Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3138
Revised: March 29, 2012
Accepted: April 27, 2012
Published online: June 28, 2012
AIM: To evaluate whether total splenic artery embolization (TSAE) for patients with hypersplenism delivers better long-term outcomes than partial splenic embolization (PSE).
METHODS: Sixty-one patients with hypersplenism eligible for TSAE (n = 27, group A) or PSE (n = 34, group B) were enrolled into the trial, which included clinical and computed tomography follow-up. Data on technical success, length of hospital stay, white blood cell (WBC) and platelet (PLT) counts, splenic volume and complications were collected at 2 wk, 6 mo, and 1, 2, 3, 4 years postoperatively.
RESULTS: Both TSAE and PSE were technically successful in all patients. Complications were significantly fewer (P = 0.001), and hospital stay significantly shorter (P = 0.007), in group A than in group B. Post-procedure WBC and PLT counts in group A were significantly higher than those in group B from 6 mo to 4 years (P = 0.001), and post-procedure residual splenic volume in group A was significantly less than that observed in group B at 1, 2, 3 and 4 years post-procedure (P = 0.001). No significant differences were observed in red blood cell counts and liver function parameters between the two groups following the procedure.
CONCLUSION: Our results indicate that TSAE for patients with hypersplenism not only delivers a better long-term outcome, but is also associated with lower complication rates and a shorter hospital stay than PSE.
- Citation: He XH, Gu JJ, Li WT, Peng WJ, Li GD, Wang SP, Xu LC, Ji J. Comparison of total splenic artery embolization and partial splenic embolization for hypersplenism. World J Gastroenterol 2012; 18(24): 3138-3144
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3138
To date, partial splenic embolization (PSE), which was first performed by Spigos et al[1] in 1979, has been considered first-line therapy for hypersplenism in many institutions, and has been proposed as an effective alternative to splenectomy for improving peripheral blood cell counts[2-4]. However, PSE is associated with many complications, including intermittent fever, abdominal pain, nausea, vomiting, postembolization syndrome, splenic abscess, splenic rupture, pneumonia, refractory ascites, pleural effusion and gastrointestinal bleeding[3-6]. To ensure a sustained and long-term increase in platelet (PLT) and leucocyte counts, the splenic infarction rate needs to be greater than 50%[6]. Thus, severe complications can ensue[1-6].
Today, total splenic artery embolization (TSAE) is widely used and has been shown to be a safe and effective method for the treatment of splenic artery aneurysms[7-9]. In addition, stainless steel spring coils are used to embolize the main branch of the splenic artery in order to improve PLT counts pre-splenectomy[10]. We have initiated a new approach, that of TSAE for the treatment of patients with hypersplenism secondary to cirrhosis of the liver, and our preliminary results demonstrate the safety and feasibility of the approach[11]. In order to determine whether this approach can ensure a sustained and long-term increase in PLT and leucocyte counts, as well as a reduction in the rate of severe complications, we undertook this nonrandomized prospective trial of endovascular treatment of hypersplenism with TSAE or PSE, with the aim of comparing clinical outcomes of both methods.
The protocol was approved by the institutional ethics committee, and all patients provided written informed consent. Between January 2006 and June 2011, consecutive patients with hypersplenism caused by cirrhosis referred for treatment with TSAE (group A) or PSE (group B) were screened for enrollment into this non-randomized, prospective, two-institution trial. They subsequently underwent computed tomography (CT) follow-up at both hospitals. The diagnosis of hypersplenism was established by review of each patient’s medical history, clinical laboratory data, ultrasonography, and CT examinations.
Eligibility criteria included: (1) documented hypersplenism caused by cirrhosis established by a review of the patient’s history, CT results or ultrasound; and (2) hepatitis B virus/hepatitis C virus-related active cirrhosis, with neutropenia (neutrophil count ≤ 2.0 × 109/L), thrombocytopenia (PLT count ≤ 50 × 109/L), or both. Patients who had severe jaundice (total serum bilirubin level ≥ 81.4 μmol/L) or spontaneous bacterial peritonitis were excluded.
