Published online Jun 21, 2012. doi: 10.3748/wjg.v18.i23.3032
Revised: February 27, 2012
Accepted: March 20, 2012
Published online: June 21, 2012
The combination of either boceprevir or telaprevir with ribavirin and interferon (triple therapy) has been shown to be more effective than ribavirin+interferon (dual therapy) for the treatment of genotype 1 hepatitis C. Since the benefit of these treatments takes place after years, simulation models are needed to predict long-term outcomes. In simulation models, the choice of different values of yearly discount rates (e.g., 6%, 3.5%, 2%, 1.5% or 0%) influences the results, but no studies have specifically addressed this issue. We examined this point by determining the long-term benefits under different conditions on the basis of standard modelling and using quality-adjusted life years (QALYs) to quantify the benefits. In our base case scenario, we compared the long-term benefit between patients given a treatment with a 40% sustained virologic response (SVR) (dual therapy) and patients given a treatment with a 70% SVR (triple therapy), and we then examined how these specific yearly discount rates influenced the incremental benefit. The gain between a 70% SVR and a 40% SVR decreased from 0.45 QALYs with a 0% discount rate to 0.22 QALYs with a 6% discount rate (ratio between the two values = 2.04). Testing the other discounting assumptions confirmed that the discount rate has a marked impact on the magnitude of the model-estimated incremental benefit. In conclusion, the results of our analysis can be helpful to better interpret cost-effectiveness studies evaluating new treatment for hepatitis C.
- Citation: Messori A, Fadda V, Maratea D, Trippoli S. Effect of discounting on estimation of benefits determined by hepatitis C treatment. World J Gastroenterol 2012; 18(23): 3032-3034
- URL: https://www.wjgnet.com/1007-9327/full/v18/i23/3032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i23.3032
The review by Tsubota et al[1] has examined the main options available for the treatment of hepatitis C, including two antiviral drugs that have recently been marketed in many countries. Focusing more thoroughly on these two innovative agents is worthwhile because boceprevir and telaprevir, along with other innovative agents, are thought to be an important advancement in the treatment of this disease[2], although at a high cost.
Hepatitis C virus (HCV) genotype 1, which accounts for 60% of all HCV-infected patients[3-5], is the target at which these two new agents are directed in combination with ribavirin + interferon. Considering that the combination of either boceprevor or telaprevir with ribavirin+interferon (triple therapy) has been shown to be more effective than ribavirin + interferon (dual therapy) in genotype 1[1-3], in the near future the dual therapy is expected to be replaced by the triple therapy in a certain proportion of these cases. The debate is still ongoing to set appropriate criteria to identify the best candidates for the triple therapy, and this selection will depend on a number of factors including pretreated vs naive condition[3] and interleukin 28B polymorphism[6] .
The economic impact of this new approach to HCV treatment can be very substantial since it has been estimated that around 120 million euros per year are needed in a country with 60 million inhabitants[5], and this figure seems to be confirmed by the recent sales in the United States where these “third” drugs have already been available[7].
The predicted expenditure for the “third” drug (irrespective of whether it is boceprevir or telaprevir) is likely to be at least 20 000 euros per patient[5]. Since this is also the typical expenditure for target therapies in oncologic patients, decision-makers will have to face the competition for the same pharmaceutical budget between oncologic innovative treatments approved recently (e.g., ipilimumab for metastatic melanoma) and the triple therapy for genotype-1 hepatitis C.
The typical benefit of the latest oncologic treatments is a gain of 2-4 mo of survival per patient[8]; their pharmacoeconomic profile suggests an expenditure of 20 000 euros to gain up to a 4-mo survival, i.e., a cost-effectiveness ratio of 5000 euros per month or 60 000 euros per year.
Contrasting the cost-effectiveness between oncologic treatment and the triple therapy implies the need to compare the short-term benefits observed in oncologic patients (e.g., survival prolongation in metastatic melanoma from 6 mo without ipilimumab to 10 mo with ipilimumab) with the benefits in HCV patients that are instead known to take place at least 10 years after treatment.
The discount rate is the typical method employed in cost-effectiveness studies to convert future clinical benefits into their present value[9-14]. In the United States, rates around 5% or 6% per year were suggested nearly 20 years ago, but later various panels of experts revised this suggestion by proposing an annual rate of 3%[9,10]. In the United Kingdom, the National Institute of Clinical Excellence initially chose to use 3.5% per year[11], but in August 2011 this value was re-determined as 1.5% per year at least in some cases[15].
