Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3035
Revised: August 14, 2011
Accepted: March 9, 2012
Published online: June 28, 2012
Acute kidney injury (AKI), defined as an abrupt increase in the serum creatinine level by at least 0.3 mg/dL, occurs in about 20% of patients hospitalized for decompensating liver cirrhosis. Patients with cirrhosis are susceptible to developing AKI because of the progressive vasodilatory state, reduced effective blood volume and stimulation of vasoconstrictor hormones. The most common causes of AKI in cirrhosis are pre-renal azotemia, hepatorenal syndrome and acute tubular necrosis. Differential diagnosis is based on analysis of circumstances of AKI development, natriuresis, urine osmolality, response to withdrawal of diuretics and volume repletion, and rarely on renal biopsy. Chronic glomerulonephritis and obstructive uropathy are rare causes of azotemia in cirrhotic patients. AKI is one of the last events in the natural history of chronic liver disease, therefore, such patients should have an expedited referral for liver transplantation. Hepatorenal syndrome (HRS) is initiated by progressive portal hypertension, and may be prematurely triggered by bacterial infections, nonbacterial systemic inflammatory reactions, excessive diuresis, gastrointestinal hemorrhage, diarrhea or nephrotoxic agents. Each type of renal disease has a specific treatment approach ranging from repletion of the vascular system to renal replacement therapy. The treatment of choice in type 1 hepatorenal syndrome is a combination of vasoconstrictor with albumin infusion, which is effective in about 50% of patients. The second-line treatment of HRS involves a transjugular intrahepatic portosystemic shunt, renal vasoprotection or systems of artificial liver support.
- Citation: Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol 2012; 18(24): 3035-3049
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3035
The best known cause of azotemia in patients with decompensated liver cirrhosis is functional vascular renal insufficiency, which is an indirect consequence of severe peripheral arterial vasodilatation with coexistent hyperstimulation of powerful vasoconstrictor systems. Acute kidney injury (AKI) linked to this mechanism may assume a prerenal form, hepatorenal syndrome (HRS) or acute tubular necrosis (ATN). Differentiation among these three main causes of AKI has important prognostic and therapeutic implications. The diagnosis may be, however, difficult because the clinical presentations are similar and one form may convert to another . Patients with cirrhosis may also have chronic renal failure resulting from different mechanisms including renal hypoperfusion (type 2 HRS) and glomerulonephritis, related to immune or metabolic factors (Table 1).
| Acute | Chronic |
| Hypovolemia (diuretics, hemorrhage, diarrhoea) | Hepatorenal syndrome - type 2 |
| Hepatorenal syndrome - type 1 | Glomerulonephritis (HCV infection) |
| Acute tubular necrosis | Glomerulonephritis (HBV infection) |
| Nephrotoxic agents (NSAIDs, aminoglycosides, radiological contrasts) | Immunoglobulin A nephropathy1 |
| Sepsis | Diabetic nephropathy2 |
This review covers the pathophysiological conditions leading to renal hypoperfusion in the setting of portal hypertension, and the entire spectrum of acute and chronic renal diseases in chronic liver injury.
AKI is defined by the AKI Network as an abrupt (within 48 h) reduction in kidney function manifested by an absolute rise of serum creatinine of at least 0.3 mg/dL (26 μmol/L) or the equivalent to a percentage increase of 50% (1.5-fold) from baseline, or a urine output < 0.5 mL/kg per hour for > 6 h[1]. In patients with cirrhosis in whom serum creatinine levels are regularly decreased, the initial stages of AKI are commonly overlooked as increases in creatinine levels occur within the range of reference values.
HRS is a specific form of AKI that may be diagnosed only in a situation when hypercreatininemia is associated with decompensated cirrhosis (mostly in patients with diuretic-resistant ascites) or acute liver failure. Other necessary diagnostic criteria (“major criteria”), updated in 2007 by the International Ascites Club[2], include: (1) serum creatinine level > 1.5 mg/dL (133 μmol/L); (2) no improvement in serum creatinine level (decrease to 1.5 mg/dL or less) evaluated after at least 2 d under diuretic withdrawal and volume expansion with albumin given at a dose of 1 g/kg per day; maximum 100 g; (3) absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/d and microhematuria > 50 red blood cells per high power field; (4) exclusion of urinary tract outflow disturbances (normal renal ultrasonography); (5) no current or recent treatment with nephrotoxic drugs or vasodilators; and (6) absence of septic or hemorrhagic shock. Less important and not regularly occurring diagnostic features of HRS (“minor criteria”) are urine volume < 400 mL/d, low sodium concentration in the serum (< 130 mEq/L) and urine (< 10 mEq/L).
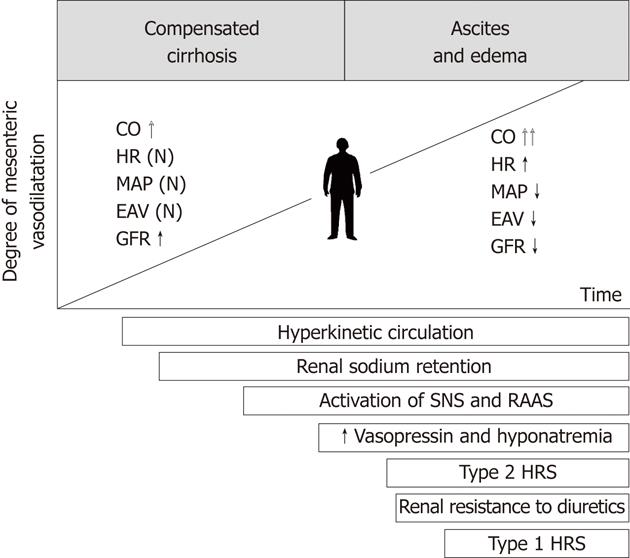
There are two types of HRS. In type 1, renal function deteriorates rapidly with doubling of the initial serum creatinine level to > 2.5 mg/dL (220 μmol/L) in < 2 wk. Development of type 1 HRS may be preceded by type 2 HRS, which is a chronic renal failure lasting several weeks to months, with serum creatinine levels in the range of 1.5-2.5 mg/dL. The annual risk of type 1 HRS development in patients with decompensated cirrhosis is about 20%, and within 5 years, it increases to 40%[3,4]. Some authors have singled out a type 3 HRS, which is an overlap of functional renal failure on an already existing chronic or acute intrinsic kidney disease. In differential diagnosis, it is important to consider that some kidney diseases are genetically linked with specific liver pathologies (e.g., autosomal dominant renal polycystic syndrome and oxalosis), and certain systemic diseases simultaneously affect the liver and kidneys (Table 2)[2].
| Drug-induced hepato-nephrotoxicity (acetaminophen, aspirin, NSAIDs) |
| Granulomatous diseases (e.g., sarcoidosis, leptospirosis) |
| Storage diseases (e.g., amyloidosis) |
| Systemic autoimmune diseases (e.g., lupus erythematosus) |
| Non-alcoholic fatty liver disease and diabetic nephropathy |
| Autosomal dominant polycystic kidney disease |
| Wilson’s disease |
| Pregnancy-induced liver diseases (pre-eclampsia /HELLP syndrome) |
| Shock (cardiac failure, sepsis, hemorrhage, dehydration) |
| Alpha1-antitrypsin deficiency |
Renal blood perfusion and the glomerular filtration rate (GFR) depend on systemic and local factors such as cardiac output, systemic mean arterial pressure, basal muscular tone of renal arterioles, renal vascular autoregulation and intra-abdominal pressure. All these factors are considerably affected by end-stage liver cirrhosis as, in this disease, the mean arterial pressure is decreased, the renal adrenergic tone is enhanced, cardiac performance is diminished as a consequence of chronic heart overload, renal synthesis of vasoprotective peptides is reduced, and intra-abdominal pressure increases due to accumulating ascites.
In patients with decompensated cirrhosis, arterial vasodilatation affects many vascular territories such as the skin, skeletal muscles, brain and lungs. However, it is most accentuated in the splanchnic area involving the intestines, pancreas and mesentery[5]. On one hand, the pathophysiological significance of splanchnic vasodilatation relies on the maintenance of a high portal pressure and, on the other hand, on disturbances of hemodynamic homeostasis in systemic circulation.