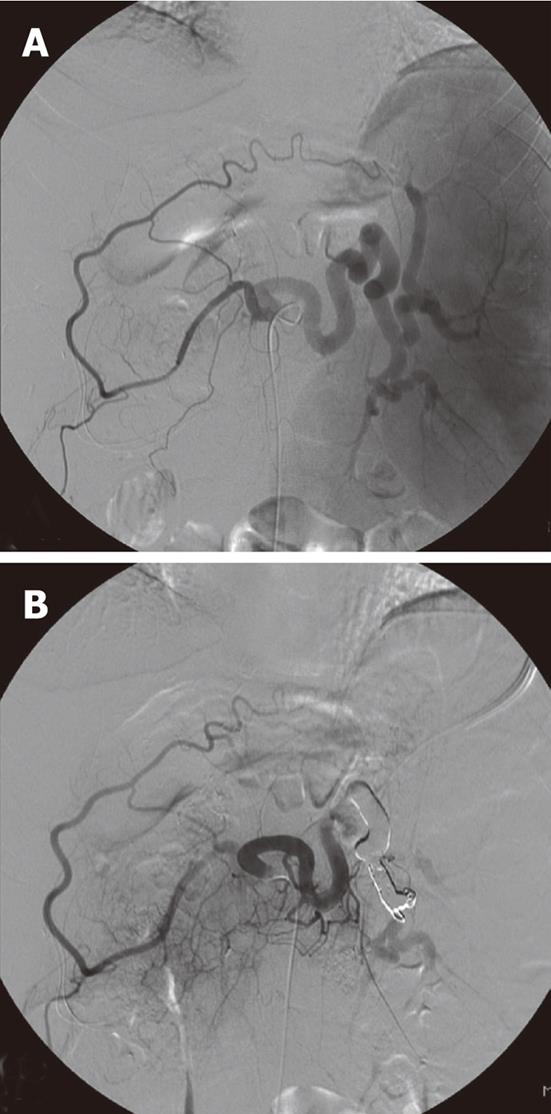
All embolization procedures were performed by two experienced interventional radiologists. Prior to embolization, selective angiography of the celiac trunk, the splenic artery and the superior mesenteric artery was performed in all patients via the right femoral artery using a 5-Fr diagnostic catheter. To avoid total splenic infarction, we confirmed patency of the collateral arteries, which were confirmed as connected to the hilar splenic artery from the left gastric artery or from the gastroepiploic artery on a celiac arteriogram (Figure 1).
Once these connections were confirmed, TSAE was performed, using details of the coiling procedure as has been previously described[11]. Coils and gelfoam were used as embolization material, either alone or in combination. In general, the embolization coils used in this series were standard 0.089 cm fibered coils, microcoils (Tornado; Cook Inc., Bloomington, IN, United States). Post-embolization check angiograms were performed using selective splenic artery, celiac and superior mesenteric artery angiograms to confirm occlusion of the main splenic artery and patency of the collateral arteries (Figure 1). Preoperative antibiotic prophylaxis was used routinely for 3 d. Following embolization, patients were monitored clinically, and antibiotics were administered after the procedure for several days in order to avoid infection.
The embolic agents most commonly used for splenic arterial embolization are gelatin sponge pledgets (Gelfoam cube, Upjohn, Kalamazoo, United States) and polyvinyl alcohol particles (PVP, lvalon, Ingenor, Paris; and Contour, Interventional Therapeutics Corp., South San Francisco, CA, United States). Details of PSE with gelatin sponge pledgets[12] or with PVP[13] have been described previously, and therefore we do not repeat the methodology here.
All patients were followed up at our outpatient clinic. Peripheral blood cell parameters, including white blood cell (WBC), PLT and red blood cell (RBC) counts, were monitored at different time-points prior to the procedure, and at day 3, 14 and 30, and subsequently at 6-monthly intervals for a total of 5 years. To determine any possible effects on liver function, serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TB), albumin (Alb), as well as the prothrombin time (PT), were measured before and after the procedures at the same time-points. The frequency and type of complications associated with the procedures were recorded.
