Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3105
Revised: February 20, 2012
Accepted: February 26, 2012
Published online: June 28, 2012
AIM: To assess the alcohol drinking patterns in a cohort of primary sclerosing cholangitis (PSC) patients and the possible influence on the development of fibrosis.
METHODS: Ninety-six patients with PSC were evaluated with a validated questionnaire about a patient’s lifetime drinking habits: the lifetime drinking history (LDH) questionnaire. In addition, clinical status, transient elastography and biochemistry values were analysed and registered. Patients were defined as having either significant or non-significant fibrosis. Significant fibrosis was defined as either an elastography value of ≥ 17.3 kPa or the presence of clinical signs of cirrhosis. Patients were divided into two groups depending on their alcohol consumption patterns; no/low alcohol consumption (one drink or unit/d) and moderate/high alcohol consumption (≥ 1 drink or unit/d). LDH data were calculated to estimate lifetime alcohol intake (LAI), current alcohol intake, drinks per year before and after diagnosis of PSC. We also calculated the number of episodes of binge-drinking (defined as consuming ≥ 5 drinks per occasion) in total, before and after the diagnosis of PSC.
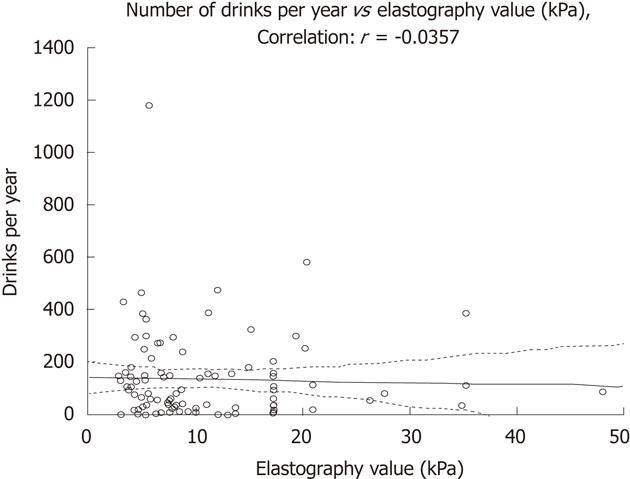
RESULTS: The mean LAI was 3882 units of alcohol, giving a mean intake after onset of alcohol consumption of 2.6 units per week. Only 9% of patients consumed alcohol equal to or more than one unit per day. Current alcohol intake in patients with significant fibrosis (n = 26) was less than in patients without significant fibrosis (n = 70), as shown by lower values of phosphatidylethanol (B-PEth) (0.1 μmol/L vs 0.33 μmol/L, respectively, P = 0.002) and carbohydrate-deficient transferrin (CDT) (0.88% vs 1.06%, respectively, P = 0.02). Self-reported LAI was similar between the two groups. Patients with significant fibrosis reduced their alcohol intake after diagnosis from 103 to 88 units per year whereas patients without fibrosis increased their alcohol intake after PSC diagnosis from 111 to 151 units/year. There were no correlations between elastography values and intake of alcohol (units/year) (r = -0.036).
CONCLUSION: PSC patients have low alcohol consumption. The lack of correlation between fibrosis and alcohol intake indicates that a low alcohol intake is safe in these patients.
- Citation: Hagström H, Stål P, Stokkeland K, Bergquist A. Alcohol consumption in patients with primary sclerosing cholangitis. World J Gastroenterol 2012; 18(24): 3105-3111
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3105
Little is known about risk factors for progression of fibrosis in primary sclerosing cholangitis (PSC) except for duration of disease and presence of symptoms[1-4]. Excessive consumption of alcohol causes liver disease[5-7], and a high intake of alcohol acts as a co-factor for progression of other chronic liver diseases, such as non-alcoholic steatohepatitis, hereditary hemochromatosis and hepatitis C (HCV). For instance, heavy episodic drinking has been shown to be associated with progression of fibrosis in non-alcoholic fatty liver disease[8], alcohol consumption of more than 60 g per day increases the risk for cirrhosis 9-fold in patients with hereditary hemochromatosis[9], and an alcohol intake of more than 210 g per week in patients with HCV has been shown to increase fibrosis[10-13]. The threshold for a safe intake of alcohol with regard to development of fibrosis in patients with concomitant liver disease is unclear and most patients with a chronic liver disease are advised to keep their alcohol intake to a minimum. The evidence for giving such advice to patients with chronic liver diseases in general is scarce. There is no evidence that alcohol in low amounts influences disease progression and there are data suggesting that a low alcohol intake, on the contrary, may have a protective effect against fibrosis. A recent study by Cheung et al[14] indicated no increased risk for fibrosis in HCV patients with alcohol intake less than 210 g per week and in a study by Moriya et al[15], low consumption of alcohol seemed to protect against non-alcoholic fatty liver disease in healthy individuals.
