Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3112
Revised: January 5, 2012
Accepted: March 9, 2012
Published online: June 28, 2012
AIM: To investigate the relationship between blood riboflavin levels and riboflavin transporter 2 (RFT2) gene expression in gastric carcinoma (GC) development.
METHODS: High-performance liquid chromatography was used to detect blood riboflavin levels in patients with GC. Real-time fluorogenic quantitative polymerase chain reaction and immunohistochemistry were used to analyze the expression of RFT2 mRNA and protein in samples from 60 GC patients consisting of both tumor and normal tissue.
RESULTS: A significant decrease in the RFT2 mRNA levels was detected in GC samples compared with those in the normal mucous membrane (0.398 ± 0.149 vs 1.479 ± 0.587; P = 0.040). Tumors exhibited low RFT2 protein expression (75%, 16.7%, 8.3% and 0% for no RFT2 staining, weak staining, medium staining and strong staining, respectively), which was significantly lower than that in the normal mucous membrane (10%, 16.7%, 26.7% and 46.7% for no RFT2 staining, weak staining, medium staining and strong staining, respectively; P < 0.05). Tumors with low RFT2 expression were significantly associated with tumor stage and histological grade. Moreover, a significantly decrease in Uyghur patients was observed compared with Han patients. However, other parameters-gender, tumor location and lymph node metastasis-showed no significant relationship with RFT2 expression. Blood riboflavin levels were reverse correlated with development of GC (1.2000 ± 0.97 569 ng/mL in high tumor stage patients vs 2.5980 ± 1.31 129 ng/mL in low tumor stage patients; P < 0.05). A positive correlation of plasma riboflavin levels with defective expression of RFT2 protein was found in GC patients (χ2 = 2.619; P = 0.019).
CONCLUSION: Defective expression of RFT2 is associated with the development of GC and this may represent a mechanism underlying the decreased plasma riboflavin levels in GC.
-
Citation: Eli M, Li DS, Zhang WW, Kong B, Du CS, Wumar M, Mamtimin B, Sheyhidin I, Hasim A. Decreased blood riboflavin levels are correlated with defective expression of
RFT2 gene in gastric cancer. World J Gastroenterol 2012; 18(24): 3112-3118 - URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3112
Gastric carcinoma (GC) is one of the most common cancers and the second leading cause of cancer deaths worldwide. In China, the incidence and mortality rates for gastric cancer in 2006 were 35.02 and 26.08 per 100 000 persons, respectively. The survival rate for gastric cancer is generally low, with a 5-year survival rate not exceeding 30%[1]. Helicobacter pylori infection of the gastric mucosa is associated with GC, infecting the gastric mucosa of 50% or more of the world’s population. However, the relatively low GC risk suggests that other factors[2] such as lifestyle factors-including diet and genetic predisposition-may play an etiologic role[3,4]. Nitrosamines have been suspected in the etiology of GC in the high incidence area of China; however, riboflavin deficiencies and other micronutrients may also be involved[5,6].
Riboflavin is a water-soluble vitamin that is a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which participate in various redox reactions, some of which are absolutely essential to the function of aerobic cells. Milk and dairy products, meats, fatty fish, certain fruit as well as vegetables are good sources of riboflavin. However, riboflavin deficiency and poor riboflavin status seem to be of most concern for the elderly and adolescents, despite the diversity of available riboflavin-rich foods[7]. Thus, whether other factors exist that may influence dietary riboflavin absorption or that may change the status of riboflavin needs to be determined.
Indeed, a recent study showed that riboflavin transporter 2 (RFT2) was found to transport riboflavin that is highly expressed in the small intestine and may be involved in such riboflavin absorption[8]. Additionally, Fujimura et al[9] demonstrated that RFT2 is a transporter involved in the epithelial uptake of riboflavin in the small intestine for its nutritional utilization. Because RFT2 is responsible for transporting riboflavin and riboflavin deficiency has been reported as a risk factor for GC, and our previous genome-wide association studies showed that genetic variations at 20p13 for RFT2 (or C20 or f54) contribute significantly to the risk for cutaneous squamous cell carcinoma and GC in Chinese Han and Uyghur populations[10], we propose that human RFT2 is likely to have an important role in gastric carcinogenesis that involves modulating riboflavin absorption. However, most previous studies on riboflavin and RFT2 were relatively small and investigated only the functional roles of riboflavin and RFT2[11-13].