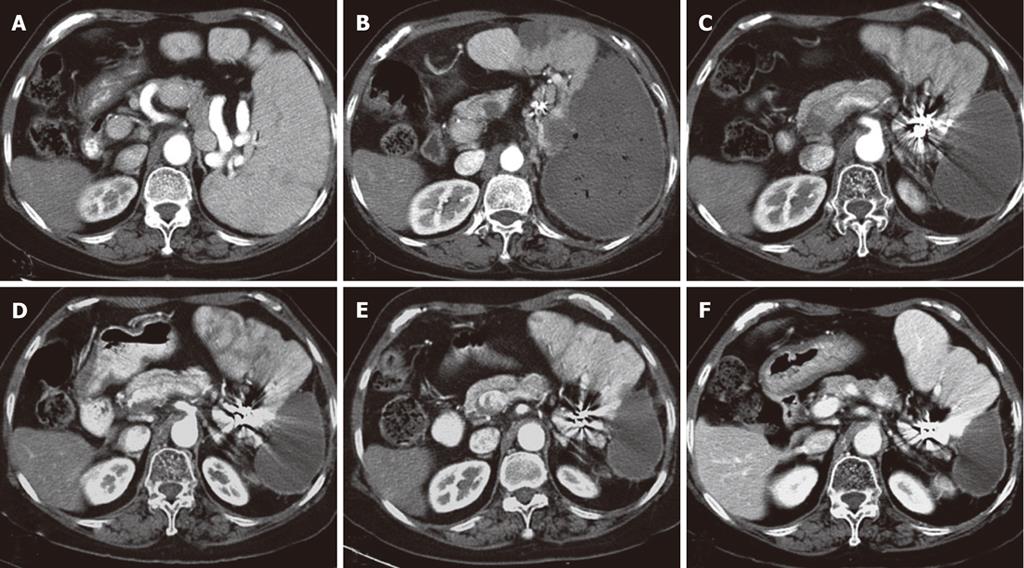
Abdominal CT scans were routinely performed before and two weeks after the procedure, and subsequently every 6 mo during follow-up (Figure 2). Based on enhanced CT images, we measured and compared the pretreatment splenic volume and the post-embolization residual splenic volume on a 3.1 workstation (GE Medical Systems, Milwaukee, WI, United States) using volumetric analysis software. The infarcted splenic volume (mL) was calculated by subtracting the noninfarcted splenic volume from the pretreatment volume. The splenic infarction rate was calculated by dividing the infarcted splenic volume by the pretreatment volume (× 100%).
Complications related to the procedures were categorized as either major or minor. A major complication was defined as a complicated disease requiring surgical or interventional treatment or a prolonged postoperative hospital stay of more than 30 d, and included splenic abscess, splenic rupture, pneumonia, refractory ascites, pleural effusions, gastrointestinal bleeding, variceal rupture and liver failure. A minor complication was defined as a complication that had minimal consequences and could be tolerated by patients or treated using conservative methods, including post-embolization syndrome, abdominal fullness, and loss of appetite.
We predicted that rates for a WBC count > 4 × 109/L at the 2-year follow-up assessment would be approximately 95% in group A and 60% in group B. We estimated that at least 25 patients with hypersplenism would need to be enrolled into each group for the study to have a statistical power of 80% with a two-sided alpha level of 0.05. This power was based on the ability to detect an absolute difference of 30% in patients with WBC counts > 4 × 109/L at the 2-year follow-up assessment.
All data are expressed as mean ± SD. Comparisons of variables between the two groups were performed by applying the Mann-Whitney test, χ2-test or the Fisher’s exact test as appropriate. All statistical analyses were performed using the SPSS package, version 13.0 (SPSS, Chicago, Illinois, United States).
A total of 109 patients with hypersplenism were enrolled. Before each procedure, patients or family members had the right to choose either TSAE or PSE. Initially, 51 and 58 patients were assigned to group A and group B, respectively. Of these, 48 patients did not meet the inclusion criteria, with a total follow-up of < 2 years in 43 patients, and five patients were lost to follow-up (two in group A and three in group B). The remaining 61 patients were enrolled. Subjects included 36 males and 25 females, with a mean age of 47.92 ± 8.00 years (range: 28-60 years). Demographic features and clinical presentation of included patients are summarized in Table 1.