The impact of alcohol on progression of fibrosis in PSC has not been previously studied, although alcohol consumption has been reported to be associated with development of cholangiocarcinoma[16]. One of the most common questions from patients with PSC is: what amount of alcohol can be considered to be a safe intake? “Safe” amounts of alcohol (in liver-healthy individuals) are usually considered to be less than 210 g (approximately 18 units or drinks) of alcohol per week[7]. The purpose of our study was to describe the alcohol consumption patterns, and to evaluate whether lifetime alcohol consumption correlates to the fibrosis stage, in patients with PSC.
All patients diagnosed with PSC at the Department of Gastroenterology and Hepatology, Karolinska University Hospital are recorded in a local PSC register.
Eligible for this study were 141 patients with PSC who were identified as currently living in the Stockholm area and who were having their regular follow-ups at our clinic.
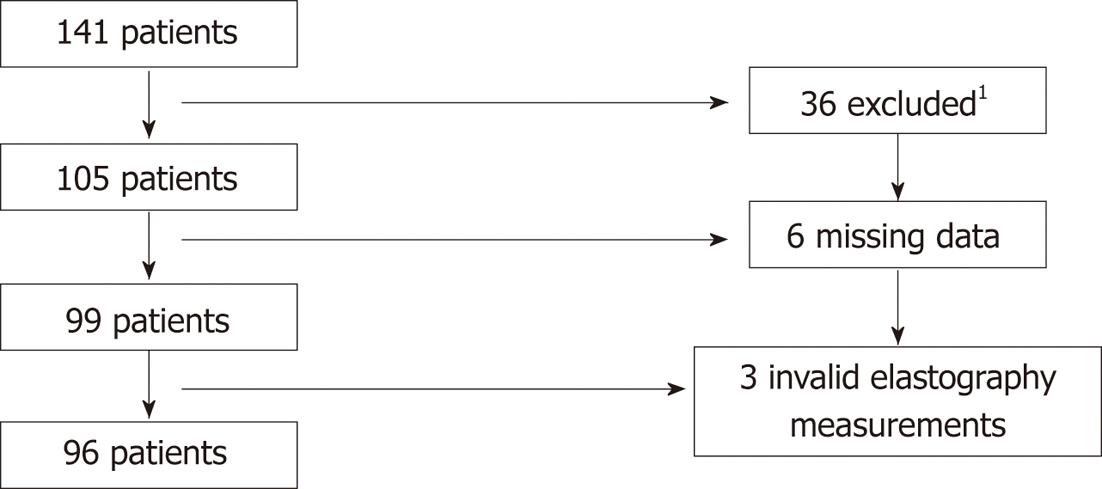
The diagnosis of PSC were made according to accepted criteria; i.e., typical cholangiographic findings of bile duct irregularities, strictures and dilatations or histological findings of cholangitis, or signs of small-duct PSC in combination with biochemical and clinical findings[4,17]. We excluded 32 patients with a recent diagnosis of cancer, not Swedish speaking, presence of Down’s syndrome, dementia, current pregnancy, severe psychiatric disease (e.g., psychosis, bipolar disease), co-existing liver diseases (e.g., hepatitis B and C or hereditary hemochromatosis). Four patients declined to participate. The study cohort and patient selection are summarized in Figure 1.
From our registry and patient charts, the following data were registered: duration of disease, age, sex, co-existing inflammatory bowel disease (IBD, diagnosed through endoscopy and histology), symptoms, smoking and body mass index (BMI). The patients were interviewed with a structured protocol for confirmation and validation of the data collected from the registry and for current symptoms and alcohol habits, including the question as to whether the patients had reduced their alcohol intake after the diagnosis of PSC was established.