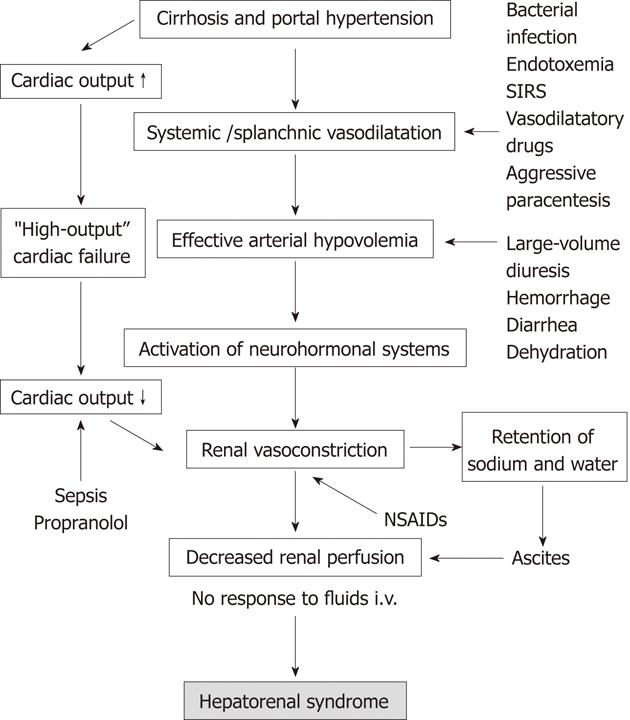
Splanchnic vasodilatation appears in the early stages of cirrhosis, however, it remains masked by increased cardiac output. At this point, the GFR is increased and glomeruli may be hypertrophied[5,6]. Splanchnic vasodilatation increases along with the progression of liver injury, portal hypertension and mesenteric angiogenesis. In advanced cirrhosis, the hyperkinetic cardiac function characterized by high stroke volume and tachycardia is no longer able to compensate for significant enlargement of the arterial vascular compartment. The disproportion between the blood volume and capacity of the arterial vascular tree leads to reduction of the effective blood volume and arterial pressure. In this setting, the baroreceptors located in the central circulation trigger hyperstimulation of the sympathetic system, renin-angiotensin-aldosterone axis and hypothalamic vasopressin secretion[7]. These adaptive mechanisms help to maintain arterial blood pressure at a safe level. Another mechanism contributing to this adaptation is renal retention of sodium and water. Endogenous vasoconstrictors have limited impact on the splanchnic arterial system, due to its intrinsic hyporeactivity[8]. By contrast, they strongly constrict renal arterioles initiating functional renal failure. The contributing mechanism to renal hypoperfusion is failure of renal vascular autoregulation, having its source in the impaired synthesis of vasodilators within the kidney (mainly prostaglandin E) and increased local release of angiotensin II and endothelin[9,10]. The chronology of pathophysiological events underlying HRS is shown in Figure 1.
Substances most commonly incriminated in splanchnic vasodilatation are the intestinal and pancreatic vasoactive peptides, which reach the systemic circulation through portosystemic collaterals. Among compounds having myorelaxing properties are glucagon, vasoactive intestinal peptide, adrenomedulin, endogenous cannabinoid receptor agonists, substance P, natriuretic factor and carbon monoxide[11]. Major attention, however, has been focused on endothelial factors; that is, nitric oxide (NO) and prostacyclin. Results of many studies have provided evidence for increased production of NO in the arterial vascular system, as in cirrhosis, both the constitutive and inducible isoforms of NO synthetases are activated[12].
Cardiac failure in cirrhosis, named portal cardiomyopathy, has a functional background and disappears after liver transplantation. It comprises systolic and diastolic dysfunctions, mainly of the left heart chamber, and electromechanical abnormalities including a prolongation of the Q-T interval. Diastolic heart dysfunction precedes abnormalities of systolic cardiac performance. Left ventricular end-diastolic pressure is elevated in patients with decompensated cirrhosis[13]. Rapid hemodynamic changes occurring, for example, after a transjugular intrahepatic portosystemic shunt (TIPS), or liver transplantation may cause a striking increase in filling pressure favoring the development of congestive heart failure.
At rest, the portal cardiomyopathy is camouflaged by a low afterload (low peripheral vascular resistance), but limited cardiac reserve may be unveiled by hemodynamic stress (e.g., physical activity, TIPS, voluminous intravenous infusion or use of vasoconstrictor), which is followed by significantly lower increments in the stroke volume than in healthy persons[14].
Severe infections resulting in septic shock syndrome are known to induce cardiodepression due to the emergence of inflammatory mediators having negative inotropic effects. Reduction of cardiac output and inadequate cardiac contractile response to inflammatory stress is an important cause of HRS development in patients with end-stage liver cirrhosis. Ruiz-del-Arbol et al[15] have studied a group of 23 patients with spontaneous bacterial peritonitis (SBP) that was treated with antibiotics. The eight patients who developed HRS had significantly lower baseline cardiac output than the 15 patients without renal dysfunction. In the subgroup of patients with HRS, the cardiac output further declined with a concurrent drop in arterial pressure.
Liver cirrhosis is associated with hypervolemia as a consequence of renal sodium retention. In conditions of splanchnic vasodilatation and increased cardiac output, the blood is preferentially pooled in the portal venous system. High hydrostatic pressure in this system accompanied by hypoalbuminemia is responsible for plasma escape from the vascular compartment to the peritoneal cavity. This phenomenon mainly occurs at the level of sinusoidal vessels and to a lesser degree at intestinal capillaries.
Ascites is responsible for a worsened quality of life, risk of SBP and renal failure[7]. The independent variables of mortality in ascitic patients are Child-Pugh class C, hyponatremia renal failure and, as has recently been suggested, β-blocker therapy[16].
Tense ascites causes an increase in intra-abdominal pressure, giving rise to abdominal compartment syndrome, which is a known risk factor for AKI developing in critically ill patients with acute abdominal disorders such as peritonitis, acute pancreatitis, ileus, trauma or after urgent abdominal surgeries[17]. Intra-abdominal pressure > 20 mmHg, measured in the urinary bladder, leads to severe impairment of renal venous blood flow and secondary disturbances in arterial perfusion of the kidneys[18].
Fluid removal in volume-overloaded patients with decompensated heart failure (refractory to intensive medical therapy) caused an improvement in renal function corresponding to a reduction of persistently elevated intra-abdominal pressure[19]. Knowledge of the actual influence of tense ascites on kidney function in cirrhosis is not complete. It has been shown in pilot studies that large-volume paracentesis (LVP) followed by intravenous administration of albumin ameliorates renal function in patients hospitalized for HRS or esophageal varices bleeding[20-22]. LVP should be followed by diuretics to prevent reaccumulation of fluid.
HRS develops spontaneously in about 50% of cases and it is triggered by identifiable factors dependent or independent of medical activities in the remaining cases. Pathogenesis of HRS with triggering factors is shown in Figure 2.
Patients with cirrhosis have a particular predisposition to bacterial infections that is mostly a result of impaired reactivity of reticuloendothelial cells and neutrophils. Bacterial infections develop in 30%-60% of patients with cirrhosis, being responsible for about 25% of overall mortality in this disease[23]. The most common site of infection is the ascitic fluid, and less frequent are the respiratory system, urinary tract or subcutaneous tissue. Gastrointestinal bleeding, irrespective of its source, is associated with increased translocation of intestinal bacteria through ischemic mucosa and very high risk of SBP.
Bacterial infection induces HRS because of aggravation of splanchnic arterial vasodilatation and deterioration of liver function by endotoxemia and cytokine overproduction. Type 1 HRS commonly develops in patients with SBP, especially in those with serum bilirubin levels > 4 mg/dL (68 μmol/L) or serum creatinine levels > 1 mg/dL (88 μmol/L) - that is, in patients with pre-damaged kidneys by type 2 HRS or by other causes[24].
In a randomized trial comparing daily norfloxacin with placebo, it was demonstrated that a low content of protein in the ascitic fluid (< 1.5 g/dL) and severe liver disease indicated a beneficial effect of this antibiotic on the development of SBP and survival[25]. It seems reasonable to use norfloxacin (or trimethoprim/sulfamethoxazole) continuously in patients that meet these criteria[26].
Inflammation developing in cirrhotic patients has been shown to favor serious complications specific for liver failure and portal hypertension, including HRS[27,28]. Systemic inflammatory response syndrome (SIRS) is mostly triggered by overt or occult bacterial infection but also may result from activation of the inflammatory pathways related to circulating endotoxins or proinflammatory mediators. Accordingly, SIRS occurs in many nonbacterial abdominal acute inflammatory diseases such as pancreatitis, alcoholic steatohepatitis or portal venous thrombosis. It is also speculated that SIRS may be induced by liver necrosis associated with a “spill over” of inflammatory mediators of hepatic origin. All these conditions generate production of proinflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α)], which enforce the endothelial cells to produce more NO, playing an important role in the pathogenesis of arterial vasodilatation.
According to the American Society of Critical Care Medicine Consensus Conference, SIRS is diagnosed if the patient fulfills at least two of the following criteria: (1) core temperature > 38 °C or < 36 °C; (2) heart rate ≥ 90 beats per minute; (3) respiratory rate ≥ 20 breaths per minute; or (4) white blood cell count ≥ 12 G/mm3 or ≤ 4 G/mm3 with a differential count showing ≥ 10% immature polymorphonuclear neutrophils[29]. An important limitation for use of these criteria is the fact that many of them may be modified by cirrhosis itself.