| Group A(n = 27) | Group B(n = 34) | P value | |
| Age (yr) | 48.48 ± 8.87 | 47.945 ± 7.32 | 0.445 |
| Female/male | 11/15 | 14/20 | 0.930 |
| Child-Pugh classification, n (%) | 0.441 | ||
| A | 19 (70.4) | 23 (676) | |
| B | 6 (22.2) | 9 (26.5) | |
| C | 2 (7.4) | 2 (5.8) | |
| Virus, n (%) | 0.463 | ||
| B | 22 (81.5) | 25 (73.5 ) | |
| C | 5 (19.5) | 9 (26.5) | |
| WBC counts × 109 | 1.50 ± 0.32 | 1.54 ± 0.30 | 0.453 |
| PLT counts × 109 | 40.33 ± 6.16 | 39.74 ± 4.63 | 0.574 |
| Splenic volume (cm3) | 769.93 ± 61.40 | 745.73 ± 50.09 | 0.201 |
The technical and initial clinical outcomes of the two groups are shown in Table 2. Both TSAE and PSE were technically successful in all patients, with no procedure-related complications. The thirty-day mortality rate was zero.
| Group A (n = 27) | Group B (n = 34) | P value | |
| Technical success, n (%) | 27 (100) | 34 (100) | 0.999 |
| Hospital stay (d) | 8.52 ± 1.91 | 15.88 ± 6.36 | < 0.001 |
| Complications, n (%) | 0.007 | ||
| Minor complications | 0.005 | ||
| Post-embolization syndrome | 21 (77.8) | 34 (100) | |
| Major complications | 0 (0) | 7 (20.6) | 0.014 |
| Splenic abscess | 0 | 2 | |
| Pleural effusion | 0 | 3 | |
| Ascites | 0 | 2 | |
| CT follow-up (mo) | 36.44 ± 12.67 | 36.00 ± 11.82 | 0.926 |
| Clinical follow-up (mo) | 40.63 ± 12.60 | 40.14 ± 11.58 | 0.965 |
| WBC count > 4 × 109/L at 2-year follow-up, n (%) | 27 (100) | 21 (62) | < 0.001 |
Post-embolization syndrome, which included abdominal pain, fever and vomiting, occurred in 21 patients (77.8%) in group A and 34 in group B (100%) (P = 0.014). Abdominal pain was severe enough to require morphine administration in 15 patients, including two in group A (7.4%) and 13 in group B (38.2%) (P < 0.01). Severe complications, which occurred in seven patients (20.6%) in group B and no patient (0%) in group A, are listed in Table 2. Three patients had pleural effusions, which resolved with thoracocentesis. Two patients with ascites were treated with abdominal paracentesis. Two patients suffered from a persistent high fever and were found to have a splenic abscess on abdominal CT scan. The abscess was drained with a catheter and had resorbed within 2 mo. The length of hospital stay was significantly less in group A, 5-13 d (mean, 8.52 ± 1.91 d), than in group B, 9-37 d (mean, 15.88 ± 6.36 d) (P < 0.001).
At the time of writing, all patients in group A had been followed up and assessed at the 2-wk, 6-mo, 1- and 2-year time-points; 35 had been assessed for 3 years and 23 had been assessed for ≥ 4 years, prospectively, with a mean follow-up of 40.36 ± 11.94 mo (range: 24-64 mo). There were no significant differences in the number of patients in each follow-up period between the two groups.
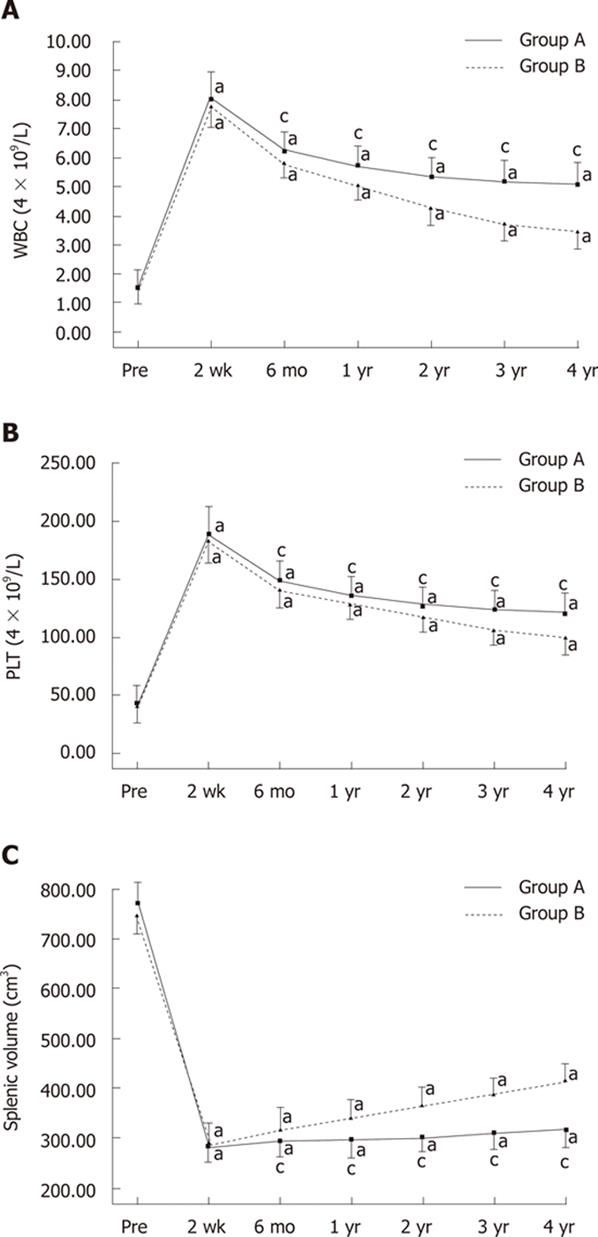
Changes in WBC and PLT counts before the procedures and between 2 wk and 4 years after the procedures are shown in Figure 3A and B. After the procedures, in both groups, WBC and PLT counts increased significantly, peaked at 2 wk, and then gradually fell during the 4-year follow-up period (Figure 3A and B). There were no significant changes in RBC counts either after PSE or during the long-term follow-up period.