At the interview, patients received the lifetime drinking history (LDH) questionnaire, a detailed and validated questionnaire about the patient’s lifetime drinking habits[18,19]. This questionnaire allows the calculation of the total number of units during the patient’s lifetime, with the possibility of calculating changes in drinking habits during life. It also allows measurement of total number of binge drinking episodes, defined as drinking 5 or more units of alcohol at one occasion. One unit of alcohol is equivalent to 12 g of alcohol. Patients were thoroughly informed about the questionnaire and later filled it out at home. When data were missing, the patient was contacted by telephone and information was supplemented through a telephone interview. Six of 105 patients (5.7%) did not return the LDH questionnaire despite being reminded and were excluded. No/low alcohol consumers were defined as drinking less than one drink per day, and moderate/high alcohol consumer as drinking equal to or more than one drink per day.
Transient elastography with FibroScan (EchoSens, Paris, France) was performed on all patients on the same occasion as the interview. The cut-off values for significant fibrosis were adopted from Corpechot et al[20] and the threshold for fibrosis stage 4 according to Ludwig (cirrhosis) was set to ≥ 17.3 kPa. At least 10 measurements were made, and only the scans where more than 60% of measurements were valid were accepted. We divided the population into two subgroups: patients with significant and non-significant fibrosis. Significant fibrosis was defined as either elastography values ≥ 17.3 kPa or a clinical diagnosis of cirrhosis diagnosed with histology[21] or typical radiological and biochemical findings of cirrhosis (such as irregular hepatic parenchyma, splenomegaly, oesophageal varices, presence of intraabdominal collaterals) or manifestation of decompensation. In nine patients elastography failed, most often due to overweight. In six of these, presence of significant fibrosis was evident from clinical data and they were included into the “significant fibrosis” group. The three patients with no available information on fibrosis from either elastography or clinical data were excluded. Twenty-six patients were found to have significant fibrosis and 70 patients had non-significant fibrosis.
Biochemical data including blood count, sodium, potassium, creatinine, alkaline phosphatase, serum transaminases, total bilirubin, PK-INR, albumin, carbohydrate-deficient transferrin (CDT) and phosphatidylethanol (B-PEth, measured by liquid chromatography-mass spectrometry) in plasma were collected and analysed at the routine biochemistry laboratory at Karolinska University Hospital.
Continuous variables were analyzed using the Mann-Whitney U-test or the Wilcoxon Signed Rank Test where appropriate. For comparison of categorical data the χ2 analysis was used or, in the case of small expected frequencies, F test. For correlation tests of linear data, the Pearson r test was used. We controlled the results for duration of disease using co-variance analysis of variance. Statistical data were analyzed using the Statistica® 9.1 software (StatSoft Inc., Tulsa OK) and SAS 9.2 software (SAS Institute Inc., Cary NC).
The local ethics committee at Karolinska University Hospital approved this study, registry No: 2009/1894-31/1. Written informed consent was obtained from all participating subjects.
Clinical characteristics and data on lifetime alcohol consumption for the 96 patients are presented in Table 1. There were 66% men, mean age was 47 ± 13 years (range: 22-75 years) and 73 patients (76%) were diagnosed with concomitant IBD. Mean elastography value was 11.1 ± 8.2 kPa (range: 2.8-48 kPa). Seven patients (7.3%) had been diagnosed with PSC before they first started drinking alcohol. There were no cases of patients with Child-Pugh score of 10 (i.e., class C) or higher.