Diarrhea and vomiting lead to dehydration and hypovolemia, which can aggravate the hemodynamic disturbances responsible for the development of HRS. Diarrhea is a common problem associated with alcoholism. Moreover, the diarrhea and vomiting occur as a consequence of infectious diseases but also may have an iatrogenic background. Diarrhea is a side effect of drugs commonly used in cirrhotic patients, such as lactulose or ursodeoxycholic acid. An increased number of bowel movements in patients with decompensated cirrhosis should prompt the withdrawal or dose reduction of these drugs.
Patients with peripheral edema tolerate aggressive diuresis because the fluid from the subcutaneous tissue is easily absorbed to the intravascular system. In such patients, the daily reductions of body weight may reach 2 kg. Using high doses of diuretics following the disappearance of peripheral edema frequently leads to hypovolemia, which initiates the development of HRS. In patients with ascites without peripheral edema, the urinary output should not be higher than 1100 mL/d, with diurnal reductions of the body weight not exceeding 0.5 kg. This principle originates from the fact that the diurnal ability of ascites to relocate from the peritoneal cavity to the vascular system is limited to 700-900 mL, and in the setting of postinflammatory peritoneal lesions, this limitation is even more severe. Diuretics should be discontinued if the serum creatinine is > 2.0 mg/dL (180 μmol/d) and serum sodium is < 120 mEq/L, despite fluid restriction, or in the case of encephalopathy[26,30]. Intravenous use of furosemide is not recommended as a dose of 80 mg has been shown to cause an acute reduction in renal blood flow and subsequent azotemia in patients with cirrhosis and ascites[26].
Patients with cirrhosis carry a significant risk of bleeding from the upper digestive tract. The most likely sources of bleeding are esophageal/gastric varices, portal gastropathy or gastroduodenal peptic ulcers. A 2-year risk of bleeding from large esophageal varices in patients who never bled was 25%-30%, and in those who had at least one episode of bleeding in the past exceeded 60%[31]. Acute bleeding from the upper digestive tract leads to hypovolemia and a decrease of renal perfusion. The probability of HRS development in bleeding patients depends on the amount of blood loss, the functional reserve of the liver, and the presence of bacterial infection. In one study, hemorrhagic shock and HRS were independent risk factors of in-hospital mortality. If HRS developed in a bleeding patient, the mortality rate was 55% (3% in patients without renal failure)[32].
LVP is considered to be a relatively safe and effective procedure for the treatment of refractory ascites. However, removal of > 5 L of fluid without blood volume expansion may lead to paracentesis-induced circulatory dysfunction (PICD). Ascitic patients show increased heart rate and cardiac output and low diastolic arterial pressure, which are accentuated after LVP[33-35]. Mechanisms of PICD are not entirely clear, but LVP causes further reduction of volemia in the central circulation with simultaneous stimulation of all vasoconstrictive systems, having a deleterious effect on renal function. According to earlier studies, HRS develops in about 10% of cirrhotic patients treated with LVP[36]. It has been recently shown that use of β-blockers may be associated with an increased risk of PICD in patients with cirrhosis and refractory ascites[37].
Recognition of PICD is based on finding a significant increase in plasma renin activity (> 50% of pretreatment values on days 5-7 after paracentesis). According to this definition, Nasr et al[38] recognized PICD in 73.3% of patients with tense ascites despite volume expansion (with dextran in 87.5% and with albumin in 38.5% of patients). In this study, PICD was a clinically silent complication with no clinical or laboratory predictive factor.
At present, salt-free human albumin is the plasma expander of choice, if at least 5 L of ascitic fluid is evacuated. The role of vasopressors in PICD escape is still uncertain. In the randomized study including 24 patients with decompensated cirrhosis, midodrine was not as effective as albumin in preventing PICD, because this syndrome developed in six patients in the midodrine group (60%) and in only four patients (31%) in the albumin group[39]. By contrast, in two randomized studies with 20 and 40 ascitic patients, terlipressin and midodrine were not less effective than albumin for prevention of PICD[40,41].
Renal perfusion in advanced cirrhosis greatly depends on vasoprotective mechanisms attributed to prostaglandin E2 and prostacyclin (“prostaglandin dependency”). Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit renal synthesis of prostaglandins, therefore, they impair vasoprotective mechanisms. Generally, NSAIDs should be avoided in patients with cirrhosis because in this population, they induce renal failure in 33% of patients (3%-5% in the population with an intact liver)[42]. Moreover, NSAIDs are responsible for the development of resistance to diuretics[43]. Renal complications are also observed after use of selective cyclo-oxygenase 2 antagonists such as celecoxib and rofecoxib. Even short-term therapy with celecoxib induces significant reduction in GFR in patients with decompensated cirrhosis[44].
In patients with advanced liver cirrhosis, the homeostasis of arterial pressure is highly dependent on adaptive neurohormonal mechanisms. Administration of drugs inhibiting these mechanisms; that is, inhibitors of angiotensin converting enzyme (ACE), angiotensin II receptor antagonists or clonidine, increases the risk of a significant reduction in arterial pressure, resulting in functional renal failure. These drugs may affect renal function irrespective of arterial hypotension. It has been shown that the administration of small doses of ACE inhibitors, having no effect on arterial pressure, cause a significant decline in GFR[45]. However, not all studies have confirmed the harmful effects of anti-angiotensin drugs on the kidneys[46,47].
Treatment of renal failure in advanced liver cirrhosis aims to abate the most important pathophysiological factors responsible for the development of HRS. The first-line treatment is focused on reduction of portal hypertension and counteracting the arterial vasodilatation and effective hypovolemia, by vasoconstrictor and albumin infusion. The second-line treatment involves a reinforcement of renal vasoprotection, kidney replacement therapy or artificial liver support.
Albumin is the preferred volume expander in patients with renal failure. Except for augmentation of oncotic pressure, the albumin binds endotoxins, bile acids, bilirubin, fatty acids and NO; therefore, it joins metabolic, immune and vasoconstrictor effects[48,49]. Albumin should be infused in high percentage solutions (20%-25% albumin) and the typical dosage is 1 g/kg (up to 100 g) on day 1, and 20-60 g/d thereafter.
Use of albumin may prevent the development of HRS. In a non-placebo-controlled study, the plasma volume expansion with albumin has been shown to reduce the incidence of renal failure in cirrhotic patients hospitalized for SBP as compared with antibiotic monotherapy[24]. Moreover, an unblinded randomized controlled trial in patients with new-onset ascites demonstrated that weekly 25-g infusions of albumin for 1 year followed by infusions every 2 wk improved survival compared to treatment with diuretics alone[50]. By contrast, treating HRS with albumin monotherapy is only minimally effective, but adding albumin to vasoconstrictors provides a supplementary benefit. In a recent study, 46 patients with HRS were randomly assigned to treatment with terlipressin plus albumin or to albumin monotherapy[51]. Renal function improved in 43.5% of patients treated with terlipressin plus albumin, and in only 8.7% of patients treated with albumin alone.
Many pharmaceutical agents, predominantly vasoconstrictors along with albumin infusion, have been studied in patients with type 1 HRS. These studies are based on controlled or uncontrolled short case series. Splanchnic vasoconstrictors improve effective arterial blood volume and paradoxically dilate renal vasculature. The optimal choice of vasoconstrictor, time for initiation and duration of therapy and most favorable dosage remains to be determined. Vasoconstrictor drugs should not be given to patients with ischemic disease of the heart, brain or lower extremities. Their use is also contraindicated in patients with heart failure, cardiac arrythmias, asthma or respiratory failure.
Terlipressin is a synthetic analog of vasopressin that is characterized by a longer half-time (i.v. administration every 4-6 h) and fewer side effects (ischemic complications occurring in 5%-12% of patients) in comparison with the parent drug[52-54]. Although terlipressin is only a partial agonist of renal vasopressin V2 receptors, an acute reduction in serum sodium concentration is a common finding during use of this drug, and is sometimes associated with reversible neurological complications[55].
Treatment with terlipressin and albumin leads to the normalization of renal function in 34%-65% of patients with type 1 HRS and the recurrence of HRS after treatment withdrawal occurs in 15%-22% of cases[52,55-60]. The most applicable predictor of HRS reversal seems to be baseline serum creatinine[61]. Terlipressin plus albumin therapy increases short-term survival by 34%-43%[56-58]. In a multivariate analysis, the predictors of improved survival at 6 wk were: a significant reduction in serum creatinine between baseline and day 4, a serum level of bilirubin < 10 mg/dL and a treatment-induced increase of the mean arterial pressure by > 5 mmHg[62]. However, not all studies have shown survival benefits for terlipressin. A European multicenter, randomized, controlled trial of terlipressin and albumin vs albumin monotherapy in 46 patients with both types of HRS demonstrated an improvement in renal function but no survival advantage at 3 mo[50]. Hence, liver transplantation is still the optimal therapy for HRS, and use of terlipressin is considered as a bridge to transplantation. According to recent recommendations of American Association for the Study of Liver Diseases, patients with type 1 HRS should have an expedited referral for liver transplantation[26].