To clarify these issues, in the present study, we examined blood riboflavin levels and their tissue riboflavin transporter gene statuses. Additionally, we further analyzed the relationship between RFT2 and riboflavin in the development of GC.
A total of 120 paraffin-embedded, formalin-fixed tissue specimens were used in this study. Sixty patients with GC who underwent surgery at the Department of General Surgery of the first affiliate Hospital of Xinjiang Medical University in 2011 were enrolled in the study. Additionally, 60 matched cases with normal mucous membranes were involved. The patient population consisted of 39 men and 21 women, with a mean age of 54.9 years (range: 39-76 years). Peripheral blood samples also collected into EDTA Vacutainer Tubes (Becton Dickinson) and immediately placed on ice. Next, the remaining samples were centrifuged (10 min at 2000 g and 4 °C) and collected plasma was stored at -80 °C until use. Control samples were obtained from subjects who underwent routine health checks, were recruited in the same area, and were matched with GC patients regarding age and gender. The selection criteria included individuals who were free from certain diseases, including neoplasms, cardiovascular diseases, hepatic diseases, renal diseases or inflammatory diseases. All patients were enrolled with written informed consent, and the study was approved by the Ethical Committee of the Medical University of Xinjiang. Patients had not received preoperative chemotherapy and/or radiotherapy. Tumor stage was determined according to the tumor node metastasis classification system of the International Union against Cancer. In addition, 60 frozen biopsies that included 30 GC and 30 matched normal mucous epithelia (5 cm away from the tumor), which were collected within 30 min after resection and kept at -80 °C, were subjected to real-time reverse transcription-polymerase chain reaction (RT-PCR) for detection of RFT2 mRNA expression. Histological diagnosis was confirmed for each specimen.
Blood plasma was analyzed for its concentration of riboflavin by high-performance liquid chromatography (HPLC) as described previously[14]. The HPLC system used was a Waters 2695 liquid chromatograph and Waters 2475 fluorescence detector with the autosampler set at 28 °C and configured for a 96-well microtiter plate. Water was generated using a Milli-Q water system. All chemicals were of analytical grade. For quality control, we used three Clin Chek serum controls, reconstituted and stored at -80 °C. Aliquots of aqueous (0.3860 g/L C2H7NO4) flavin stock solutions (5 mmol/L) were stored at -20 °C in the dark. An excitation wavelength of 450 nm was used and riboflavin was detected at an emission wavelength of 520 nm. The peak area was measured and used for quantification.
RFT2 mRNA expression was detected by quantitative real-time RT-PCR. Total RNA was extracted from fresh frozen tissue using Trizol (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions and treated with TURBO DNA-free™ DNase (Ambion, Austin, TX, United States) to remove the genomic DNA. Reverse transcription was performed using a reverse transcription system kit (Takara Bio, Tokyo, Japan) as previously described[15]. Real-time PCR for RFT2 was performed in a 10-μL reaction volume using the Platinum SYBR Green q PCR Super Mix-UDG (Invitrogen Life Technologies, Carlsbad, CA, United States) and the Light Cycler 480 system (Roche Diagnostics, Penzberg, Germany). The following hRFT2 and β-actin (as a reference) primers were used for RT-PCR: hRFT2 forward primer: AATCTAGAGCACTTGGACCTTTCC; hRFT2 reverse primer GGGTTCAGGGACAGGTCTAAAGA; β-actin forward primer: GGCACCCAGCACAATGAAG; β-actin reverse primer: CCGATCCACACGGAGTACTTG. The thermal cycle conditions were 95 °C for 10 s for one cycle, followed by 40 cycles of amplification at 95 °C for 5 s, and 60 °C for 45 s. The expression level of RFT2 mRNA was obtained using the 2-ΔΔCT calculation method. All PCR products were analyzed on a 2% agarose gel with ethidium bromide staining.