| Mean (range) | |
| Age at inclusion (yr) | 47 (22-75) |
| Male sex | 63/96 |
| Duration of primary sclerosing cholangitis (yr) | 12 (0-30) |
| Age at primary sclerosing cholangitis diagnosis (yr) | 35 (11-65) |
| Fibroscan value (kPa) | 11.1 (2.8-48) |
| Undergone orthotopic liver transplantation, n (%) | 12 (12.5) |
| Bilirubin (μmol/L) | 15.3 (3-82) |
| Alkaline phosphatase (μkat/L) | 2.66 (0.5-12.4) |
| Phosphatidylethanol (μmol/L) | 0.28 (0.1-8.4) |
| Carbohydrate-deficient transferrin (%) | 1.01 (0.5-3) |
| Body mass index (kg/m2) | 24.1 (17.2-34.3) |
| Inflammatory bowel disease (%) | 73/96 (76) |
| Smoking-current user, n (%) | 4 (4.2) |
| Smoking-ex user, n (%) | 16 (16.7) |
| Smoking-never used, n (%) | 76 (79.2) |
| Lifetime drinking habits | |
| Age at first alcohol intake (yr) | 17 (12-28) |
| Lifetime alcohol intake (unit) | 3882 (0-20 270) |
| Yearly alcohol intake, total (unit) | 137 (0-1180) |
| Yearly alcohol intake, before diagnosis (unit) | 109 (0-674) |
| Yearly alcohol intake, after diagnosis (unit) | 134 (0-1110) |
| Total occasions of binge-drinking | 294 (0-4054) |
| Yearly occasions of binge-drinking, total | 11.3 (0-165) |
| Yearly occasions of binge-drinking, before diagnosis | 12.0 (0-295) |
| Yearly occasions of binge-drinking, after diagnosis | 8.0 (0-83) |
Mean age at onset of alcohol consumption was 17 ± 3 years (range: 12-28 years). The mean lifetime alcohol intake (LAI) was 3882 units of alcohol (median: 2275 units, range: 0-20 270 units), giving a yearly mean alcohol intake of 137 units per drinking year and a mean number of 2.6 units per week during the years of alcohol consumption. Nine percent (9/96) drank equal to or more than one unit per day, and one percent (1/96) had a mean consumption of more than two units per day. The mean number of total episodes of binge drinking was 294 (median: 96, range: 0-4054), equalling eleven binges per year or 0.2 binges per week on average.
Twenty-eight percent (24/87) of no/low alcohol consumers (< 1 unit per day) had significant fibrosis compared to 22% of consumers with moderate/high alcohol intake (> 1 unit per day, P = 0.57). Moderate/high consumers had significantly more episodes of binge drinking. There were no significant differences in biochemical data or BMI (data not shown) between the moderate/high and the no/low alcohol consumption groups.
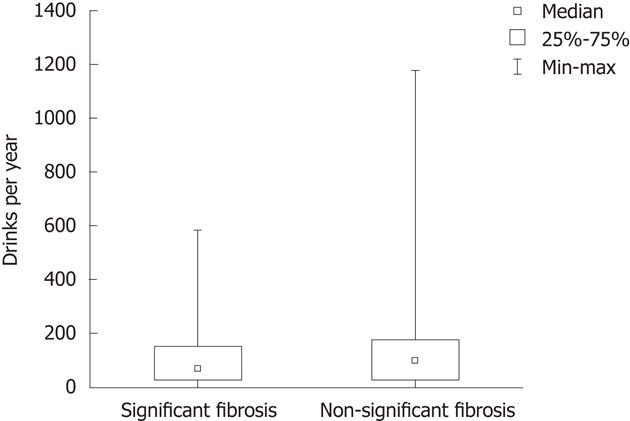
There were no significant differences in mean units of alcohol consumed per year between patients with significant and non-significant fibrosis, as shown in Figure 2. There was no correlation between yearly alcohol intake (units/year) and elastography values (Figure 3). Thirty-eight percent of all patients (36/96) reported a decreased alcohol intake after the diagnosis of PSC. This figure was similar in patients with and without significant fibrosis (39% vs 37%). To further evaluate if the drinking habits changed after diagnosis, the LDH data were compared before and after PSC diagnosis. A total increase from 109 to 134 units per year, and a decrease in binge drinking of twelve to eight binges per year was found in all patients (not signitficant). Among patients with non-significant fibrosis, we found an increase in total alcohol consumption after PSC diagnosis (111 units per year vs 151 units per year, P = 0.07) whereas a decrease in total alcohol consumption after PSC diagnosis (103 units per year vs 88 units per year, P = 0.59) was found in the significant fibrosis group. Binge-drinking before and after PSC diagnosis was 14.9 binges per year vs 9.6 binges per year (P = 0.24) in the non-significant fibrosis group and 4.3 binges per year vs 3.6 binges per year (P = 0.5) in the significant fibrosis group. The significant fibrosis group had higher bilirubin values (24.8 μmol/L vs 11.8 μmol/L, P = 0.015), lower CDT values (0.88% vs 1.06%, P = 0.02) and lower PEth values (0.1 vs 0.33, P = 0.0016) than the non-significant fibrosis group. Comparison of clinical variables and alcohol consumption between patients with significant and non-significant fibrosis are summarized in Table 2. We also performed a similar analysis with different cut-off values; 12.5 and 14.5 kPa respectively, which are the suggested cut-off values for cirrhosis (stage 4 fibrosis) in HCV[22,23], with similar results as when the cut-off level of ≥ 17.3 kPa was used (data not shown).