Somatostatin is a hormone that, in pharmacological doses, decreases splanchnic arterial blood flow. This effect is not a result of intrinsic vasoconstrictive properties, but rather comes from inhibitory effects on the release of vasoactive intestinal and pancreatic peptides. Compared with terlipressin, somatostatin exerts a less beneficial effect on renal sodium excretion in patients with or without ascites[63]. Octreotide is a synthetic analog of somatostatin, with similar hemodynamic effects. Two studies, including one with randomization and a crossover design, demonstrated that octreotide alone is not effective for type 1 HRS[64,65]. Acute administration of octreotide to cirrhotic patients, with or without ascites, did not produce any change in the GFR or in the estimated renal plasma blood flow[66]. Unfortunately, octreotide significantly decreased the free water clearance and fractional excretion of filtered sodium[67]. Also chronic use of octreotide had no renoprotective effects, because improvement in renal blood flow was associated with a reduced GFR[68].
Midodrine is an oral α1-adrenergic agonist with vasoconstrictive properties. In three pilot studies involving 79 patients with type 1 HRS, short-term administration of midodrine in conjunction with octreotide caused significant changes in systemic hemodynamics and normalization of renal function in 49% of patients[69-71]. It is unknown whether these beneficial effects are attributable to midodrine alone or also to the potentiating effects of octreotide. However, favorable renal effects of this regimen may disappear under chronic treatment. In 1 mo therapy of refractory ascites with midodrine, octreotide and albumin, significant reductions in the plasma renin and aldosterone concentrations were found, but renal function tests were not improved[72]. In a retrospective study involving 60 patients with type 1 HRS treated with midodrine, octreotide plus albumin and 21 nonrandomized albumin-treated controls, the 30-d mortality was reduced in the treatment group (43% vs 71%, respectively, P < 0.05)[69].
The regimen composed of midodrine, octreotide and albumin may be used outside of an intensive care unit. This is not the case for norepinephrine, which requires continuous intravenous infusion and hemodynamic monitoring. This drug is infrequently used in treatment of HRS, although efficacy of norepinephrine in reversal of renal failure may be not inferior to terlipressin[73].
Apart from systemic causes, the development of HRS in decompensated cirrhosis is dependent on the failure of local vasoprotective factors. Until now, no drugs enforcing renal vasoprotection have been used as routine treatment for HRS. Although earlier studies have suggested the reversal of HRS during treatment with misoprostol, a prostaglandin E1 analog[74], the high doses and side effects have prevented this drug from use in cirrhotic patients.
Pentoxifylline is an inhibitor of TNF-α, which plays a major role in the pathogenesis of alcoholic hepatitis and endotoxin-mediated liver injury[75]. Meta-analysis of five trials, with a total of 336 randomized participants with alcoholic hepatitis, showed a positive effect of pentoxifylline on mortality associated with HRS[76]. The usefulness of pentoxifylline in other liver diseases is unclear. In a recent French study, the chronic peroral use of pentoxifylline (400 mg, 3 times daily) in patients with advanced cirrhosis of a different etiology did not decrease short-term mortality, however, it reduced the risk of complications (including AKI) at 2 mo and 6 mo[77].
Implantation of TIPS augments the return of blood to the right heart, thus it counteracts the central hypovolemia and neurohormonal activation. In several studies, the plasma renin activity, norepinephrine concentrations and excretion of sodium improved gradually after insertion of the TIPS[78-80]. In a large study involving 129 cirrhotic patients with varying degrees of baseline renal function, TIPS has been shown to improve renal function. Patients with pre-treatment serum creatinine levels between 1.2 and 1.9 mg/dL had reduced mean levels of creatinine, from 1.5 to 1.1 mg/dL, and those with pre-TIPS creatinine levels > 2.0 mg/dL showed a reduction from 2.8 to 1.5 mg/dL. This means that patients with poorer renal function benefit the most from TIPS[81]. A meta-analysis including five randomized controlled trials (330 refractory ascites patients) showed that TIPS improved survival when compared with repetitive paracenteses[82].
Decision on insertion of TIPS must be balanced against the risk of complications, which include: (1) portal encephalopathy; (2) liver insufficiency; (3) loss of stent function; (4) cardiac failure; (5) bacteremia; (6) hemolysis; or (7) bacterial infection of the stent[83,84].
Continuous or repeated dialysis may be considered as rescue therapy for patients with type 1 HRS, in whom pharmacological treatment is ineffective and there are no contraindications for liver transplantation. Independent indications for use of dialysis are hypervolemia not responding to diuretics, metabolic acidosis and refractory hyperkalemia. Dialysis is a bridging therapy aimed at keeping the patient alive until receiving the graft. A more recent study has reported that eight of 30 patients with HRS survived 30 d with use of dialysis or continuous venovenous hemodialysis in the intensive care unit setting[85].
In patients with cirrhosis, the hypotensive reactions and blood clotting abnormalities are more frequent during dialysis than in patients with an intact liver. Peritoneal dialysis may be better tolerated by cirrhotic patients than hemodialysis, with no increase in the number of complications[86]. This procedure enables removal of the ascites fluid and does not expose patients to anticoagulants.
Patients who are on continuous renal replacement therapies require anticoagulation to prevent thrombosis. Different anticoagulants have been used to ensure the patency of hemodialysis catheters. In patients who are at high risk for bleeding, nonstandard preventive options are applied, such as minimum-dose or no-heparin regimens or regional citrate anticoagulation, which are associated with low risks of bleeding and severe dialyzer clotting[87]. Sodium citrate is metabolized by the liver and the body clearance of this compound has been shown to be significantly reduced in critically ill cirrhotic patients. In addition, citrate clearance cannot be predicted by standard liver function tests and is not influenced by renal function[88]. It is, therefore, advisable to minimize the dose of citrate anticoagulation and monitor serum ionized calcium level and blood pH in hemodialyzed cirrhotic patients.
At present, there are a few systems of artificial liver support, such as: Molecular Adsorbent Recirculating System (MARS), Fractionated Plasma Separation, Adsorption and Dialysis System and Single-Pass Albumin Dialysis Extended. These systems are designed to remove from the blood toxins, which are soluble in water and are adsorbed on albumin molecules. It seems that this kind of therapy may only achieve a temporary improvement in metabolic abnormalities that result from liver and kidney insufficiencies.
In pilot studies, MARS caused a statistically significant decrease of serum bilirubin and creatinine levels in patients with type 1 HRS who were disqualified from other forms of treatment. In addition, in a randomized study, the use of MARS led to increases of arterial blood pressure and urinary sodium output[89]. However, in a recent small study comprising six patients with type 1 HRS not responding to vasoconstrictor treatment, MARS was shown to be ineffective regarding systemic hemodynamics and renal function[90]. Experience with other artificial liver support systems in HRS treatment is limited[91]. Patients undergoing treatment with adsorbent recirculating systems can experience declines in blood pressure, hypothermia, bradycardia, tissue hypoxia or blood clotting abnormalities associated with use of anticoagulants.
Epidemiological data suggest that AKI in cirrhosis has a prerenal origin in 60% of cases, and only one third of these patients fulfills the criteria of type 1 HRS[92]. Intrinsic renal injury in the form of ATN may be responsible for about 40% of AKI in cirrhotic patients with ascites[93]. Type 2 HRS is the most common form of chronic renal disease. The Multidisciplinary Working Party has defined chronic renal disease as an estimated GFR < 60 mL/min for > 3 mo[94]. Chronic glomerular injury related to viral, immune and metabolic factors is usually an asymptomatic disease with mild proteinuria and hematuria. Occurrence of arterial hypertension in liver cirrhosis is a rare finding indicating a possibility of chronic glomerulonephritis. Among hospitalized patients with decompensated cirrhosis, chronic glomerulonephritis is responsible for about 1% of all causes of azotemia[92].
ATN should be suspected in all cases of AKI preceded by septic or posthemorrhagic shock, prolonged dehydration, severe pancreatitis, exposure to nephrotoxic substances (aminoglycosides, contrast agents) or major surgical interventions[95].
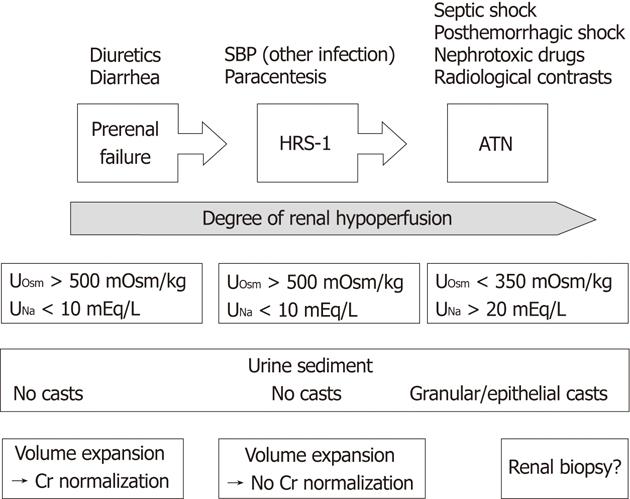
In clinical practice, the distinction between ATN and type 1 HRS may be difficult (Figure 3). Usually, in patients with HRS, the urinary concentration of sodium does not exceed 10 mEq/L (preserved tubular ability for sodium reabsorption and urine concentration), whereas in patients with ATN, it is > 20 mEq/L with urine osmolality < 350 mOsm/kg. However, these criteria may be misleading in certain cases. Another finding favoring a diagnosis of ATN is the presence of bile-stained granular or epithelial casts in the urine, but this finding is also not definitive[95]. It is expected that a positive response to a vasoconstrictor plus albumin should rule out a diagnosis of ATN, however, a recent study has demonstrated some benefit of terlipressin in patients with cirrhosis and ATN[55]. Studies on using urine biomarkers for ATN are underway and the most promising candidates are interleukin-18, urinary kidney injury molecule 1 and urinary neutrophil gelatinase-associated lipocalin[96,97].
Reduction in GFR in ischemic ATN results from injury to the tubular epithelium and impaired glomerular capillary pressure. An overlapping factor may be tubular obstruction from casts composed of detached epithelial cells and cellular debris as well as hemoglobin and myoglobin deposits[98]. ATN caused by ischemia lasts 7-21 d[99] and, in most patients, renal function returns to normal or near normal levels following regeneration of the tubular epithelium. Regenerating cells derive from the stem cells that have survived the ischemic insult. It is also likely that migrating bone marrow stem cells contribute to this process[100,101]. During ATN-related renal failure, patients should be treated with renal replacement therapy, which may last 6-8 wk if tubular damage is caused by toxic agents[92].
Aminoglycoside antibiotics still constitute an important therapeutic alternative against germs that are insensitive to other antibiotics[102]. Nephrotoxicity is the most important therapeutic limitation of aminoglycoside antibiotics, especially gentamicin. The typical manifestation of aminoglycoside toxicity is nonoliguric or even polyuric renal dysfunction accompanied by proteinuria, hypercalciuria, hypermagnesemia, aminoaciduria, glycosuria, and serum electrolyte alterations[103]. Despite rigorous patient monitoring, kidney injury appears in 10%-25% of therapeutic courses. Traditionally, aminoglycoside nephrotoxicity has been considered to result mainly from tubular damage, but recently the role of reduced glomerular filtration has also been raised[104].
For patients with chronic liver diseases, the use of gentamicin or tobramycin considerably enhances the risk of damaging the tubules and onset of acute renal insufficiency[103,105]. The particular sensitivity of cirrhotic patients to aminoglycosides may be related to sodium depletion, concomitant use of diuretics and reduced renal function. Other drugs that can lead to AKI are amphotericin, acyclovir, penicillin and cytostatics.
Use of contrast media is a well-known cause of tubular injury, particularly in the presence of predisposing conditions like dehydration or diabetic nephropathy. Mechanisms of contrast-induced nephropathy (CIN) are complex and not fully explained. It has been shown that renal blood flow remains 45% below baseline level for at least 4 h following contrast administration[106]. Renal vasoconstriction with medullary hypoxia may be mediated by high osmolality and viscosity of contrast agents, as well as the release of endothelin and adenosine[107]. The cytotoxic effects including the generation of oxygen free radicals are also considered. Currently used iodinated contrasts are either ionic and hyperosmotic (1400-1800 mosmol/kg) or nonionic iso- or low-osmolal, characterized by lower nephrotoxicity[108]. Application of contrast media in cirrhotic patients with hypercreatininemia is not recommended, because it is associated with a high risk of AKI. This applies to use of contrast in both diagnostic and therapeutic procedures[109]. Patients with cirrhosis and normal renal function do not seem to be hypersensitive to CIN. In a retrospective case-control study including 72 patients with cirrhosis and 72 patients without cirrhosis, the intravenous administration of 100-150 mL of low osmolality radiocontrast medium induced AKI in two patients with cirrhosis and one patient in the control group (difference not significant). The cirrhotic patients who developed CIN had received high-dose diuretics and were hypovolemic[110].
The most common form of glomerular disease found in patients with chronic hepatitis C is membranoproliferative glomerulonephritis (MPGN)[111,112], followed by membranous nephropathy, focal glomerulosclerosis and immunoglobulin A (IgA) nephropathy[113-115]. In autopsy studies, the glomerular pathology was detected in more than half of hepatitis C patients. Histopathological features of MPGN were present in 11.2%, membranous nephropathy in 2.7%, mesangioproliferative glomerulonephritis in 17.6% and mesangial expansion without proliferation in 23.4% of deceased hepatitis C patients. Cirrhosis was associated with a higher percentage of inflammatory changes in glomeruli compared with the precirrhotic stage of liver disease (59.2% vs 32.3%)[116]. MPGN is associated with mixed essential (type II) cryoglobulinemia. Renal biopsy revealed the presence of cryoglobulins in about 70% of hepatitis C patients with MPGN[117]. This finding strongly indicated an etiological relationship between cryoglobulinemia and MPGN.
Successful treatment of noncirrhotic hepatitis C patients with interferon-α is probably paralleled with a favorable effect on MPGN, because a positive response to treatment was associated with a significant reduction in serum levels of cryoglobulin and creatinine[118]. One study has shown the efficacy of rituximab in the treatment of type-II-cryoglobulinemia-related MPGN in patients with hepatitis C[119].
MPGN, membranous nephropathy and polyarteritis nodosa create a wide spectrum of glomerular lesions found in patients infected with hepatitis B virus (HBV). The prevalence of glomerulonephritis among patients with chronic hepatitis B is significantly higher in endemic regions, where the hepatitis B surface antigen carrier state is common[120,121]. Chronic carriage of HBV leads to the development of nephrotic syndrome in some individuals (particularly children), with a strong male predominance. Studies showing expression of HBV viral antigens in kidney tissue confirm the role of the virus in the development of glomerulonephritis[120,122].
In children, the membranous nephropathy often retreats spontaneously, whereas in adults it may show rapid progression[120]. The main symptom of membranous nephropathy is nephrotic syndrome, with the cause not revealed until a renal biopsy is done. Retrospective analysis of clinical studies involving small groups of hepatitis B patients with membranous nephropathy or MPGN has indicated a beneficial role of antiviral therapy. Positive virological responses to treatment with interferon-α2-A or lamivudine were associated with significant improvement in renal function[123,124].
IgA nephropathy is the most common form of primary glomerulonephritis in developed countries[125,126]. The disease may appear at any age with the highest prevalence in the second and third decade of life. The incidence of mesangial IgA deposition in apparently healthy individuals ranges from 3% to 16%[127]. Usually, IgA nephropathy is recognized on routine urine screening with asymptomatic hematuria and/or proteinuria. The diagnosis may be established only by renal biopsy that discloses globular IgA deposits in the mesangium, and to a lesser degree in the glomerular capillary walls (immunofluorescence microscopy)[125,128].
Class A immunoglobulins (with or without small quantities of other immunoglobulins and C3 complement) are found in glomeruli of 35%-90% of patients with liver cirrhosis[129]. In these patients, IgA nephropathy is usually subclinical and the features of nephrotic syndrome occur in about 1.5% of cases. Hematuria is found less frequently than in the primary form of IgA nephropathy. This type of nephropathy may be particularly common in patients with alcoholic cirrhosis, in whom IgA serum levels are regularly increased[130].
Isoform IgA2 is cleared from circulation with the participation of the hepatic asialoglycoprotein receptor, whereas IgA1 only partially uses this elimination pathway[131]. The pathogenesis of IgA nephropathy coexisting with liver cirrhosis is unknown, however, a hypothesis of reduced hepatic clearance of immunological complexes, due to impaired asialoglycoprotein receptor function and phagocytosis by Kupffer cells, is very likely. The direct influence of immunological mechanisms should also be considered, as they play an important role, not only in autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis, but also in hepatitis B and C, alcoholic liver disease, primary hemochromatosis and α-1 antitrypsin deficiency[132-136].