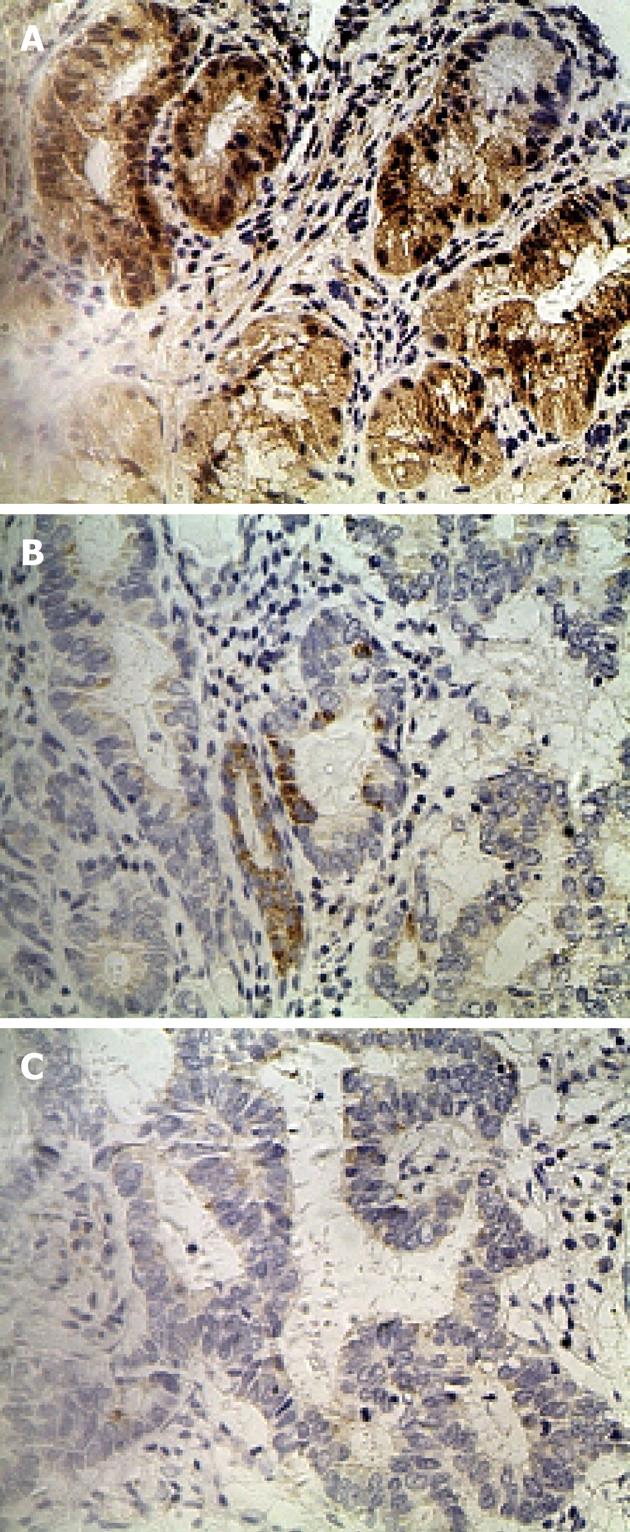
Immunohistochemistry (IHC) was performed using Histostain-SP kits (Zhongshan Golden Bridge, Beijing, China) according to the manufacturer’s recommendations and as described previously[16]. Sections of paraffin embedded tissue with a thickness of 3 μm were deparaffined in xylene, and then rehydrated in graded concentrations of ethyl alcohol (100%, 95%, 80% and 70%), and samples were pretreated using a microwave for 15 min on high mode in Tris/EDTA buffer (pH 9.0). After cooling and rinsing in distilled water, endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min at room temperature. Subsequently, the slides were pretreated with 1% bovine serum albumin in phosphate-buffered saline (PBS; pH 7.4) for 10 min. Samples were then preincubated with a protein blocking solution for 15 min and incubated with an RFT2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States; dilution: 1:300) diluted in PBS at 4 °C overnight in a humid chamber. Slides were washed three times in PBS, and then incubated with a secondary biotinylated antibody for 15 min at room temperature. Thereafter, sections were washed with PBS, and treated with peroxidase-conjugated streptavidin for 15 min. Finally, the sections were lightly stained with hematoxylin, and PBS was used instead of the primary antibody as a negative control. All immunostained sections were coded and independently examined by two investigators using light microscopy. Results were scored for each antibody separately and semi-quantitatively by assessing the staining intensity and the percentage of stained cells in the tumors. Staining intensity was scored as 0 (no staining), 1+ (weak), 2+ (medium), or 3+ (strong). The percentage of stained cells was categorized as follows: (1) 0% to 10% stained cells; (2) 11% to 50% stained cells; and (3) 50% stained cells or greater. The final score was obtained by multiplying the two scores. Cases with a score of 0 to 4 were classified as negative, and those with a final score of 5 to 9 were classified as positive.