Before PSE, there were no significant differences between the two groups with respect to sex, age, splenic volume, Child-Pugh class, and peripheral blood cell counts (Table 1). In both group A and group B, WBC and PLT counts increased significantly and remained higher than the pre-procedure counts from the second week to the fourth year after the procedures (P < 0.05, Figure 3A and B). Although there were no significant differences in WBC and PLT counts between the two groups 2 wk after the procedures, both WBC and PLT counts in group A were significantly higher than those observed in group B at 6 mo, 1, 2, 3 and 4 years after the procedures (P < 0.05, Figure 3A and B). A WBC count > 4 × 109/L at the 2-year follow-up assessment was observed in all 27 (100%) patients in group A and in 21 (62%) patients in group B (P < 0.001).
In comparison with pre-procedure levels of liver function parameters values, including AST, ALT, TB, Alb and PT, in each of the two groups, there were no significant short- and long-term changes after the procedures.
Splenic volume changes before the procedures and between one month and 4 years after the procedures are shown in Figure 3C. All patients in group A were followed up and assessed at 2 wk, 6 mo, 1 and 2 years; 35 were assessed for 3 years and 23 were assessed for ≥ 4 years, prospectively, with a mean follow-up of 40.36 ± 11.94 mo (range: 24-64 mo). After the procedures, splenic volume decreased significantly, peaked one month after the procedures, and then gradually increased during the 4-year follow-up period (Figure 3C). In groups A and B, splenic volume gradually increased and remained lower than the pre-procedure volume from 1 mo to four years after the procedures (P < 0.05, Figure 3C). There were no significant differences in splenic volume between the two groups before embolization, and at 1 and 6 mo post-procedure, but the residual splenic volume in group A was significantly smaller than that observed in group B 1, 2, 3 and 4 years after the procedures (P < 0.05, Figure 3C).
This nonrandomized trial was designed to compare the clinical outcomes of two endovascular regimens in patients with hypersplenism. The results demonstrate a sustained and long-term increase in WBC and PLT counts in group A, with no major complications, compared with group B. The significant increase in WBC and PLT counts in group A during follow-up appears to be predominantly attributable to the complete occlusion of the main splenic artery.
Hypersplenism is a well-known complication of portal hypertension in cirrhosis, which can result in thrombocytopenia and/or leukocytopenia. There are several possible approaches to management, for example splenectomy[14-16], PSE[1-6], ligation and banding of the splenic artery[17] and percutaneous placement of a narrowed stent into the splenic artery[18]. Surgical splenectomy can eliminate hypersplenism-induced blood cell destruction, but the morbidity of severe complications after splenectomy, including laparoscopic and open splenectomy, still ranges from 9.6% to 26.6%[14,15]. In addition, splenectomy is often associated with an increased long-term risk of septic events[14,15]. Ligation and banding of the splenic artery are also used to treat hypersplenism, but complications such as portal vein thrombosis, sepsis and multi-organ failure may occur with these methods[17]. Although percutaneous placement of a narrowed stent into the splenic artery is a promising new technique for treating hypersplenism[18], it is difficult to ensure a sustained and long-term increase in WBC and PLT counts as the splenic artery is not completely occluded, which cannot provide a sustained splenic infarction rate (> 50%).
Today, PSE is the most commonly used alternative to splenectomy for patients with hypersplenism, with the aim of improving the peripheral blood cell count[2-4], but it is often associated with minor and major complications, such as post-embolization syndrome, splenic abscess, splenic rupture, pneumonia, refractory ascites, pleural effusions and gastrointestinal bleeding[5,6], as reported in our study and in many other series[2-6]. Post-embolization syndrome is the most frequent minor complication, which is usually well-tolerated by patients and controlled with conservative therapy[3]. Major complications, on the other hand, such as splenic abscess, splenic rupture and pleural effusions, require surgical or other interventional treatment. Many reports have demonstrated a close association between the incidence of complications and the splenic infarction rate, and it appears that complications occur more frequently in patients with a splenic embolization > 50%[6,12,19,20]. In this study, the embolized splenic volume rate was the same for both groups, but we observed that both minor and major complications were significantly less in group A than in group B post-procedure (P < 0.05). Moreover, no major complications occurred. Therefore, we do not believe that there is a close relationship between the rate of complications and splenic infarction rates in patients with hypersplenism treated with TSAE. This may be explained by the collateral arteries, which connect to the hilar splenic artery from the left gastric artery or from the gastroepiploic artery, and provide a small amount of blood to the spleen, thereby avoiding complete splenic infarction.
In this study, both WBC and PLT counts in group A were significantly higher than those observed in group B at 6 mo, 1, 2, 3 and 4 years post-procedures. Although WBC and PLT counts in both groups decreased with time, the counts in group A revealed a sustained and long-term increase above normal levels and decreased very slowly, whereas those in group B decreased more quickly. The relatively high WBC and PLT counts in group A were mainly attributable to differences in treatment behavior, which may provide one explanation. As PSE embolizes the peripheral artery in the spleen, leaving the main splenic artery open, it is relatively easy for relapse to occur with resumption of blood flow, which leads to the residual splenic volume increasing relatively quickly. On the contrary, when the main splenic artery is completely embolized, the blood flow supply to the spleen from the splenic artery is stopped, with only a small amount of blood supplied from collateral arteries. This ensures that the residual splenic volume increases very slowly. Our study demonstrated that the residual splenic volume in group A increased very slowly and was significantly smaller than that observed in group B (P < 0.05), whereas the residual splenic volume in group B increased much more quickly.
The study has the following limitations. Firstly, a major weakness remains the lack of randomization, and the patient population was relatively small, which restricted our ability to detect possible differences between the two groups. As it was a preliminary prospective clinical study, and many patients were unwilling to choose TSAE at the outset, it was difficult to randomly select patients. Moreover, the two approaches varied with regard to different pathologic changes. The pathologic changes developed in distinctive patterns during follow-ups and were not affected by individual or group. Furthermore, our results revealed a sustained and long-term increase in WBC and PLT counts in group A compared with group B, for all follow-up time-points. Therefore, we believe that the non-randomized allocation of patients into two groups did not affect the results. Secondly, severe complications may occur when collateral arteries are not present or patent. Confirmation of the patency of the collateral arteries, which were connected to the hilar splenic artery from the left gastric artery or from the gastroepiploic artery, was a necessary step prior to TSAE. Lastly, the clinical indications for splenic embolization can be bleeding from varices or hypersplenism, and therefore great attention needs to be directed at these patients after embolization in order to prevent bleeding.
In conclusion, the results of this nonrandomized, prospective trial indicate that TSAE for patients with hypersplenism not only delivers better long-term outcomes but is also associated with lower rates of complications and a shorter hospital stay than PSE. Further clinical trials and longer-term follow-up studies are needed.
Partial splenic embolization (PSE) has been proposed as an effective alternative to splenectomy to improve peripheral blood cell counts. However, PSE is often associated with complications such as intermittent fever, abdominal pain, nausea and vomiting.