Diabetic nephropathy (DN) occurs in types 1 and 2 diabetes mellitus. The epidemiology of DN has been studied in type 1 diabetes. Twenty to thirty percent of this population presents with microalbuminuria 15 years after diabetes diagnosis and almost half of those patients will progress to overt nephropathy[137]. Development of DN is strongly dependent on the control of glycemia and arterial blood pressure. The aggressive treatment of arterial hypertension and use of ACE inhibitors have been shown to reduce the rate of DN progression[138]. In Caucasians with type 2 diabetes, the prevalence of progressive renal disease seems to be lower than in type 1[139], however, there are also some data suggesting that the risk of renal injury is currently equivalent in both types of diabetes[140,141].
The mesangial expansion, thickening of glomerular basement membrane and glomerular sclerosis belong to major histological changes of DN. The late abnormality is deposition of hyaline in the glomerular arterioles, which may assume a nodular appearance[142]. DN usually leads to arterial hypertension, renal arteriosclerosis, proteinuria and fluid retention. Patients with DN may tolerate less spironolactone because of hyperkalemia.
Among patients with cirrhosis related to HCV infection, diabetes occurs in about 25% of cases. Even more frequent is the occurrence of diabetes in patients with cirrhosis related to nonalcoholic steatohepatitis, which like diabetes, is a feature of the metabolic syndrome. The percentage of glucose intolerance in the population of patients with liver cirrhosis of different etiology approaches 60%[143,144]. For these reasons, DN is one of the most common causes of chronic renal failure in patients with cirrhosis. Diabetes promotes vascular pathology, hence, advanced atherosclerosis of the coronary arteries is recognized in 20%-25% of candidates for liver transplantation[145].
An obstruction in urine outflow as the cause of renal failure is found in < 1% of patients with liver cirrhosis. Postrenal causes of renal insufficiency are similar to those occurring in the general population. They include nephrolithiasis, benign prostatic hyperplasia in men, ureter infiltration by tumors of the reproductive organs in women, kidney tumors, or neurogenic urinary bladder. Patients with alcoholic liver disease seem to have an increased risk of renal papillary necrosis that, after sequestration, obstructs urine outflow[146]. The diagnosis of postrenal failure is based on imaging studies (i.e., ultrasound and computed tomography), which show urine retention or hydronephrosis. The aim of the treatment is removal or bypassing of the obstacle.
Parameters of renal function are strong predictors of survival in liver cirrhosis, because vascular-mediated renal failure is one of the latest events in the natural history of chronic liver disease. The median survival time in patients with type 2 HRS is about 5 mo, but with type 1 of this syndrome, is as short as 2-4 wk[25] .
Generally, the creatinine level is not a perfect indicator of renal function as it is influenced by many nonrenal factors (e.g., body weight, age, sex, race and blood volume), shows a nonlinear relationship with GFR and does not distinguish between functional and organic renal diseases. Still, a less reliable indicator of renal function is the serum level of blood urea nitrogen, which is strongly modified by the diet or the presence of blood within the digestive tract.
The creatinine levels in patients with end-stage cirrhosis regularly overestimate the actual GFR because of decreased hepatic production of creatine and muscle wasting[24]. An additional factor responsible for low serum creatinine in decompensated cirrhosis is hypervolemia, and accordingly, the increased volume of distribution for this substance. For these reasons, the early stages of AKI usually remain unrecognized in cirrhotic patients. Moreover, in jaundiced patients, the measurement of creatinine may provide erroneous results due to chromogenic interference with bilirubin in a spectrophotometric assay[147]. Recently, it has been shown that, in cirrhotic patients, the serum concentration of creatinine being higher than 0.97 mg/dL (88 μmol/L) is tantamount to impairment in renal function, as this level is equivalent to the GFR of 50 mL/min[148].
Mathematical models based upon creatinine levels, such as Cockroft-Gault or modification of diet in renal disease, introduce a systematic error to calculations of the GFR. It particularly regards the Cockroft-Gault formula that estimates GFR from serum creatinine level and body mass, which in cirrhotic patients is highly dependent on body water content. On the basis of existing studies, the significance of cystatin C in the measurement of renal function in cirrhosis cannot be definitely determined.
Serum creatinine is one of three variables that form the model of end stage liver disease (MELD) score that predicts 3-mo survival in patients with end-stage cirrhosis. Therefore, MELD score is an important tool to set up the priority for access to the graft among patients waiting for a liver transplant. In the MELD score, creatinine has been assigned a much higher, and probably excessive, weight than bilirubin[149].
Total muscle mass is one of the factors influencing the serum creatinine concentration. It is the reason why men and women having the same GFR may differ with respect to serum creatinine levels. Therefore, it can be assumed that equal MELD values in both sexes may mean a worse prognosis for women. This assumption has been confirmed by finding a higher mortality rate among women listed for liver transplant in comparison with the pre-MELD era[27]. This finding challenges the belief that MELD realizes the principle that “the sickest goes first”.
Development of type 1 HRS is an unfavorable moment to conduct a liver transplantation because an elevated creatinine level is a predictor of perioperative complications and death. It also predicts the duration of stay in the intensive care unit and probability of renal replacement therapy[150,151]. Therefore, improvement of renal function should be the goal before liver transplantation. Nonetheless, 3-year survival of patients undergoing liver transplantation during HRS is approximately 60%[150], thus clearly worse than in patients with normal renal function, but still much better than in those without transplantation.
Development of ATN before liver transplantation denotes maintenance of renal failure after this procedure. Diagnosis of ATN before the scheduled hepatic transplantation may enforce the decision on simultaneous liver and kidney transplantation (SLKT). A reliable diagnosis of ATN in such circumstances requires renal biopsy, preferably in a transcaval way, because coagulopathy or large ascites precludes percutaneous biopsy. Indications for SLKT in patients with end-stage liver cirrhosis are: (1) AKI (including HRS) with persisting hypercreatininemia ≥ 2 mg/dL for a period of at least 8 wk (despite renal replacement therapy); (2) chronic renal insufficiency with GFR < 30 mL/min; and (3) chronic renal failure with histopathological evidence of sclerosis or fibrosis of > 30% of glomeruli[86].
From 1984 to 2008, SLKT was reported in 3536 patients. Apart from liver cirrhosis and chronic or acute renal failure, the main indications were oxalosis and polycystic liver and kidney disease. The cumulative 5-year survival of both organs was 60.9%, and patient survival was 42.6%[152].
With improvements in operative techniques, patient survival following liver transplantation has substantially increased with more common occurrence of chronic renal disease resulting from nephrotoxicity of immunosuppressive drugs, rendering kidney transplantation inevitable. After 10 years from liver transplantation, renal failure occurs in about 20% of patients[153].
Hypercreatinemia is a common finding in patients with end-stage liver disease and it may be a form of AKI or chronic renal failure; for example, DN or glomerular nephritis associated with nonalcoholic fatty liver disease and viral hepatitis, respectively. Knowledge of the nature of renal disease is essential for therapeutic decisions, especially in patients eligible for liver transplantation. AKI is an ominous manifestation of circulatory dysfunction in patients with late cirrhosis, generally identified with HRS. However, HRS may be only part of the pathophysiological continuum of renal hypoperfusion-related diseases, ranging from prerenal insufficiency to ischemic kidney damage. Moreover, HRS is probably not the most common form of renal disease associated with end-stage cirrhosis. At present, the diagnosis of HRS is based on exclusion of contact with nephrotoxic agents and the spectrum of factors leading to hypovolemia. In the near future, the diagnosis of HRS will probably require the absence of urinary markers specific for injury to tubular epithelial cells.
Peer reviewers: Virendra Singh, MD, DM, Additional Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India; Valentin Fuhrmann, MD, Department of Internal Medicine 4, Intensive Care Unit, Medical University Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; Mauro Bernardi, MD, Professor of Internal Medicine, Department of Clinical Medicine, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy
S- Editor Shi ZF L- Editor Kerr C E- Editor Xiong L
| 1. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4986] [Article Influence: 277.0] [Reference Citation Analysis (0)] |
| 2. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] |
| 3. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [PubMed] |
| 4. | Yeung E, Yong E, Wong F. Renal dysfunction in cirrhosis: diagnosis, treatment and prevention. MedGenMed. 2004;6:9. [PubMed] |
| 5. | Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 217] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Wong F, Massie D, Colman J, Dudley F. Glomerular hyperfiltration in patients with well-compensated alcoholic cirrhosis. Gastroenterology. 1993;104:884-889. [PubMed] |
| 7. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750-1754. [PubMed] |
| 9. | Laffi G, La Villa G, Pinzani M, Marra F, Gentilini P. Arachidonic acid derivatives and renal function in liver cirrhosis. Semin Nephrol. 1997;17:530-548. [PubMed] |
| 10. | Ros J, Clària J, Jiménez W, Bosch-Marcé M, Angeli P, Arroyo V, Rivera F, Rodés J. Role of nitric oxide and prostacyclin in the control of renal perfusion in experimental cirrhosis. Hepatology. 1995;22:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Laleman W, Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int. 2005;25:1079-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 17. | De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Am J Kidney Dis. 2011;57:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Kashtan J, Green JF, Parsons EQ, Holcroft JW. Hemodynamic effect of increased abdominal pressure. J Surg Res. 1981;30:249-255. [RCA] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 272] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, Tang WH. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail. 2008;14:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | El-Ashry N, El-Damarawy M, Salem M, Mogawer S. Large volume abdominal paracentesis effect on some humoral factors and cardiac performance in patients with liver cirrhosis and tense ascities. J Egypt Soc Parasitol. 2007;37:571-584. [PubMed] |
| 21. | Umgelter A, Reindl W, Wagner KS, Franzen M, Stock K, Schmid RM, Huber W. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care. 2008;12:R4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Savino JA, Cerabona T, Agarwal N, Byrne D. Manipulation of ascitic fluid pressure in cirrhotics to optimize hemodynamic and renal function. Ann Surg. 1988;208:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Garcia-Tsao G. Bacterial infections in cirrhosis. Can J Gastroenterol. 2004;18:405-406. [PubMed] |
| 24. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 25. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 26. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6211] [Cited by in RCA: 6522] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 30. | Leung W, Wong F. Medical management of ascites. Expert Opin Pharmacother. 2011;12:1269-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 32. | Cárdenas A, Ginès P, Uriz J, Bessa X, Salmerón JM, Mas A, Ortega R, Calahorra B, De Las Heras D, Bosch J. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Saló J, Ginès A, Ginès P, Piera C, Jiménez W, Guevara M, Fernández-Esparrach G, Sort P, Bataller R, Arroyo V. Effect of therapeutic paracentesis on plasma volume and transvascular escape rate of albumin in patients with cirrhosis. J Hepatol. 1997;27:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Sola-Vera J, Such J. Understanding the mechanisms of paracentesis-induced crculatory dysfunction. Eur J Gastroenterol Hepatol. 2004;16:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Peltekian KM, Wong F, Liu PP, Logan AG, Sherman M, Blendis LM. Cardiovascular, renal, and neurohumoral responses to single large-volume paracentesis in patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol. 1997;92:394-399. [PubMed] |
| 36. | Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, Angeli P, Ruiz-Del-Arbol L, Planas R, Solà R. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 37. | Sersté T, Francoz C, Durand F, Rautou PE, Melot C, Valla D, Moreau R, Lebrec D. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol. 2011;55:794-799. [PubMed] |
| 38. | Nasr G, Hassan A, Ahmed S, Serwah A. Predictors of large volume paracantesis induced circulatory dysfunction in patients with massive hepatic ascites. J Cardiovasc Dis Res. 2010;1:136-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Appenrodt B, Wolf A, Grünhage F, Trebicka J, Schepke M, Rabe C, Lammert F, Sauerbruch T, Heller J. Prevention of paracentesis-induced circulatory dysfunction: midodrine vs albumin. A randomized pilot study. Liver Int. 2008;28:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Moreau R, Asselah T, Condat B, de Kerguenec C, Pessione F, Bernard B, Poynard T, Binn M, Grangé JD, Valla D. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Singh V, Dheerendra PC, Singh B, Nain CK, Chawla D, Sharma N, Bhalla A, Mahi SK. Midodrine versus albumin in the prevention of paracentesis-induced circulatory dysfunction in cirrhotics: a randomized pilot study. Am J Gastroenterol. 2008;103:1399-1405. [PubMed] |
| 42. | Arroyo V, Gines P, Rimola A, Gaya J. Renal function abnormalities prostaglandins and effects of nonsteroidal anti-inflammatory drugs in cirrhosis with ascites. An overview with emphasis on pathogenesis. Am J Med. 1986;81:104-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Runyon BA. Refractory ascites. Semin Liver Dis. 1993;13:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Guevara M, Abecasis R, Terg R. Effect of celecoxib on renal function in cirrhotic patients with ascites. A pilot study. Scand J Gastroenterol. 2004;39:385-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Schepke M, Werner E, Biecker E, Schiedermaier P, Heller J, Neef M, Stoffel-Wagner B, Hofer U, Caselmann WH, Sauerbruch T. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Schepke M, Wiest R, Flacke S, Heller J, Stoffel-Wagner B, Herold T, Ghauri M, Sauerbruch T. Irbesartan plus low-dose propranolol versus low-dose propranolol alone in cirrhosis: a placebo-controlled, double-blind study. Am J Gastroenterol. 2008;103:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Tripathi D, Therapondos G, Lui HF, Johnston N, Webb DJ, Hayes PC. Chronic administration of losartan, an angiotensin II receptor antagonist, is not effective in reducing portal pressure in patients with preascitic cirrhosis. Am J Gastroenterol. 2004;99:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 640] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 49. | Arroyo V. Human serum albumin: not just a plasma volume expander. Hepatology. 2009;50:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, Boddi V, Tarquini R, Pantaleo P, Gentilini P. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403-1407. [PubMed] |
| 51. | Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 401] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 52. | Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichaï P, Abergel A, Halimi C, Pauwels M, Bronowicki JP. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Fabrizi F, Dixit V, Martin P. Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006;24:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Solà E, Lens S, Guevara M, Martín-Llahí M, Fagundes C, Pereira G, Pavesi M, Fernández J, González-Abraldes J, Escorsell A. Hyponatremia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertension. Hepatology. 2010;52:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Triantos CK, Samonakis D, Thalheimer U, Cholongitas E, Senzolo M, Marelli L, Leandro G, Patch D, Burroughs AK. Terlipressin therapy for renal failure in cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Fabrizi F, Dixit V, Messa P, Martin P. Terlipressin for hepatorenal syndrome: A meta-analysis of randomized trials. Int J Artif Organs. 2009;32:133-140. [PubMed] |
| 58. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 59. | Dobre M, Demirjian S, Sehgal AR, Navaneethan SD. Terlipressin in hepatorenal syndrome: a systematic review and meta-analysis. Int Urol Nephrol. 2011;43:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 61. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 62. | Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219-226. [PubMed] |
| 63. | Kalambokis G, Economou M, Paraskevi K, Konstantinos P, Pappas C, Katsaraki A, Tsianos EV. Effects of somatostatin, terlipressin and somatostatin plus terlipressin on portal and systemic hemodynamics and renal sodium excretion in patients with cirrhosis. J Gastroenterol Hepatol. 2005;20:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Kiser TH, Fish DN, Obritsch MD, Jung R, MacLaren R, Parikh CR. Vasopressin, not octreotide, may be beneficial in the treatment of hepatorenal syndrome: a retrospective study. Nephrol Dial Transplant. 2005;20:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Silva G, Segovia R, Backhouse C, Palma M, Márquez S, Iturriaga H. [Effects of acute octreotide infusion on renal function in patients with cirrhosis and portal hypertension]. Rev Med Chil. 2004;132:144-150. [PubMed] |
| 67. | Güney Duman D, Tüney D, Bilsel S, Benli F, Karan S, Avsar E, Ozdogan O, Tözün N. Octreotide in liver cirrhosis: a salvage for variceal bleeding can be a gunshot for kidneys. Liver Int. 2005;25:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, Tsianos EV. The effects of chronic treatment with octreotide versus octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, Amodio P, Sticca A, Caregaro L, Maffei-Faccioli A. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 325] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 71. | Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Tandon P, Tsuyuki RT, Mitchell L, Hoskinson M, Ma MM, Wong WW, Mason AL, Gutfreund K, Bain VG. The effect of 1 month of therapy with midodrine, octreotide-LAR and albumin in refractory ascites: a pilot study. Liver Int. 2009;29:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 74. | Fevery J, Van Cutsem E, Nevens F, Van Steenbergen W, Verberckmoes R, De Groote J. Reversal of hepatorenal syndrome in four patients by peroral misoprostol (prostaglandin E1 analogue) and albumin administration. J Hepatol. 1990;11:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Tyagi P, Sharma P, Sharma BC, Puri AS, Kumar A, Sarin SK. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: a pilot randomized control trial between pentoxifylline and placebo. Eur J Gastroenterol Hepatol. 2011;23:210-217. [PubMed] |
| 76. | Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev. 2009;2009:CD007339. [PubMed] |
| 77. | Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, Carbonell N, Renard P, Ramond MJ. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 79. | Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med. 1995;122:816-822. [PubMed] |
| 80. | Quiroga J, Sangro B, Núñez M, Bilbao I, Longo J, García-Villarreal L, Zozaya JM, Betés M, Herrero JI, Prieto J. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology. 1995;21:986-994. [PubMed] |
| 81. | Anderson CL, Saad WE, Kalagher SD, Caldwell S, Sabri S, Turba UC, Matsumoto AH, Angle JF. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: a 7-year, single-center experience. J Vasc Interv Radiol. 2010;21:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Deltenre P, Mathurin P, Dharancy S, Moreau R, Bulois P, Henrion J, Pruvot FR, Ernst O, Paris JC, Lebrec D. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Martinet JP, Fenyves D, Legault L, Roy L, Dufresne MP, Spahr L, Lafortune M, Pomier-Layrargues G. Treatment of refractory ascites using transjugular intrahepatic portosystemic shunt (TIPS): a caution. Dig Dis Sci. 1997;42:161-166. [PubMed] [DOI] [Full Text] |
| 84. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am. 2009;93:855-869, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Schwab SJ, Honorato JJ, Sharar LR, Dennis PA. Hemodialysis without anticoagulation. One-year prospective trial in hospitalized patients at risk for bleeding. Am J Med. 1987;83:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Kramer L, Bauer E, Joukhadar C, Strobl W, Gendo A, Madl C, Gangl A. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 90. | Wong F, Raina N, Richardson R. Molecular adsorbent recirculating system is ineffective in the management of type 1 hepatorenal syndrome in patients with cirrhosis with ascites who have failed vasoconstrictor treatment. Gut. 2010;59:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Rifai K, Ernst T, Kretschmer U, Hafer C, Haller H, Manns MP, Fliser D. The Prometheus device for extracorporeal support of combined liver and renal failure. Blood Purif. 2005;23:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 93. | Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 95. | Davenport A. Management of acute kidney injury in liver disease. Contrib Nephrol. 2010;165:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405-414. [PubMed] |
| 97. | Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, Parving HH, Rossing P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 98. | Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kidney Dis. 1982;2:363-374. [PubMed] |
| 99. | Myers BD, Moran SM. Hemodynamically mediated acute renal failure. N Engl J Med. 1986;314:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 165] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 101. | Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 102. | Leibovici L, Vidal L, Paul M. Aminoglycoside drugs in clinical practice: an evidence-based approach. J Antimicrob Chemother. 2009;63:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352-357. [PubMed] |
| 104. | Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 445] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 105. | Hampel H, Bynum GD, Zamora E, El-Serag HB. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001;96:2206-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Tumlin JA, Wang A, Murray PT, Mathur VS. Fenoldopam mesylate blocks reductions in renal plasma flow after radiocontrast dye infusion: a pilot trial in the prevention of contrast nephropathy. Am Heart J. 2002;143:894-903. [PubMed] |
| 107. | Pflueger A, Larson TS, Nath KA, King BF, Gross JM, Knox FG. Role of adenosine in contrast media-induced acute renal failure in diabetes mellitus. Mayo Clin Proc. 2000;75:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 696] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 109. | Becker CR, Davidson C, Lameire N, McCullough PA, Stacul F, Tumlin J, Adam A. High-risk situations and procedures. Am J Cardiol. 2006;98:37K-41K. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Najjar M, Hamad A, Salameh M, Agarwal A, Feinfeld DA. The risk of radiocontrast nephropathy in patients with cirrhosis. Ren Fail. 2002;24:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 861] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 112. | Johnson RJ, Willson R, Yamabe H, Couser W, Alpers CE, Wener MH, Davis C, Gretch DR. Renal manifestations of hepatitis C virus infection. Kidney Int. 1994;46:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Uchiyama-Tanaka Y, Mori Y, Kishimoto N, Nose A, Kijima Y, Nagata T, Umeda Y, Masaki H, Matsubara H, Iwasaka T. Membranous glomerulonephritis associated with hepatitis C virus infection: case report and literature review. Clin Nephrol. 2004;61:144-150. [PubMed] |
| 114. | Altraif IH, Abdulla AS, al Sebayel MI, Said RA, al Suhaibani MO, Jones AA. Hepatitis C associated glomerulonephritis. Am J Nephrol. 1995;15:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | McGuire BM, Julian BA, Bynon JS, Cook WJ, King SJ, Curtis JJ, Accortt NA, Eckhoff DE. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144:735-741. [PubMed] |
| 116. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kobayashi M. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. 1997;25:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Misiani R, Bellavita P, Fenili D, Vicari O, Marchesi D, Sironi PL, Zilio P, Vernocchi A, Massazza M, Vendramin G. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med. 1994;330:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 394] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 119. | Roccatello D, Baldovino S, Rossi D, Mansouri M, Naretto C, Gennaro M, Cavallo R, Alpa M, Costanzo P, Giachino O. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2004;19:3054-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 120. | Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 169] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Levy M, Chen N. Worldwide perspective of hepatitis B-associated glomerulonephritis in the 80s. Kidney Int Suppl. 1991;35:S24-S33. [PubMed] |
| 122. | Takekoshi Y, Tochimaru H, Nagata Y, Itami N. Immunopathogenetic mechanisms of hepatitis B virus-related glomerulopathy. Kidney Int Suppl. 1991;35:S34-S39. [PubMed] |
| 123. | Conjeevaram HS, Hoofnagle JH, Austin HA, Park Y, Fried MW, Di Bisceglie AM. Long-term outcome of hepatitis B virus-related glomerulonephritis after therapy with interferon alfa. Gastroenterology. 1995;109:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 125. | Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 542] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 126. | D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 127. | Waldherr R, Rambausek M, Duncker WD, Ritz E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant. 1989;4:943-946. [PubMed] |
| 128. | Galla JH. IgA nephropathy. Kidney Int. 1995;47:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 129. | Newell GC. Cirrhotic glomerulonephritis: incidence, morphology, clinical features, and pathogenesis. Am J Kidney Dis. 1987;9:183-190. [PubMed] |
| 130. | Lomax-Smith JD, Zabrowarny LA, Howarth GS, Seymour AE, Woodroffe AJ. The immunochemical characterization of mesangial IgA deposits. Am J Pathol. 1983;113:359-364. [PubMed] |
| 131. | Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2000;191:2171-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 132. | Singri N, Gleason B, Flamm SL, Kanwar YS, Ghossein C. Secondary IgA nephropathy presenting as nephrotic syndrome with glomerular crescentic changes and acute renal failure in a patient with autoimmune hepatitis. J Nephrol. 2004;17:125-129. [PubMed] |
| 133. | Lai KN, Lai FM, Tam JS, Vallance-Owen J. Strong association between IgA nephropathy and hepatitis B surface antigenemia in endemic areas. Clin Nephrol. 1988;29:229-234. [PubMed] |
| 134. | Cecchin E, De Marchi S. Alcohol misuse and renal damage. Addict Biol. 1996;1:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Gouet D, Fort E, Roblot P, Maréchaud R, Sudre Y, Touchard G. [Glomerulopathy with mesangial IgA deposits in primary hemochromatosis]. Rev Med Interne. 1987;8:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 136. | Szönyi L, Dobos M, Vásárhelyi B, Héninger E, Vas T, Nagy J, Kovács T. Prevalence of alpha1-antitrypsin phenotypes in patients with IgA nephropathy. Clin Nephrol. 2004;62:418-422. [PubMed] |
| 137. | Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124. [PubMed] [DOI] [Full Text] |
| 138. | Costacou T, Ellis D, Fried L, Orchard TJ. Sequence of progression of albuminuria and decreased GFR in persons with type 1 diabetes: a cohort study. Am J Kidney Dis. 2007;50:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 139. | Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 332] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 140. | Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996;27:167-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 484] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 142. | Adler S. Diabetic nephropathy: Linking histology, cell biology, and genetics. Kidney Int. 2004;66:2095-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 143. | Letiexhe MR, Scheen AJ, Gérard PL, Bastens BH, Pirotte J, Belaiche J, Lefèbvre PJ. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab. 1993;77:1263-1268. [PubMed] [DOI] [Full Text] |
| 144. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 145. | Kalaitzakis E, Rosengren A, Skommevik T, Björnsson E. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci. 2010;55:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 146. | Longacre AM, Popky GL. Papillary necrosis in patients with cirrhosis: a study of 102 patients. J Urol. 1968;99:391-395. [PubMed] |
| 147. | Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 148. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 149. | Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 150. | Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 280] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 151. | Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, Markmann JF, Olthoff KM, Shaked A, Reddy KR. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 152. | Mehrabi A, Fonouni H, Ayoub E, Rahbari NN, Müller SA, Morath Ch, Seckinger J, Sadeghi M, Golriz M, Esmaeilzadeh M. A single center experience of combined liver kidney transplantation. Clin Transplant. 2009;23 Suppl 21:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Gonwa TA, Morris CA, Goldstein RM, Husberg BS, Klintmalm GB. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation. 1991;51:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 194] [Article Influence: 5.7] [Reference Citation Analysis (0)] |