Several years ago, the pharmacoeconomic studies comparing dual therapy vs interferon alone led to the development of numerous models[16-19] based on the Markov technique that were aimed at predicting the natural history of the disease with or without achievement of post-treatment sustained virologic response (SVR). Although the number of simulation models for hepatitis C published in the past is exceedingly high, the systematic review by Hartwell et al[19] confirms that the models initially developed by Bennett et al[16] and by Shepherd et al[17,18] remain still valid to carry out a thorough comparative assessment of the new vs old treatments.
The choice of specific values of yearly discount rates is the key factor influencing the model’s outcome (Table 1). For this reason, we have summarized the different effects determined by the choice of different discount rates using a single simulation model among those reported in the literature.
| Authors | Modelling details (base case) | Expected outcome | ||
| No treatment | Interferon monotherapy | Dual treatment | ||
| Bennett et al[16] 1997 | Age = 35 yr; time horizon = lifetime; discount rate = 0% per year | 36.2 LYs | 37.7 LYs | NR |
| Bennett et al[16] 1997 | Age = 35 yr; time horizon = lifetime; discount rate = 5% per year | 16.2 LYs | 16.4 LYs | NR |
| Bennett et al[16] 1997 | Age = 35 yr; time horizon = lifetime; discount rate = 0% per year | 28.0 QALYs | 31.7 QALYs | NR |
| Shepherd et al[17] 2004 | Age = 36 yr; time horizon = 30 yr; discount rate = 1.5% per year | 21.464 QALYs | NR | 23.417 QALYs |
| Shepherd et al[18] 2007 | Age = 40 yr; time horizon = lifetime; discount rate = 1.5% per year | 20.17 QALYs | NR | From 20.94 to 22.48 QALYs |
| Hartwell et al[19] 20112 | Age = 40 yr; time horizon = lifetime; discount rate = 3.5% per year, dual therapy with PEG-interferon alpha-2α | NR (naïve patients), 10.74 QALYs (previously treated patients) | NR | 15.68 QALYs (naïve patients), 11.05 QALYs (previously treated patients) |
| Hartwell et al[19] 20112 | Age = 40 yr; time horizon = lifetime; discount rate = 3.5% per year, dual therapy with PEG-interferon alpha-2β | NR (naïve patients), 10.74 QALYs (previously treated patients) | NR | 13.89 QALYs (naïve patients), 11.14 QALYs (previously treated patients) |
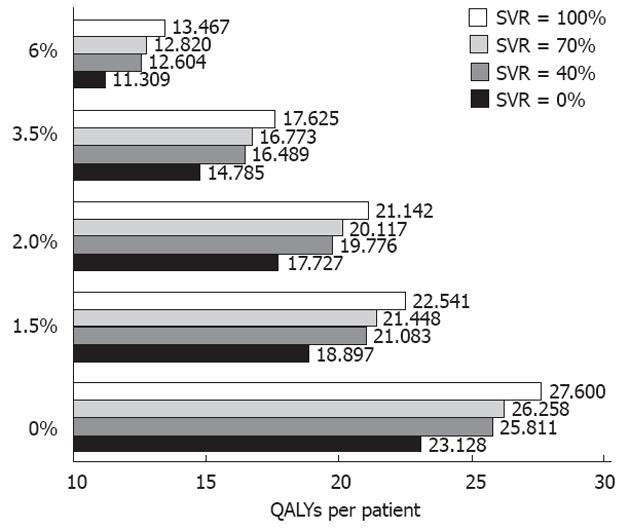
The results of our analysis are shown in Figure 1. The values of quality-adjusted life years (QALYs) per patient have been calculated by examining five different assumptions of yearly discount rates (6%, 3.5%, 2%, 1.5% and 0%) and four SVR rates (0%, 40%, 70% and 100%). With regard to the SVR rates, the assumption of a 100% SVR has, of course, a purely hypothetical function, whereas the assumption of 0% SVR represent the option of no treatment. More importantly, the assumption of 40% SVR represents the typical outcome of dual treatment while 70% SVR is used to estimate the outcome of triple treatment, as well as other treatments that are currently under investigation, but will become available quite soon[2].
The information shown in Figure 1 clearly indicates that the effect of choosing different discount rates is very substantial. The gain between 100% SVR and 0% SVR (a purely hypothetical comparison) decreases from 4.47 QALYs with a 0% discount rate to 2.16 QALYs with a 6% discount rate (ratio between the two values =2.07). On the other hand, the gain between 70% SVR and 40% SVR decreases from 0.45 QALYs with a 0% discount rate to 0.22 QALYs with a 6% discount rate (ratio between the two values =2.04). These simulations have a general validity because they are only based on the clinical end-point of SVR, and therefore do not rely on specific assumptions in the patients whether are naive or pretreated. As shown in Figure 1, we could compute the value of QALYs per patient for any intermediate value of SVR (SVRNN%) in a range from 0% to 100% according to the equation: QALYs SVRNN% = [QALYSVR100%× NN + QALYsSVR0%× (100-NN)]/100. It should be noted that, in real practice as well as in model-based estimations, the favorable economic results of these treatments do not result only from the economic counter-value of the clinical benefit, but also from the savings derived from reduced morbidity. However, the latter factor was beyond the purposes of the present study.
In conclusion, our analysis has exclusively focused on the consequences of choosing different discount rates in estimating the magnitude of the clinical benefit of treatments for hepatitis C. Our results indicate that varying the discount rate within commonly accepted values can produce more than 2-fold variations in the estimates of the incremental benefit. This point should be kept in mind when regulatory agencies or third-part payers will be asked to determine the value-based price for the new treatments in this area.
We thank Professor Americo Cicchetti and Professor Antonio Gasbarrini (both from the Università Cattolica, Policlinico Gemelli, Rome, Italy), coordinators of the WEF group (Workshop in Epatologia e Farmacoeconomia).
Peer reviewers: Chao-Hung Hung, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Niao Sung, Kaohsiung 833, Taiwan, China; Faisal M Sanai, Hepatobiliary Sciences, King Abdulaziz Medical City, King Abdulaziz Medical City, Riyadh 11462, Saudi Arabia
S- Editor Gou SX L- Editor Ma JY E- Editor Li JY
| 1. | Tsubota A, Fujise K, Namiki Y, Tada N. Peginterferon and ribavirin treatment for hepatitis C virus infection. World J Gastroenterol. 2011;17:419-432. [PubMed] |
| 2. | Chung RT. A watershed moment in the treatment of hepatitis C. N Engl J Med. 2012;366:273-275. [PubMed] |
| 3. | Butt AA, Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin Infect Dis. 2012;54:96-104. [PubMed] |
| 4. | Messori A, Del Santo F, Maratea D. First-line treatments for hepatitis C. Aliment Pharmacol Ther. 2011;33:1383-1385. [PubMed] [DOI] [Full Text] |
| 5. | Maratea D, Messori A, Fadda V. Nationwide prediction of future expenditure for protease inhibitors in chronic hepatitis C. Dig Liver Dis. 2012;44:86-87. [PubMed] |
| 6. | Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617. [PubMed] |
| 7. | Cohen B. Vertex score big with hepatitis drug in America (September 2011). Available from: http: //www.hepctrust.org.uk/News_Resources/news/2011/September/Vertex score big with hepatitis drug in America accessed on 20 December 2011.. |
| 8. | Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044-1048. [PubMed] |
| 9. | Krahn M, Gafni A. Discounting in the economic evaluation of health care interventions. Med Care. 1993;31:403-418. [PubMed] |
| 10. | Siegel JE, Torrance GW, Russell LB, Luce BR, Weinstein MC, Gold MR. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost Effectiveness in Health and Medicine. Pharmacoeconomics. 1997;11:159-168. [PubMed] |
| 12. | West RR, McNabb R, Thompson AG, Sheldon TA, Grimley Evans J. Estimating implied rates of discount in healthcare decision-making. Health Technol Assess. 2003;7:1-60. [PubMed] |
| 13. | Brouwer WB, Niessen LW, Postma MJ, Rutten FF. Need for differential discounting of costs and health effects in cost effectiveness analyses. BMJ. 2005;331:446-448. [PubMed] |
| 14. | Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ. 2011;20:2-15. [PubMed] [DOI] [Full Text] |
| 15. | Claxton K; Anonymous. NICE changes discount rate methods (7 August 2011). Available from: http: //scharrheds.blogspot.com/2011/08/nice-changes-discount-rate-methods.html accessed on 20 December 2011. |
| 16. | Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855-865. [PubMed] |
| 17. | Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8:iii-iv, 1-125. [PubMed] |
| 18. | Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1-205, iii. [PubMed] |
| 19. | Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15:i-xii, 1-210. [PubMed] |