Total splenic artery embolization (TSAE) has been widely used for the treatment of splenic artery aneurysms, but treatment of patients with hypersplenism with TSAE has rarely been reported. The authors present the comparative clinical outcomes of TSAE and PSE in patients with hypersplenism.
The use of TSAE for the treatment of hypersplenism was devised for the management of patients with thrombocytopenia or leukocytopenia associated with cirrhosis of the liver. All procedures were performed under fluoroscopic control. This is the first study comparing TSAE and PSE in patients with hypersplenism.
TSAE is a safe and feasible procedure and may serve as supplemental treatment for hypersplenism with thrombocytopenia or leukocytopenia accompanying cirrhosis of the liver, as a result of low complication rates and good medium-term clinical efficacy.
The authors present a nonrandomized prospective trial of the endovascular treatment of hypersplenism with TSAE or PSE, with the aim of comparing the clinical outcomes of these two treatment approaches. The results reveal that treatment with TSAE ensured a sustained and long-term increase in white blood cell and platelet counts, as well as lower complication rates and a shorter hospital stay compared to PSE. In addition, post-procedure residual splenic volume in patients treated with TSAE was significantly smaller than that of PSE during follow-up. These results suggest an alternative approach for the management of patients with hypersplenism.
Peer reviewers: Dr. Hussein Mousa Atta, Professor, Department of Surgery, Faculty of Medicine, Minia University, 25 El-Badia Street, Cairo 11341, Egypt; Dr. Radan Bruha, 4th Department of Internal Medicine, First Faculty of Medicine, Charles University in Prague, 12808 Prague, Czech Republic
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Spigos DG, Jonasson O, Mozes M, Capek V. Partial splenic embolization in the treatment of hypersplenism. AJR Am J Roentgenol. 1979;132:777-782. [PubMed] |
| 2. | Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatol Res. 2008;38:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Sangro B, Bilbao I, Herrero I, Corella C, Longo J, Beloqui O, Ruiz J, Zozaya JM, Quiroga J, Prieto J. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology. 1993;18:309-314. [PubMed] |
| 4. | N'Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L, Grando-Lemaire V, Ganne-Carrie N, Sellier N, Trinchet JC. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol. 2005;17:179-184. [PubMed] |
| 5. | Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Zhu K, Meng X, Qian J, Huang M, Li Z, Guan S, Jiang Z, Shan H. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Loffroy R, Guiu B, Cercueil JP, Lepage C, Cheynel N, Steinmetz E, Ricolfi F, Krausé D. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short- and long-term results. Ann Vasc Surg. 2008;22:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Laganà D, Carrafiello G, Mangini M, Fontana F, Dizonno M, Castelli P, Fugazzola C. Endovascular treatment of splenic artery aneurysms. Radiol Med. 2005;110:77-87. [PubMed] |
| 9. | Laganà D, Carrafiello G, Mangini M, Dionigi G, Caronno R, Castelli P, Fugazzola C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Takahashi T, Arima Y, Yokomuro S, Yoshida H, Mamada Y, Taniai N, Kawano Y, Mizuguchi Y, Shimizu T, Akimaru K. Splenic artery embolization before laparoscopic splenectomy in children. Surg Endosc. 2005;19:1345-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | He XH, Li WT, Peng WJ, Li GD, Wang SP, Xu LC. Total embolization of the main splenic artery as a supplemental treatment modality for hypersplenism. World J Gastroenterol. 2011;17:2953-2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Liu JD, Chang CC, Chen YY. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;13:619-622. [PubMed] |
| 13. | Zhu K, Meng X, Li Z, Huang M, Guan S, Jiang Z, Shan H. Partial splenic embolization using polyvinyl alcohol particles for hypersplenism in cirrhosis: a prospective randomized study. Eur J Radiol. 2008;66:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Winslow ER, Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery. 2003;134:647-653; discussion 654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623-2634. [PubMed] |
| 16. | Watanabe Y, Horiuchi A, Yoshida M, Yamamoto Y, Sugishita H, Kumagi T, Hiasa Y, Kawachi K. Significance of laparoscopic splenectomy in patients with hypersplenism. World J Surg. 2007;31:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Nüssler NC, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P. Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 2003;9:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Firat A, Boyvat F, Moray G, Aytekin C, Karakayali H, Haberal M. Comparison of two different percutaneous splenic artery interventions in the treatment of hypersplenism: preliminary report. Transplant Proc. 2005;37:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |