred in the gastric fundus, while ghrelin is secreted predominantly by enteroendocrine cells in the gastric fundus, although ghrelin gene transcripts and ghrelin-producing cells are found throughout the gastrointestinal tract[13]. Whether the ghrelin-secreting fundus is ulcer-resistant or only the acid-secreting areas (gastric body and antrum) are vulnerable to stress, deserves further investigation. It is possible that locally released ghrelin may have a protective action on the fundic gastric mucosa.
In conclusion, this study demonstrates that vision-triggered psychosomatic responses do not play an important role in WRS-induced gastric mucosal lesions; however, skin sensation-induced increase of vagal outflow and subsequent increase of gastric acid secretion do play a leading role. Skin receptors cannot differentiate different liquids, and immersion with different liquids induced the same gastric injury as WI does. GHRP-6 protects against WRS-induced gastric lesions mainly by suppressing the vagal effect on gastric mucosa, and this protection is likely peripheral. The protective effect of GHRP-6 on gastric stress ulceration suggests a clinical application in treating stress-related gastric injury.
Gastric ulcers are among the most frequently occurring stomach diseases across the world and stress is an important inducer of this disease. Therefore, an understanding of the key mechanism of gastric stress ulceration and the development of preventive/therapeutic drugs are important in treating this disease.
How stress induces gastric ulcers is an old question that needs a new answer. Most previous studies only looked at restricted areas, especially at the physiological and molecular levels. Exploring the key mechanism and developing therapeutic drugs for gastric stress ulcer are urgently required.
In contrast to other mechanistic studies on gastric stress ulceration, this investigation focuses on the psychosomatic mechanisms of water immersion and restraint stress (WRS)-induced gastric bleeding erosions, and found that increased outflow from the vagal center is the leading cause of WRS-induced gastric injury. Skin sensation, but not vision, triggers the stress reaction via vago-vagal reflex. The study also found that growth hormone releasing peptide-6 (GHRP-6), a synthetic agonist for growth hormone secretagogues receptor, prevents the occurrence of gastric mucosal lesions, mainly by suppressing the vagal effect on the stomach.
The study demonstrates the key signaling pathway by which water immersion induces gastric mucosal damage in the rat, and provides the first evidence that GHRP-6 can prevent this damage. The study suggests a clinical application of GHRP in treating gastric stress ulceration.
Discovering the mechanism of gastric stress ulceration is a prerequisite for the prevention and treatment of this disease. This study shows that skin sensation and the subsequent vago-vagal reflex play a key role in the development of water immersion-induced gastric mucosal damage in the rat. GHRP-6 prevents this damage, probably by suppressing the vagal effect on the stomach. The study is innovative and with potential therapeutic interest.
Peer reviewers: Stéphane Supiot, MD, PhD, Department of Radiation Oncology, Centre René Gauducheau, St-Herblain, 44800 Nantes, France; Jose Liberato Ferreira Caboclo, Professor, Department of Surgery, FAMERP, Av Bady Bassit, 15025-900 Rio de Janeiro, Brazil; Jackie Wood, PhD, Department of Physiology and Cell Biology, College of Medicine and Public Health, The Ohio State University, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210-1218, United States
S- Editor Gou SX L- Editor Stewart GJ E- Editor Xiong L
| 1. | Brodie DA, Hooke KF. The effect of vasoactive agents on stress-induced gastric hemorrhage in the rat. Digestion. 1971;4:193-204. [PubMed] |
| 2. | Ernst H, Konturek PC, Brzozowski T, Lochs H, Hahn EG, Konturek SJ. Adaptation of gastric mucosa to stress. Effect of ranitidine. J Physiol Pharmacol. 1998;49:405-419. [PubMed] |
| 3. | Uramoto H, Ohno T, Ishihara T. Gastric mucosal protection induced by restraint and water-immersion stress in rats. Jpn J Pharmacol. 1990;54:287-298. [PubMed] |
| 4. | Robles TF, Carroll JE. Restorative biological processes and health. Soc Personal Psychol Compass. 2011;5:518-537. [PubMed] |
| 5. | Lin HP, Lin HY, Lin WL, Huang AC. Effects of stress, depression, and their interaction on heart rate, skin conductance, finger temperature, and respiratory rate: sympathetic-parasympathetic hypothesis of stress and depression. J Clin Psychol. 2011;67:1080-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Xu XB, Cao JM, Pang JJ, Xu RK, Ni C, Zhu WL, Asotra K, Chen MC, Chen C. The positive inotropic and calcium-mobilizing effects of growth hormone-releasing peptides on rat heart. Endocrinology. 2003;144:5050-5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin's role on gastrointestinal tract cancer. Surg Oncol. 2010;19:e2-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Cao JM, Ong H, Chen C. Effects of ghrelin and synthetic GH secretagogues on the cardiovascular system. Trends Endocrinol Metab. 2006;17:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Broglio F, Arvat E, Gottero C, Benso A, Prodam F, Destefanis S, Aimaretti G, Papotti M, Muccioli G, Deghenghi R. Natural and synthetic growth hormone secretagogues: do they have therapeutic potential? Treat Endocrinol. 2003;2:153-163. [PubMed] |
| 10. | Rossoni G, De Gennaro Colonna V, Bernareggi M, Polvani GL, Müller EE, Berti F. Protectant activity of hexarelin or growth hormone against postischemic ventricular dysfunction in hearts from aged rats. J Cardiovasc Pharmacol. 1998;32:260-265. [PubMed] |
| 11. | King MK, Gay DM, Pan LC, McElmurray JH, Hendrick JW, Pirie C, Morrison A, Ding C, Mukherjee R, Spinale FG. Treatment with a growth hormone secretagogue in a model of developing heart failure: effects on ventricular and myocyte function. Circulation. 2001;103:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H1643-H1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Jeffery P, McDonald V, Tippett E, McGuckin M. Ghrelin in gastrointestinal disease. Mol Cell Endocrinol. 2011;340:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Brzozowski T, Konturek PC, Drozdowicz D, Konturek SJ, Pawlik M, Sliwowski Z, Pawlik WW, Hahn EG. Role of central and peripheral ghrelin in the mechanism of gastric mucosal defence. Inflammopharmacology. 2005;13:45-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Konturek PC, Brzozowski T, Burnat G, Szlachcic A, Koziel J, Kwiecien S, Konturek SJ, Harsch IA. Gastric ulcer healing and stress-lesion preventive properties of pioglitazone are attenuated in diabetic rats. J Physiol Pharmacol. 2010;61:429-436. [PubMed] |
| 17. | Xie YF, Jiao Q, Guo S, Wang FZ, Cao JM, Zhang ZG. Role of parasympathetic overactivity in water immersion stress-induced gastric mucosal lesion in rat. J Appl Physiol. 2005;99:2416-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Said SA, El-Mowafy AM. Role of endogenous endothelin-1 in stress-induced gastric mucosal damage and acid secretion in rats. Regul Pept. 1998;73:43-50. [PubMed] |
| 19. | Kitagawa H, Fujiwara M, Osumi Y. Effects of water-immersion stress on gastric secretion and mucosal blood flow in rats. Gastroenterology. 1979;77:298-302. [PubMed] |
| 20. | Tanida M, Shen J, Kubomura D, Nagai K. Effects of anserine on the renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Physiol Res. 2010;59:177-185. [PubMed] |
| 21. | Murison R, Overmier JB. Some psychosomatic causal factors of restraint-in-water stress ulcers. Physiol Behav. 1993;53:577-581. [PubMed] |
| 22. | Lin WC, Yano S, Watanabe K. Stimulation of gastric acid secretion by microinjection of pentobarbital into the ventromedial hypothalamus. Res Commun Chem Pathol Pharmacol. 1988;60:269-272. [PubMed] |
| 23. | Ender F, Labancz T, Rosivall L. Protective effects of the inhibition of the renin-angiotensin system against gastric mucosal lesions induced by cold-restraint in the rat. Acta Physiol Hung. 1993;81:13-18. [PubMed] |
| 24. | Duan YM, Li ZS, Zhan XB, Xu GM, Tu ZX, Gong YF. Changes in endothelin-1 gene expression in the gastric mucosa of rats under cold-restraint-stress. Chin J Dig Dis. 2004;5:28-34. [PubMed] |
| 25. | Kitagawa H, Kurahashi K, Fujiwara M. Gastric mucosal erosion due to a mucosal ischemia produced by thromboxane A2-like substance in rats under water-immersion stress. J Pharmacol Exp Ther. 1986;237:300-304. [PubMed] |
| 26. | Arai I, Muramatsu M, Aihara H. Body temperature dependency of gastric regional blood flow, acid secretion and ulcer formation in restraint and water-immersion stressed rats. Jpn J Pharmacol. 1986;40:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Shichijo K, Ito M, Taniyama K, Sekine I. The role of sympathetic neurons for low susceptibility to stress in gastric lesions. Life Sci. 1993;53:261-267. [PubMed] |
| 28. | Alboni P, Alboni M, Gianfranchi L. Diving bradycardia: a mechanism of defence against hypoxic damage. J Cardiovasc Med (Hagerstown). 2011;12:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol. 2001;84:254-271. [PubMed] |
| 30. | Gooden BA. Mechanism of the human diving response. Integr Physiol Behav Sci. 1994;29:6-16. [PubMed] |
| 31. | Andersson JP, Linér MH, Fredsted A, Schagatay EK. Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J Appl Physiol. 2004;96:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Fahlman A, Bostrom BL, Dillon KH, Jones DR. The genetic component of the forced diving bradycardia response in mammals. Front Physiol. 2011;2:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Bülbül M, Babygirija R, Zheng J, Ludwig K, Xu H, Lazar J, Takahashi T. Food intake and interdigestive gastrointestinal motility in ghrelin receptor mutant rats. J Gastroenterol. 2011;46:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Sakata I, Sakai T. Ghrelin cells in the gastrointestinal tract. Int J Pept. 2010;2010:945056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Baek YH, Lee KN, Jun DW, Yoon BC, Kim JM, Oh TY, Lee OY. Augmenting Effect of DA-9601 on Ghrelin in an Acute Gastric Injury Model. Gut Liver. 2011;5:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Adami M, Pozzoli C, Leurs R, Stark H, Coruzzi G. Histamine H(3) receptors are involved in the protective effect of ghrelin against HCl-induced gastric damage in rats. Pharmacology. 2010;86:259-266. [PubMed] |
| 37. | Torsello A, Locatelli V, Melis MR, Succu S, Spano MS, Deghenghi R, Müller EE, Argiolas A. Differential orexigenic effects of hexarelin and its analogs in the rat hypothalamus: indication for multiple growth hormone secretagogue receptor subtypes. Neuroendocrinology. 2000;72:327-332. [PubMed] |
| 38. | Sibilia V, Muccioli G, Deghenghi R, Pagani F, De Luca V, Rapetti D, Locatelli V, Netti C. Evidence for a role of the GHS-R1a receptors in ghrelin inhibition of gastric acid secretion in the rat. J Neuroendocrinol. 2006;18:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Qiu WC, Wang ZG, Wang WG, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J Gastroenterol. 2008;14:1419-1424. [PubMed] |
| 40. | Li YM, Lu GM, Zou XP, Li ZS, Peng GY, Fang DC. Dynamic functional and ultrastructural changes of gastric parietal cells induced by water immersion-restraint stress in rats. World J Gastroenterol. 2006;12:3368-3372. [PubMed] |
| 41. | Rokutan K. Role of heat shock proteins in gastric mucosal protection. J Gastroenterol Hepatol. 2000;15 Suppl:D12-D19. [PubMed] |