SPSS 15.0 (SPSS, Inc., Chicago, IL, United States) was used for statistical analysis. The Pearson χ2 test or Fisher exact test was used to compare qualitative variables. The Wilcoxon two-sample test was used to compare the mean RFT2 expression in fresh frozen GC tissues with that in normal samples, as determined by real-time quantitative RT-PCR. Results were presented as mean ± SE. P-values less than 0.05 were considered to be statistically significant.
IHC staining of 60 primary GC lesions and samples adjacent to the tumor was performed using RFT2 antibodies (Table 1). Representative staining patterns for RFT2 are shown in Figure 1. IHC staining demonstrated that RFT2 was localized to the cytoplasm. Positive staining for RFT2 was generally observed within normal gastric epithelium adjacent to the tumor, but weak or no RFT2 staining was detected in GC cells. RFT2 expression was undetectable (75%) in gastric cancer tissue. By contrast, no strong IHC staining (3+) was detected in gastric cancer tissue. The positive rates (strong staining, medium staining and weak staining) of RFT2 expression in the normal gastric surface epithelium were 46.7%, 26.7% and 16.7%, respectively. These values sharply decreased to 0%, 8.3% and 16.7%, respectively, in GC lesions (P < 0.001, compared with the positive rate in healthy tissue). We also evaluated the possible relationship between the expression of RFT2 in tumor cells and the clinicopathologic characteristics of GC, including tumor stage, histological grade, lymph node metastasis, and other parameters such as gender, tumor location, and ethnic groups. RFT2 expression was significantly decreased in GC with poor differentiation and high tumor stage. Defective expression of RFT2 in tumor cells was significantly associated with poor differentiation and high tumor stage. However, other parameters such as gender, tumor location, and lymph node metastasis had no significant relationship with RFT2 expression (Table 1). Additionally, lower expression was observed in Uyghur patients compared with Han patients, but the difference was not statistically significant.
| Characteristics | n | RFT2 expression | Z | P value | |||
| − | + | ++ | +++ | ||||
| Normal mucous epithelia | 60 | 6 (10.0) | 10 (16.7) | 16 (26.7) | 28 (46.7) | -7.937 | < 0.001 |
| Gastric cancer | 60 | 45 (75.0) | 10 (16.7) | 5 (8.3) | 0 | ||
| Gender | |||||||
| Male | 39 | 31 (79.5) | 6 (15.4) | 3 (7.7) | 0 | -0.621 | 0.534 |
| Female | 21 | 14 (66.7) | 4 (19.0) | 2 (9.5) | 0 | ||
| Ethnic groups | |||||||
| Han | 32 | 21 (55.0) | 8 (25.0) | 3 (20.0) | 0 | -1.943 | 0.052 |
| Uyghur | 28 | 24 (81.82) | 2 (13.6) | 2 (4.5) | 0 | ||
| Tumor location | |||||||
| Cardia of stomach | 10 | 2 (20.0) | 4 (40.0) | 4 (40.0) | 0 | -3.127 | 0.209 |
| Body of stomach | 11 | 8 (72.1) | 2 (18.2) | 1 (8.7) | 0 | ||
| Antrum of stomach | 39 | 35 (89.7) | 4 (10.3) | 0 | 0 | ||
| Differentiation | |||||||
| Moderate/well | 19 | 10 (52.6) | 5 (26.3) | 4 (21.1) | 0 | -2.834 | 0.005 |
| Poor | 41 | 35 (85.4) | 5 (12.2) | 1 (2.3) | 0 | ||
| L/N metastasis | |||||||
| Negative | 10 | 9 (90.0) | 1 (10.0) | 0 | 0 | -1.245 | 0.28 |
| Positive | 50 | 36 (72.0) | 9 (18.0) | 5 (10.0) | |||
| Stage | |||||||
| II and IIIa | 41 | 27 (65.9) | 9 (22.0) | 5 (12.1) | 0 | -2.414 | 0.019 |
| IIIb and IV | 19 | 18 (94.7) | 1 (5.3) | 0 | 0 | ||
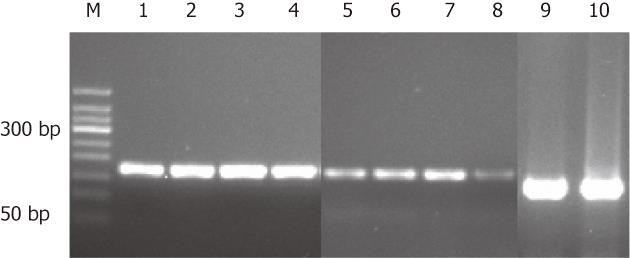
To confirm the IHC results, RFT2 mRNA expression in gastric biopsies was detected by RT-PCR (Figure 2). Similar to the IHC results, the RFT2 mRNA expression levels were significantly lower in GC than in normal counterpart tissue (0.398 ± 0.149 ng/mL vs 1.479 ± 0.587 ng/mL; P = 0.040) (Figure 2).
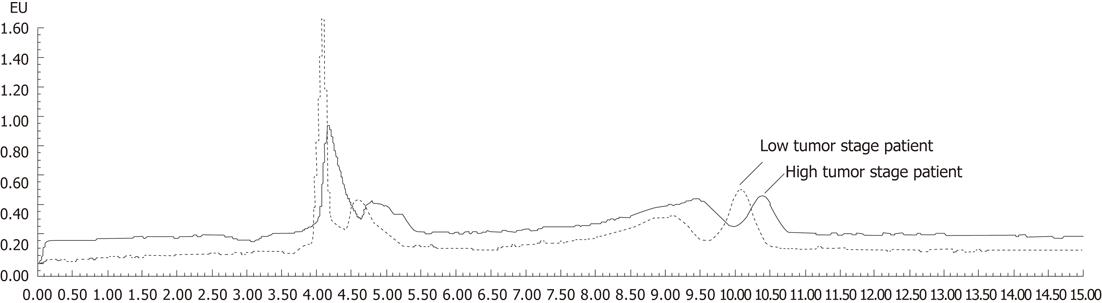
Riboflavin concentrations were determined in samples obtained from gastric cancer patients who were not taking vitamin supplements. The average blood concentration of riboflavin was 1.7632 ± 0.9836 ng/mL in GC patients. The average blood riboflavin level was 1.2000 ± 0.97 569 ng/mL in high tumor stage patients, which was significantly lower than in patients with low tumor stage (2.5980 ± 1.31 129 ng/mL; P = 0.019). Riboflavin chromatograms of plasma samples from a low tumor stage patient and a high tumor stage patient are shown in Figure 3. A tendency of decreasing blood riboflavin level was found to be associated with GC development. However, no significant difference was observed among other parameters such as gender, tumor location and ethnic groups and patients with GC. We also analyzed the relationship between the blood riboflavin level and expression of the RFT2 gene and development of GC. A positive association was found between changes in the blood riboflavin levels and changes in RFT2 protein expression as well as development of malignant GC (Table 2).
| RFT2 protein expression | Riboflavin level | χ2 | P value |
| − | 0.7012 ± 0.68 778 | -2.619 | 0.019 |
| + | 1.6425 ± 1.01 783 | ||
| ++ | 2.9691 ± 1.30 345 |
In the present study, we investigated the association between plasma levels of riboflavin and the RFT2 mRNA and protein expression status in patients with gastric adenocarcinoma. The results showed a tendency for an inverse association between riboflavin levels and GC risk, and an inverse association between RFT2 expression status and GC risk. Additionally, a positive association was found between riboflavin levels and RFT2 with GC risk. The results indicate that RFT2 likely plays an important role in gastric carcinogenesis that involves modulating riboflavin absorption.
Previous studies have reported that dietary habits and intake of nutrients play an important role in the prevention and causation of GC. High consumption of dark-green vegetables, fruits, and milk and dairy products (riboflavin is a water soluble vitamin present in various foods) has also been suggested to decrease the risk of GC. Several epidemiological studies have reported[17,18] that riboflavin deficiency is linked to an increased risk of GC because riboflavin is involved in essential oxidation-reduction reactions and its deficiency leads to skin and mucosal disorders. Therefore, measurement of plasma riboflavin can be used to assess vitamin B2 status in individuals at high risk for GC. Although riboflavin supplements can significantly reduce the risk of GC, different individual intervention effects were observed after dietary supplementation with riboflavin[19]. Therefore, RFT2 may be the key target of environmental and genetic factors because the RFT2 gene also has been reported as a susceptibility gene for GC using a genome-wide association study approach[10].
RFT2 is a transmembrane protein, which may function biologically as a transporter of riboflavin in the small intestine, and the role of riboflavin in cellular homeostasis has been well documented. Therefore, we speculate that mutation of the riboflavin transporter gene might cause riboflavin deficiency, resulting in an increased risk of GC, and such characteristics of the riboflavin transporter gene were also consistent with those of the carrier-mediated riboflavin transport system in the Caco-2 cell line as an intestinal epithelial model. Thus, it is likely that hRFT2 is the molecular entity of the riboflavin transport system in the Caco-2 cell line[20].
Riboflavin (vitamin B2) has been confirmed to serve as a co-factor in fat, amino acid, carbohydrate and vitamin metabolism as well as to reduce oxidative stress, affect cell proliferation, and affect angiogenesis[21,22]. Few epidemiological studies have investigated plasma concentrations of riboflavin in relation to GC risk[23]. The present study observed riboflavin concentrations in samples obtained from GC patients by HPLC, and the results showed a tendency of decreasing blood riboflavin level with development of GC. Use of HPLC as a convenient method for separation and measurement of vitamins in plasma has been reported in many medical institutions[14,24].
Riboflavin is essential for synthesis of FAD and FMN, which function as cofactors for several biological processes involved in energy metabolism. The most important dietary sources of riboflavin are milk and dairy products. In Xinjiang, a multi-ethnic residential area in China, the Chinese Han and Uyghur populations are the main ethnic groups and they have different dietary habits. The Uyghur population tends to consume milk, dairy products, and meat as well as fruits and vegetables. They should not lack intake of riboflavin. Therefore, riboflavin deficiency in blood is expected to be related to a disturbance in riboflavin absorption. If inadequate intake of riboflavin exists, disturbances in the steps in intermediary metabolism may occur.
In conclusion, we have identified down-regulated expression of RFT2 mRNA and protein as being closely related to the progression of GC lesions and also found a positive relationship between blood riboflavin levels and RFT2 protein expression as well as between blood riboflavin levels and development of GC. The RFT2 gene may be the key target of environmental and genetic factors in the development of GC.
Epidemiological and etiological research has confirmed that environmental and genetic factors play an important role in gastric carcinogenesis. The riboflavin transporter 2 (RFT2) gene as a susceptibility gene for gastric carcinoma (GC) is likely to have an important role in gastric carcinogenesis that involves transporting riboflavin and modulating riboflavin absorption. However, riboflavin deficiency has been reported as a risk factor for GC.
Riboflavin deficiency of is a risk factor for GC, and riboflavin supplements can significantly reduce the risk of GC. However, different individual intervention effects have been observed after dietary supplementation with riboflavin. In the present study, defective expression of RFT2 was associated with development of GC and may be a potential mechanism underlying the decreased plasma riboflavin levels in GC.
Recent reports have highlighted that genetic susceptibility combined with exposure to environmental risk factors contributes to high rates of cancer. In particular in GC, RFT2 is down-regulated. This is the first study to report that RFT2 is down-regulated in GC. Furthermore, their studies suggest that this protein may be the cause of decreased plasma riboflavin levels in GC.
By understanding how RFT2 affects the absorption of riboflavin and by enhancing its expression, this study may offer a future strategy for the treatment of patients with GC.
RFT2 is a transmembrane protein that functions biologically as a transporter of riboflavin and in maintenance of cellular homeostasis. Riboflavin is a water-soluble vitamin that participates in various redox reactions, some of which are essential for the function of aerobic cells.
The authors performed a study on riboflavin levels as well as on RFT2 gene mRNA levels in esophageal squamous cell carcinoma samples and normal counterpart tissue. The level of RFT2 was found to be inversely related to tumor stage. Down-regulation and loss of RFT2 protein expression were important in the pathogenesis of gastric cancer. This study was well designed and the molecular experiments were well done and interpreted. The data seem convincing.
Peer reviewers: Simon Law, Professor, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China; Shin Maeda, Professor, Yokohama City University, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 2. | Schlemper RJ, van der Werf SD, Vandenbroucke JP, Biemond I, Lamers CB. Seroepidemiology of gastritis in Japanese and Dutch working populations: evidence for the development of atrophic gastritis that is not related to Helicobacter pylori. Gut. 1995;37:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Yang CS, Miao J, Yang W, Huang M, Wang T, Xue H, You S, Lu J, Wu J. Diet and vitamin nutrition of the high esophageal cancer risk population in Linxian, China. Nutr Cancer. 1982;4:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1035] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] |
| 6. | Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77:1352-1360. [PubMed] |
| 7. | Jusko WJ, Levy G. Absorption, metabolism, and excretion of riboflavin-5'-phosphate in man. J Pharm Sci. 1967;56:58-62. [PubMed] |
| 8. | Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol. 2008;295:C632-C641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Fujimura M, Yamamoto S, Murata T, Yasujima T, Inoue K, Ohta KY, Yuasa H. Functional characteristics of the human ortholog of riboflavin transporter 2 and riboflavin-responsive expression of its rat ortholog in the small intestine indicate its involvement in riboflavin absorption. J Nutr. 2010;140:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, Li JM, Kong GQ, Qi H, Cui J. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 11. | Kaaks R, Tuyns AJ, Haelterman M, Riboli E. Nutrient intake patterns and gastric cancer risk: a case-control study in Belgium. Int J Cancer. 1998;78:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, Raymond FL, Childs AM, Sheridan E, Edwards S. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am J Hum Genet. 2010;86:485-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem. 2009;145:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Petteys BJ, Frank EL. Rapid determination of vitamin B₂ (riboflavin) in plasma by HPLC. Clin Chim Acta. 2011;412:38-43. [PubMed] [DOI] [Full Text] |
| 15. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [PubMed] |
| 16. | Sheyhidin I, Nabi G, Hasim A, Zhang RP, Ainiwaer J, Ma H, Wang H. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:3745-3751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Manthey KC, Chew YC, Zempleni J. Riboflavin deficiency impairs oxidative folding and secretion of apolipoprotein B-100 in HepG2 cells, triggering stress response systems. J Nutr. 2005;135:978-982. [PubMed] |
| 18. | Eussen SJ, Vollset SE, Hustad S, Midttun Ø, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Ferrari P. Vitamins B2 and B6 and genetic polymorphisms related to one-carbon metabolism as risk factors for gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2010;19:28-38. [PubMed] |
| 19. | Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507-518. [PubMed] |
| 20. | Zielińska-Dawidziak M, Grajek K, Olejnik A, Czaczyk K, Grajek W. Transport of high concentration of thiamin, riboflavin and pyridoxine across intestinal epithelial cells Caco-2. J Nutr Sci Vitaminol (Tokyo). 2008;54:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Rivlin RS. Riboflavin metabolism. N Engl J Med. 1970;283:463-472. [PubMed] |
| 22. | Henriques BJ, Olsen RK, Bross P, Gomes CM. Emerging roles for riboflavin in functional rescue of mitochondrial β-oxidation flavoenzymes. Curr Med Chem. 2010;17:3842-3854. [PubMed] |
| 23. | Sierra R, Chinnock A, Ohshima H, Pignatelli B, Malaveille C, Gamboa C, Teuchmann S, Muñoz N, Bartsch H. In vivo nitrosoproline formation and other risk factors in Costa Rican children from high- and low-risk areas for gastric cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:563-568. [PubMed] |