| Variable | Non-significant fibrosis (n = 70) | Significant fibrosis (n = 26) | P value |
| Duration of disease (yr) | 10.1 ± 6.8 | 15.6 ± 7.9 | 0.019 |
| Age at primary sclerosing cholangitis diagnosis (yr) | 36 ± 14 | 34 ± 13 | 0.29 |
| Lifetime alcohol intake (unit) | 3896 ± 4441 | 3845 ± 4457 | 0.44 |
| Yearly alcohol intake, mean (unit) | 144 ± 178 | 117 ± 135 | 0.27 |
| Total occasions of binge-drinking | 331 ± 692 | 194 ± 341 | 0.23 |
| Yearly occasions of binge-drinking | 12.7 ± 24.7 | 7.1 ± 13 | 0.41 |
| Yearly alcohol intake, before diagnosis (unit) | 111 ± 129 | 103 ± 141 | 0.24 |
| Yearly alcohol intake, after diagnosis (unit) | 151 ± 200 | 88 ± 86 | 0.26 |
| Yearly binge drinking episodes, before diagnosis | 14.9 ± 38.1 | 4.3 ± 7.3 | 0.07 |
| Yearly binge drinking episodes, after diagnosis | 9.6 ± 18.6 | 3.6 ± 4.7 | 0.23 |
| Bilirubin (μmol/L) | 11.8 ± 6.8 | 24.8 ± 23.1 | 0.015 |
| Alkaline phosphatase (μkat/L) | 2.66 ± 2.65 | 2.66 ± 2.22 | 0.45 |
| Phosphatidyl ethanol (μmol/L) | 0.33 ± 1 | 0.1 ± 0 | 0.0016 |
| Carbohydrate deficient transferrin (%) | 1.06 ± 0.38 | 0.88 ± 0.2 | 0.02 |
| Body mass index (kg/m²) | 24.12 ± 3.12 | 24.02 ± 4.24 | 0.43 |
This study describes for the first time the alcohol consumption patterns in a large cohort of PSC patients before and after PSC diagnosis. The majority of the PSC patients were shown to have low alcohol consumption. The mean LAI was 3882 units of alcohol, giving a mean intake after onset of alcohol consumption of 2.6 units per week, and only 9% drank more than one unit per day and 1% more than two units per day. In comparison, to develop alcohol-induced liver cirrhosis, subjects need to drink at least 30 g of alcohol per day, equaling around 3 drinks per day[5-7] over several years.
The lifetime alcohol consumption did not correlate with the presence of significant fibrosis, although the current alcohol intake in fibrotic patients was less than in patients without significant fibrosis, shown by lower values of PEth and CDT. There was also a trend that patients with significant fibrosis had reduced their alcohol intake after the diagnosis of PSC whereas patients without significant cirrhosis actually increased their consumption after diagnosis. This is consistent with findings from studies of the effect of alcohol on other chronic liver diseases implicating that a low intake of alcohol seems to be harmless[14,15]. One may speculate whether or not low alcohol consumption actually protects against more progressive development of cirrhosis. Although no such conclusions can be drawn from the present study, our data support that a low consumption is harmless for fibrosis progression. The alcohol consumption among our PSC patients was lower than we expected, which has influenced our ability to evaluate the impact of more marked alcohol consumption for the progression of fibrosis. We were unable to evaluate whether a moderate/high consumption was harmful or safe since the number of patients with this pattern of consumption was too low.
CDT values can be affected by factors other than alcohol, such as end-stage liver disease (Child-Pugh score ≥ 10)[24,25]. None of our patients had a Child-Turcotte-Pugh score of more than 10 and patients with significant fibrosis had lower CDT values than patients with non-significant fibrosis. CDT has not been validated in patients with PSC; however, it has been studied in primary biliary cirrhosis and not been implicated to produce false-positive results[26]. B-PEth[27] measured by mass spectrometry is an even more sensitive marker than CDT for detecting alcohol consumption over the previous 1-2 wk. It has been reported to be stable in patients with concomitant liver disease[28], but has not been studied in detail in patients with PSC.
The low alcohol consumption seen among our patients may be an effect of the general advice these patients are given in clinical practice, which is to keep alcohol intake at a minimum level. Patients with significant fibrosis also had higher bilirubin levels indicating a more severe disease, which in itself inhibits alcohol consumption. Thus, the knowledge of significant fibrosis in a patient contributes to decreased alcohol consumption. It is well known that smoking is associated with high alcohol consumption[29]; PSC patients have a low smoking frequency[30], also seen in this study. The correlation between a low smoking frequency and small total alcohol consumption in this cohort further validates our results.
Binge drinking decreased in both patients with significant and non-significant fibrosis after PSC diagnosis. This finding may reflect a change in drinking pattern with age, rather than the presence of PSC. Also, there is a general trend in Sweden towards less binge drinking[31]. The total alcohol consumption in Sweden has increased by approximately 15% since the mid 1990s[31,32]. Thirty-eight percent of all our patients reported that they had reduced their alcohol intake after the PSC diagnosis; however, the trend when looking at the LDH data was an increase in the total yearly alcohol intake. The perception of having reduced the consumption may be related to a reduction in the occasions of binge drinking episodes, which was confirmed in the questionnaire.
One limitation of the present study is the retrospective self-reported alcohol intake, which may be impaired by recollection bias. However, the LDH questionnaire is well validated and has a high test-retest correlation[19,33]. We also had a high response rate to the LDH questionnaire which is why we believe that our data are reliable. In addition, there is a risk that we have underestimated the presence of significant fibrosis since we did not perform liver biopsies to measure fibrosis. Liver biopsy is not mandatory for the diagnosis of PSC and we chose to refrain from biopsies for ethical reasons due to risk of complications. The role of transient elastography in cholestatic liver disease is not well established, although it has been shown to be a good alternative to histology for evaluating fibrosis, mainly in HCV, but also in other chronic liver diseases[20,34-36].
Chalasani et al[16] reported in 2000 that alcohol was a risk factor for developing cholangiocarcinoma (CCA) in PSC; however, they were unable to quantify the amount of alcohol consumed. Our data can at present not evaluate whether alcohol intake is important for CCA since none of our patients have developed CCA. However, we have obtained solid data regarding alcohol consumption in a large cohort of PSC patients which is being prospectively followed. This allows future studies exploring the role of alcohol as a risk factor for developing CCA in this cohort.
In conclusion, patients with PSC have low alcohol consumption. Only 9% consumed an amount of alcohol equal to or more than one unit per day. There was a trend towards increased alcohol consumption after the PSC diagnosis in patients without significant fibrosis, and these patients have significantly increased plasma levels of CDT and PEth as compared to those having significant fibrosis or cirrhosis. We found no correlation between alcohol consumption and significant fibrosis. In summary, our results indicate that low alcohol consumption is safe in patients with PSC.
Per Näsman provided the excellent statistical support.
Fibrosis progression in primary sclerosing cholangitis (PSC) is a heterogeneous process with large individual variations before significant fibrosis develops.
Alcohol, in high amounts, is known to be a risk factor for progression of fibrosis in other chronic liver diseases. The role of alcohol intake for progression of fibrosis has not previously been studied in PSC.
This is the first study of the alcohol consumption pattern in a large cohort of PSC patients and they aimed to correlate this to the occurrence and degree of fibrosis.
By increasing their knowledge of risk factors for progression of fibrosis in PSC, this study can help give doctors relevant information to patients regarding their alcohol habits.
The paper is an interesting paper assessing the lifetime drinking history. The paper is quite well written but the reader needs reason for using certain biochemical variables in this context and the statistical analysis has to be better explained. Also most readers of this paper do not know what is the amount of drinks used by patients with alcohol dependency and alcoholic cirrhosis.
Peer reviewer: Kiichi Tamada, MD, Division of Gastroenterology, School of Medicine, Jichi Medical University, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Talwalkar JA, Lindor KD. Natural history and prognostic models in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Singal AK, Stanca CM, Clark V, Dixon L, Levy C, Odin JA, Fiel MI, Friedman SL, Bach N. Natural history of small duct primary sclerosing cholangitis: a case series with review of the literature. Hepatol Int. 2011;5:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Corrao G, Bagnardi V, Zambon A, Torchio P. Meta-analysis of alcohol intake in relation to risk of liver cirrhosis. Alcohol Alcohol. 1998;33:381-392. [PubMed] |
| 6. | Kamper-Jørgensen M, Grønbaek M, Tolstrup J, Becker U. Alcohol and cirrhosis: dose--response or threshold effect? J Hepatol. 2004;41:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Crocè L, Sasso F, Pozzato G, Cristianini G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 405] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, Kechagias S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366-374. [PubMed] |
| 9. | Fletcher LM, Dixon JL, Purdie DM, Powell LW, Crawford DH. Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology. 2002;122:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, Dhillon AP, Norkrans G, Wejstål R. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J Viral Hepat. 2002;9:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Serfaty L, Poujol-Robert A, Carbonell N, Chazouillères O, Poupon RE, Poupon R. Effect of the interaction between steatosis and alcohol intake on liver fibrosis progression in chronic hepatitis C. Am J Gastroenterol. 2002;97:1807-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Monto A, Patel K, Bostrom A, Pianko S, Pockros P, McHutchison JG, Wright TL. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Cheung O, Sterling RK, Salvatori J, Williams K, Hubbard S, Luketic VA, Stravitz TR, Sanyal AJ, Contos MJ, Mills S. Mild alcohol consumption is not associated with increased fibrosis in patients with chronic hepatitis C. J Clin Gastroenterol. 2011;45:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Moriya A, Iwasaki Y, Ohguchi S, Kayashima E, Mitsumune T, Taniguchi H, Ikeda F, Shiratori Y, Yamamoto K. Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 513] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157-1170. [PubMed] |
| 19. | Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs. 2009;70:296-303. [PubMed] |
| 20. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 847] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 23. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 24. | Fleming MF, Anton RF, Spies CD. A review of genetic, biological, pharmacological, and clinical factors that affect carbohydrate-deficient transferrin levels. Alcohol Clin Exp Res. 2004;28:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | DiMartini A, Day N, Lane T, Beisler AT, Dew MA, Anton R. Carbohydrate deficient transferrin in abstaining patients with end-stage liver disease. Alcohol Clin Exp Res. 2001;25:1729-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Arndt T, Meier U, Nauck M, Gressner AM. Primary biliary cirrhosis is not a clinical condition for increased carbohydrate-deficient transferrin: experience with four independent CDT analysis methods. Clin Chim Acta. 2006;372:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Isaksson A, Walther L, Hansson T, Andersson A, Alling C. Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal. 2011;3:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Stewart SH, Reuben A, Brzezinski WA, Koch DG, Basile J, Randall PK, Miller PM. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Craig TJ, Van Natta PA. The association of smoking and drinking habits in a community sample. J Stud Alcohol. 1977;38:1434-1439. [PubMed] |
| 30. | Loftus EV, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, Melton LJ. Primary sclerosing cholangitis is associated with nonsmoking: a case-control study. Gastroenterology. 1996;110:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Ramstedt M, Boman U, Engdahl B, Sohlberg T, Svensson J. Tal om Alkohol 2010. Accessed 2011-06-21. 129 screens. Available from: http: //www.sorad.su.se/content/1/c6/04/86/13/Tal_om_Alkohol_2010.pdf. |
| 32. | Källmén H, Wennberg P, Leifman H, Bergman H, Berman AH. Alcohol habits in Sweden during 1997-2009 with particular focus on 2005 and 2009, assessed with the AUDIT: a repeated cross-sectional study. Eur Addict Res. 2011;17:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Jacob T, Seilhamer RA, Bargeil K, Howell DN. Reliability of Lifetime Drinking History among alcohol dependent men. Psychol Addict Behav. 2006;20:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 35. | Friedrich-Rust M, Müller C, Winckler A, Kriener S, Herrmann E, Holtmeier J, Poynard T, Vogl TJ, Zeuzem S, Hammerstingl R. Assessment of liver fibrosis and steatosis in PBC with FibroScan, MRI, MR-spectroscopy, and serum markers. J Clin Gastroenterol. 2010;44:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Obara N, Ueno Y, Fukushima K, Nakagome Y, Kakazu E, Kimura O, Wakui Y, Kido O, Ninomiya M, Kogure T. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J Gastroenterol. 2008;43:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |