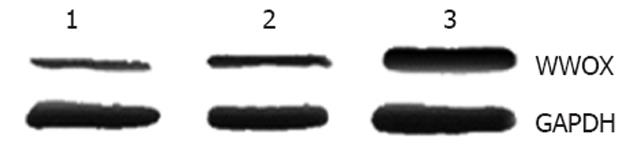Published online Jun 21, 2012. doi: 10.3748/wjg.v18.i23.3020
Revised: September 28, 2011
Accepted: January 7, 2012
Published online: June 21, 2012
AIM: To investigate the effects of the WWOX gene on the human hepatic carcinoma cell line SMMC-7721.
METHODS: Full-length WWOX cDNA was amplified from normal human liver tissues. Full-length cDNA was subcloned into pEGFP-N1, a eukaryotic expression vector. After introduction of the WWOX gene into cancer cells using liposomes, the WWOX protein level in the cells was detected through Western blotting. Cell growth rates were assessed by methyl thiazolyl tetrazolium (MTT) and colony formation assays. Cell cycle progression and cell apoptosis were measured by flow cytometry. The phosphorylated protein kinase B (AKT) and activated fragments of caspase-9 and caspase-3 were examined by Western blotting analysis.
RESULTS: WWOX significantly inhibited cell proliferation, as evaluated by the MTT and colony formation assays. Cells transfected with WWOX showed significantly higher apoptosis ratios when compared with cells transfected with a mock plasmid, and overexpression of WWOX delayed cell cycle progression from G1 to S phase, as measured by flow cytometry. An increase in apoptosis was also indicated by a remarkable activation of caspase-9 and caspase-3 and a dephosphorylation of AKT (Thr308 and Ser473) measured with Western blotting analysis.
CONCLUSION: Overexpression of WWOX induces apoptosis and inhibits proliferation of the human hepatic carcinoma cell line SMMC-7721.
-
Citation: Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X, Sun ZQ.
WWOX induces apoptosis and inhibits proliferation of human hepatoma cell line SMMC-7721. World J Gastroenterol 2012; 18(23): 3020-3026 - URL: https://www.wjgnet.com/1007-9327/full/v18/i23/3020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i23.3020
The tumor suppressor gene WWOX is localized in a common fragile site FRA16D (locus 16q23.3-24.1). Protein encoded by WWOX is an oxidoreductase containing two WW protein interaction domains. The biological role of the protein is not yet defined, although there are hypotheses that it may play a part in steroid hormones metabolism and ErbB4 receptor signaling pathway[1,2]. Low expression level of the WWOX gene has been observed in many types of cancers[3-15], possibly due to the loss of heterozygosity or epigenetic changes, such as methylation of CpG islands in promoter region. Several researches have revealed loss of heterozygosity of WWOX locus in gastric[7], pancreatic[6], esophageal[3] and lung[4] cancers. The role of WWOX in hepatic carcinoma is not well understood, and few studies have reported the effects of WWOX on hepatic carcinoma. In this study, we investigated the apoptotic effects of the WWOX gene on the human hepatic carcinoma cell line SMMC-7721.
The eukaryotic expression vector pEGFP-N1 and Escherichia coli DH5α competent cells are routinely maintained by the central laboratory at our hospital. The hepatoma cell line SMMC-7721 was obtained from the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modification of Eagle’s medium Dulbecco (DMEM) culture medium was purchased from Gibco BRL (Gaithersburg, United States). Fetal bovine serum was obtained from Sijiqing Biological Engineering Material (Hangzhou, China). The following materials were used: RNeasy Protect Mini-kit (QIAGen Co., Germany), SMARTTM PCR cDNA synthesis kit (Clontech Co., United States), DNA gel extraction kit (Dalian TaKaRa Co., China), plasmid mini-preparation kit (Shanghai Huasun Biotechnology Co., China), KOD-Plus DNA polymerase (TOYOBO Co., United States), T4 DNA ligase and the HindIII and KpnI restriction enzymes (New England Biolabs, United States), Lipofectamine 2000 (Invitrogen, United States), anti-WWOX, anti-phospho-AKT(pThr308 and Ser473), cleaved caspasse-9 and caspase-3 monoclonal antibodies (Santa Cruz Co., United States), and horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (Zhongshan Co., China). Nucleic acid sequencing was performed by Shanghai Yingjun Bioengineering Co., China. The WWOX and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were synthesized by Shanghai Yingjun Bioengineering Co., China.
SMMC-7721 cells were cultured in DMEM medium (HyClone Inc, United States) supplemented with 10% new calf bovine serum in a 37 °C and 5% CO2 incubator.
The WWOX open reading frame was amplified from a cDNA clone using the forward primer 5’GGAAGCTTTTGGAGCGGGAGTGAG-3’ and the reverse primer 5’GGATCCCAGCAGTTGTTGAAGTACA-3’, which introduced KpnI and HinDIII restriction endonuclease sites. WWOX cDNA digested with KpnI and HindIII was cloned into a pEGFP-N1 eukaryotic expression vector. The resulting vector was transfected into SMMC-7721 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). An empty vector of pEGFP-N1 was used as a negative control. After 24-48 h, the transient transfection efficiency was determined under an Olympus fluorescence microscope. The cells were then passaged at appropriate ratios to six-well plates. The next day, the cells were cultured in the presence of 1000-2000 g/mL G418 (Life Technology, Paisley, Scotland), which was increased in concentration in a stepwise manner over 14 d. Cells highly expressing green fluorescent protein (GFP) were selected.
Protein was extracted from cultured cells using lysis buffer. After a 30-min incubation on ice, the lysates were heated at 100 °C for 15 min and centrifuged at 12 000 ×g for 15 min at 4 °C. Lysates containing an equal amount of protein (25 μg) were dissolved in sodium dodecyl sulphate (SDS) sample buffer, separated on 12% SDS slab gels, and transferred electrophoretically onto polyvinylidene difluoride membranes. Equal protein loading and transfer were confirmed by Ponceau S staining. After being blocked with 5% non-fat dry milk in Tris-Buffered Saline and Tween 20 (10 mmol Ttris-HCl, pH 8.0, 100 mmol/L NaCl and 0.05% Tween), the membrane was incubated at 4 °C overnight with the appropriate primary antibodies. Following washing, horseradish peroxidase conjugated secondary antibody was applied to the membrane. Proteins bound by the secondary antibody were visualized by electrochemiluminescence (Amersham Bioscience) according to the manufacturer’s instructions. The expression of GAPDH was measured as a control, and each experiment was performed in triplicate.
Cell growth was determined by the methyl thiazolyl tetrazolium (MTT) assay (Sigma, United States). Briefly, 1 × 104 cells were seeded onto 96-well plates with four replicates for each condition. Approximately 72 h later, MTT reagent was added to each well at 5 mg/mL in a 20 μL volume, and the reaction was incubated for another 4 h. The formazan crystals formed by viable cells were subsequently solubilized in dimethyl sulfoxide, and the absorbance (A) at 490 nm was measured.
Approximately 100 cells were added to each well of a six-well culture plate. After incubation at 37 °C for 15 d, cells were washed twice with phosphate-buffered saline (PBS) and stained with Giemsa solution. The number of colonies containing ≥ 50 cells was counted under microscope [plate clone formation efficiency = (number of colonies/number of cells inoculated) × 100%]. Each experiment was performed in triplicate.
Forty-eight hours after treatment, logarithmically growing cells were collected and washed with PBS three times and fixed with 75% ethanol at -20 °C for at least 1 h. After extensive washing with PBS, the cells were suspended in Hank’s balanced salt solution containing 50 mg/mL RNase A (Boehringer Mannheim) and 50 mg/mL propidium iodide (PI) (Sigma-Aldrich), incubated for 1 h at room temperature, and were analyzed by FACScan (Becton Dickinson).
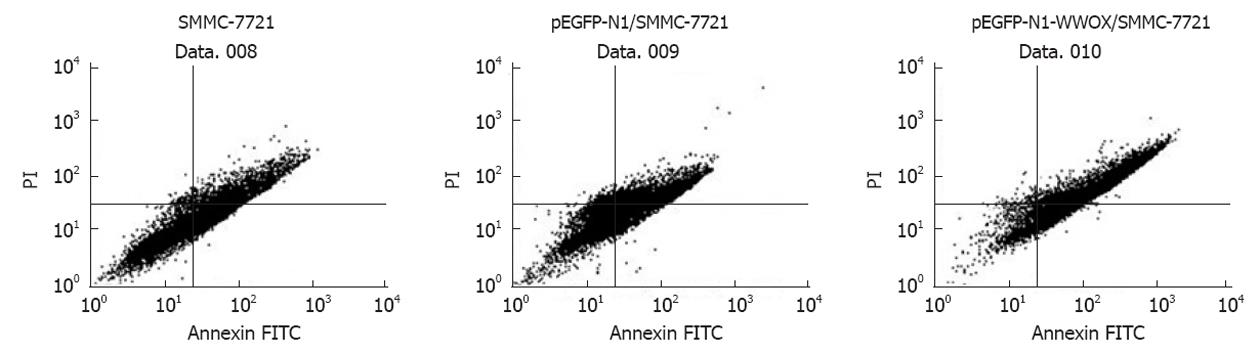
Apoptosis was analyzed 48 h after treatment using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions.
Data were presented as the mean ± SD. Comparisons of experimental values between cisplatin-treated cells and untreated controls were conducted using analysis of variance or the Kruskal-Wallis rank test. Statistical significance was defined as P < 0.05.
To study the biological functions of WWOX, we introduced WWOX into SMMC-7721 cells using a pEGFP-N1 eukaryotic expression vector containing the WWOX gene. Seven stably transfected cell clones were obtained. Western blotting analysis with anti-GFP antibodies showed that WWOX-pGFP fusion protein in the SMMC-7721 cell clones was highly expressed compared with control cells and control-vector cells (Figure 1).
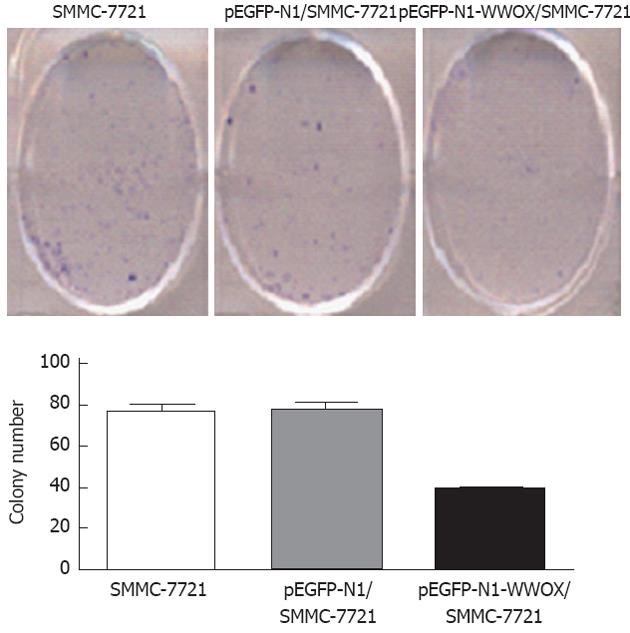
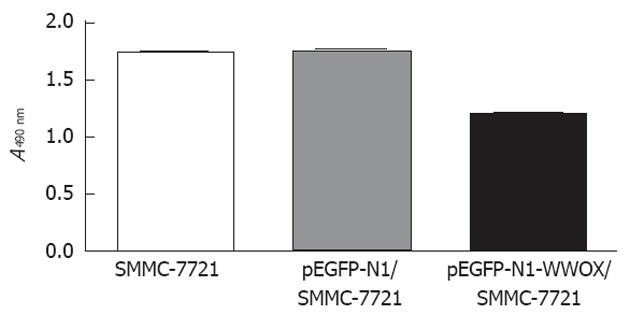
To analyze the function of WWOX, we studied the rate of cell growth in the WWOX-expressing SMMC-7721 cells. The results from the colony formation assay indicated that SMMC-7721 cells overexpressing WWOX formed significantly fewer colonies than did the control clone cells and the control-vector cells (P < 0.05) (Figure 2). Cells transfected with WWOX also showed significantly decreased cell proliferation compared with control cells and control-vector cells when examined by the MTT assay (Table 1, Figure 3).
| Cell line | Optical density value |
| SMMC-7721 | 1.77 ± 0.20 |
| pEGFP-Nl/SMMC-7721 | 1.78 ± 0.13 |
| pEGFP-Nl-WWOX/SMMC-7721 | 1.12 ± 0.23 |
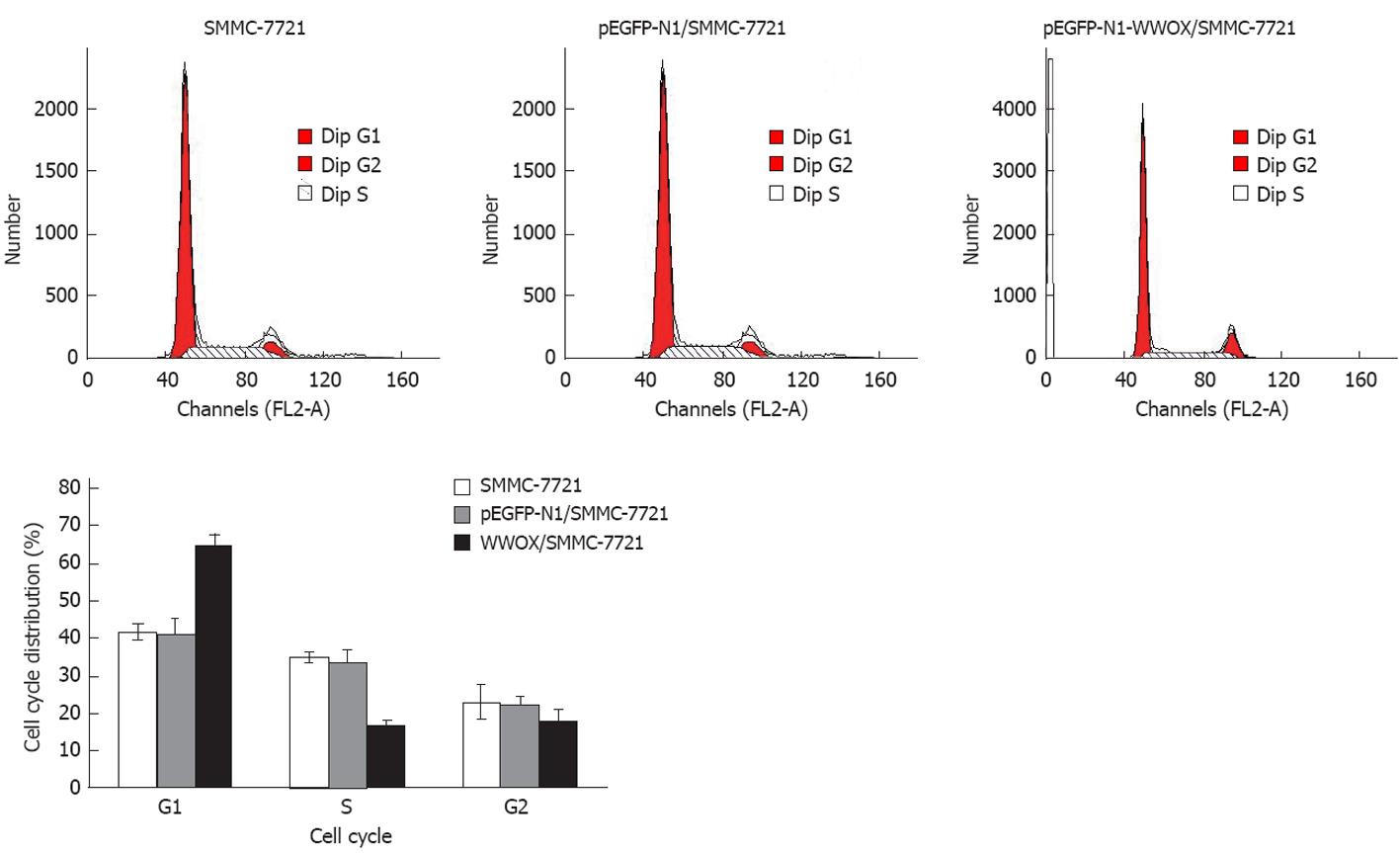
To detect the effect of WWOX overexpression on the cell cycle, we measured the cell cycle distribution in WWOX-expressing SMMC-7721 cells. In these lines, there was a marked decrease in the S-phase population, while the G1 population was significantly increased compared with the control vector and wild type SMMC-7721 cells (P < 0.05). Neither cell lines showed significant changes in the G2 population (Figure 4, Table 2). Cells transfected with pEGFP-N1-WWOX demonstrated more apoptosis than did cells transfected with the mock plasmid or the parent cells (P < 0.05) (Figure 5).
| Group | Cell cycle | ||
| G1 | S | G2 | |
| SMMC-7721 | 41.23 ± 2.12 | 34.52 ± 4.13 | 22.54 ± 3.12 |
| pEGFP-Nl/SMMC-7721 | 40.45 ± 1.32 | 33.3 ± 3.11 | 21.24 ± 1.31 |
| WWOX/SMMC-7721 | 64.23 ± 4.34 | 16.13 ± 2.65 | 17.12 ± 3.24 |
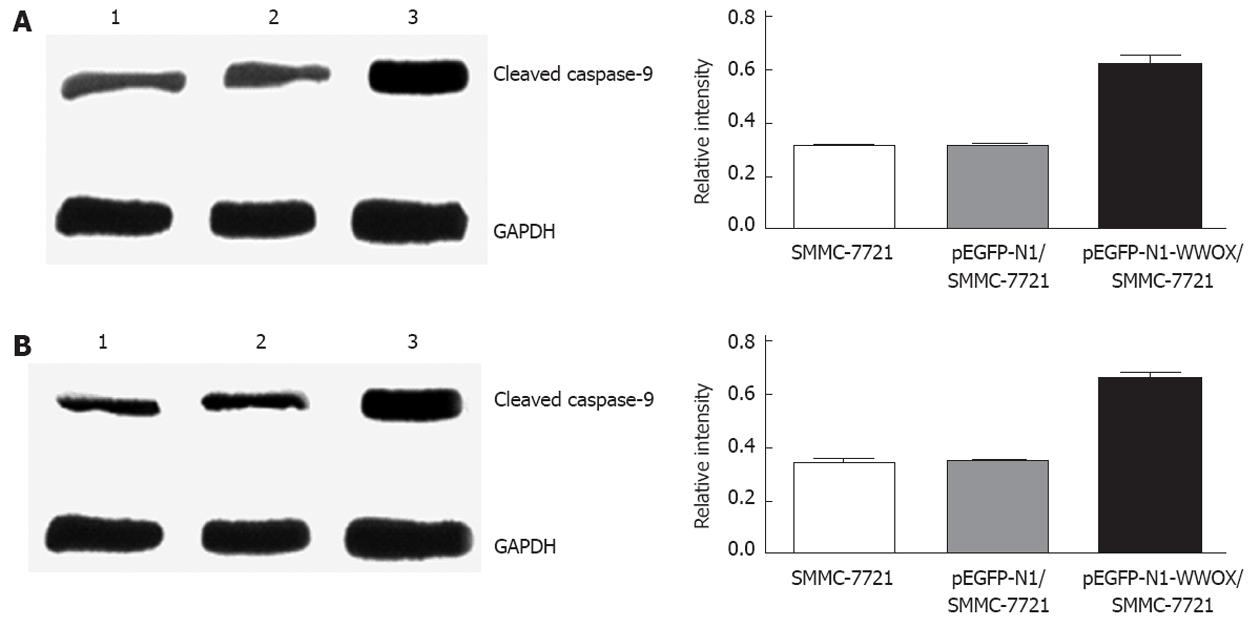
Expression of cleaved caspase-9 and caspase-3 was upregulated, as measured by Western blotting in cells that were transfected with pEGFP-N1, compared with either the cells transfected with a control vector or parental wild-type cells (Figure 6).
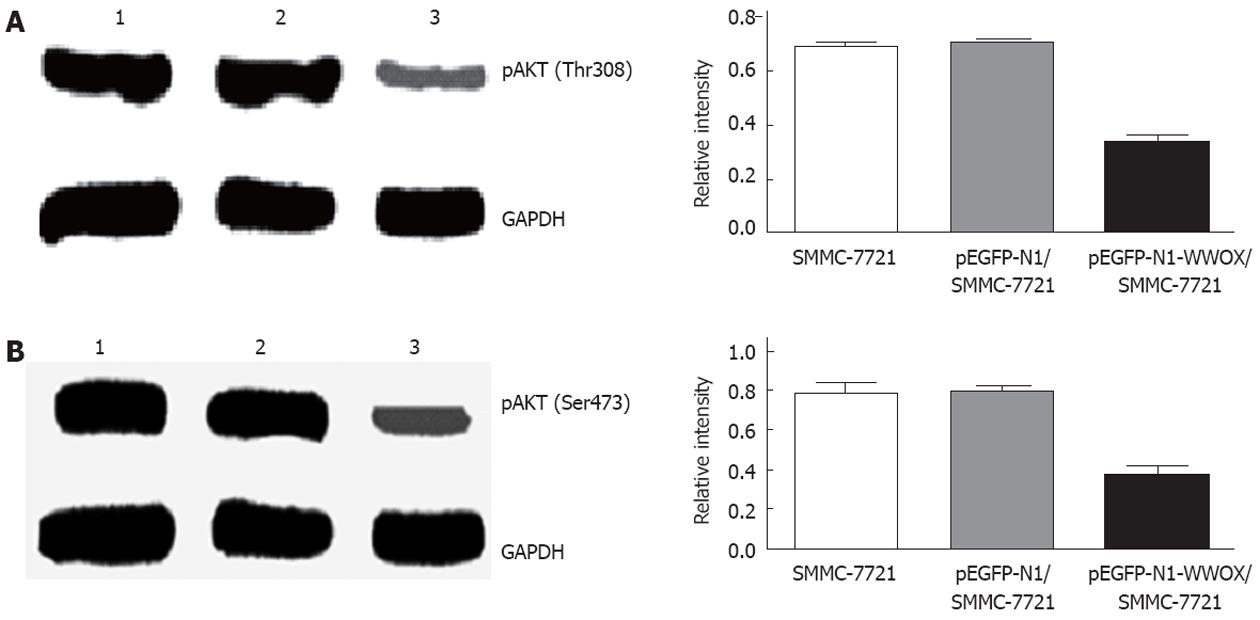
To evaluate the effect of WWOX on Akt/PKB activity, the phosphorylation level at Akt Thr308 and Ser473 was examined with specific phospho-Akt antibodies. Western blot analysis showed that WWOX significantly reduced the level of Akt/PKB phosphorylation (Figure 7).
Hepatic carcinoma is a highly invasive and clinically challenging tumor, and its molecular basis remains poorly understood. We used a gain-of-function approach by introducing WWOX into wild-type cells to investigate the effect of the WWOX gene on SMMC-7721, the human hepatic carcinoma cell line. Our data suggest that WWOX can significantly inhibit cell proliferation and induce cell apoptosis of the hepatic carcinoma cell line SMMC-7721. Overexpression of WWOX delayed the cell cycle progression from G1 into S phase, as demonstrated by flow cytometry.
Apoptosis plays a central role in tumor development, and a lack or failure of apoptosis leads to the development of many tumors, including hepatocarcinoma[16,17]. This suggests that induction of apoptosis in tumor cells might be an effective approach for delaying tumor progression. In this study, we found that overexpression of WWOX induces apoptosis in the hepatic carcinoma cell line SMMC-7721.
There are, at least, two broad extrinsic and intrinsic pathways that lead to apoptosis[18-20]. The extrinsic pathway begins with the binding of Fas ligand (FasL or CD95L) to the Fas receptor (CD95) and results in the recruitment of Fas-associated protein with death domain and pro-caspase-8 to the Fas complex. This increase in the local concentration of pro-caspase-8 leads to its autocatalysis and activation. Activated caspase-8 cleaves pro-caspase-3, which then undergoes autocatalysis to form active caspase-3, a principle effector caspase of apoptosis. The intrinsic apoptosis pathway always begins with mitochondrial damage, which results in the release of cytochrome C from the damaged mitochondria. In the cytosol or on the surface of the mitochondria, cytochrome C is bound to the protein Apaf-1 (apoptotic protease activating factor), which activates the initiating caspase, caspase-9, which then activates caspase-3[21,22]. The caspase families play an important role in the apoptosis-signaling pathway. The caspases are present in the cytoplasm under normal conditions as inactive pro-enzymes, and most of them are activated by proteolytic cleavage when the cell undergoes apoptosis[23,24]. Both caspase-8 and caspase-9 can activate the effector caspase, caspase-3, by proteolytic cleavage, and the subsequent processes result in nuclear DNA fragmentation and the formation of apoptotic bodies. This indicates that activation of caspase-3 is a central event for the process of apoptosis. Based on these results, we speculate that WWOX allows the release of cytochrome C from mitochondria, resulting in the activation of caspase-9 and caspase-3 in sequence and finally induces apoptosis of HCC cells. Consistent with this hypothesis, the results of Western blotting showed that WWOX overexpression induced the activation of caspase-9 and caspase-3.
Our work also shows that WWOX downregulates the phosphorylation of Akt/PKB at Thr308. Akt/PKB[25,26], the major downstream effector of the PI3-kinase, is a Ser/Thr protein kinase that plays a crucial role in the regulation of several cellular signaling pathways. Akt/PKB is a regulator of cell survival and apoptosis, and its activation in a variety of cells can protect against apoptosis. Akt/PKB is phosphorylated at two regulatory sites, Thr308 and Ser473, which are essential for its activation. Activated Akt/PKB can phosphorylate BAD, IκB kinase, glycogen synthase kinase-3b, and the forkhead transcription factors[27,28], leading to their inactivation and cell survival. It has been reported that the phosphorylation of caspase-9 can regulate its activity[29]. Akt phosphorylates pro-caspase-9 at Ser196, which inhibits the proteolytic processing of pro-caspase-9.
WWOX blocks the activation of Akt, thereby attenuating the activity of a major anti-apoptotic pathway and inducing cell apoptosis. It remains unclear how WWOX affects Akt phosphorylation, as it does not affect PI3-kinase activity directly[30]. Other potential consequences of WWOX inhibition, such as the modulation of the RAS-signaling pathway, the expression of p53 and other members of the B-cell lymphoma 2 family, such as myeloid cell leukemia-1[31,32], the activation of the sphingomyelin-ceramide pathway, and interference with nuclear factor-κB[33] merit further investigation in the future.
In conclusion, WWOX may play a key role in tumor cell proliferation and carcinogenesis. Overexpression of WWOX can suppress the growth of HCC cells by inhibiting cell growth and inducing cell apoptosis. Apoptosis is induced by WWOX through the activation of the caspase cascade, which is correlated with the phosphorylation of Akt/PKB. These results suggest a potential role for WWOX as an effective chemotherapeutic and chemopreventive strategy against human liver cancer.
Several researches have revealed loss of heterozygosity of WWOX locus in gastric, pancreatic, esophageal and lung cancers. The role of WWOX in hepatic carcinoma is not well understood, and few studies have reported the effects of WWOX on hepatic carcinoma. In this study, the authors investigated the apoptotic effects of the WWOX gene on the human hepatic carcinoma cell line SMMC-7721.
This is the first report about WWOX gene relevant to human hepatoma cell line SMMC-7721.
By cloning the WWOX gene and transferring it into hepatocellular carcinoma cell line SMMC-7721, the authors investigated the growth-inhibiting and apoptosis-inducing effects of WWOX gene on human hepatoma cell line SMMC-7721 and concluded that the over-expression of WWOX gene could induce apoptosis and inhibit the growth of hepatic carcinoma cell line SMMC-7721.
WWOX may have a potential role in development of chemotherapeutic and chemopreventive strategies against liver cancer.
The tumor suppressor gene WWOX is localized in a common fragile site FRA16D (locus 16q23.3-24.1). Protein encoded by WWOX is an oxidoreductase containing two WW protein interaction domains.
The manuscript describes the results of studies on the effect of tumor suppressor gene, WWOX, expression in SMMC-7721. This is an interesting and well presented study, with thorough introduction and the succinct discussion of the topic.
Peer reviewer: Bronislaw L Slomiany, PhD, Professor, Research Center, C-875, University of Medicine and Dentistry, New Jersey-New Jersey Dental School, 110 Bergen Street, PO Box 1709, Newark, NJ 07103-2400, United States
S- Editor Shi ZF L- Editor Ma JY E- Editor Li JY
| 1. | Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H. Association of Wwox with ErbB4 in breast cancer. Cancer Res. 2007;67:9330-9336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Aqeilan RI, Hagan JP, de Bruin A, Rawahneh M, Salah Z, Gaudio E, Siddiqui H, Volinia S, Alder H, Lian JB. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology. 2009;150:1530-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res. 2002;62:2258-2260. [PubMed] |
| 4. | Yendamuri S, Kuroki T, Trapasso F, Henry AC, Dumon KR, Huebner K, Williams NN, Kaiser LR, Croce CM. WW domain containing oxidoreductase gene expression is altered in non-small cell lung cancer. Cancer Res. 2003;63:878-881. [PubMed] |
| 5. | Ishii H, Vecchione A, Furukawa Y, Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M, Saito Y. Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic malignancies. Mol Cancer Res. 2003;1:940-947. [PubMed] |
| 6. | Kuroki T, Yendamuri S, Trapasso F, Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML, Williams NN. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res. 2004;10:2459-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, Huebner K, Edmonds P, Croce CM. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res. 2004;10:3053-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene. 2002;21:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Gourley C, Paige AJ, Taylor KJ, Scott D, Francis NJ, Rush R, Aldaz CM, Smyth JF, Gabra H. WWOX mRNA expression profile in epithelial ovarian cancer supports the role of WWOX variant 1 as a tumour suppressor, although the role of variant 4 remains unclear. Int J Oncol. 2005;26:1681-1689. [PubMed] |
| 10. | Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer. 2004;100:1605-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Ishii H, Furukawa Y. Alterations of common chromosome fragile sites in hematopoietic malignancies. Int J Hematol. 2004;79:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA. 2001;98:11417-11422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Sbrana I, Veroni F, Nieri M, Puliti A, Barale R. Chromosomal fragile sites FRA3B and FRA16D show correlated expression and association with failure of apoptosis in lymphocytes from patients with thyroid cancer. Genes Chromosomes Cancer. 2006;45:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J, Weller M, Meyermann R. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 1999;67:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. J Clin Invest. 1995;95:2633-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 194] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10002] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 17. | Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1047] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3677] [Cited by in RCA: 3688] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 19. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5480] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 20. | Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 958] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 21. | Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3727] [Cited by in RCA: 3770] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 22. | Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2262] [Cited by in RCA: 2263] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 23. | Sakai T, Liu L, Teng X, Mukai-Sakai R, Shimada H, Kaji R, Mitani T, Matsumoto M, Toida K, Ishimura K. Nucling recruits Apaf-1/pro-caspase-9 complex for the induction of stress-induced apoptosis. J Biol Chem. 2004;279:41131-41140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22:4385-4399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1833] [Cited by in RCA: 1928] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 26. | Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1304] [Cited by in RCA: 1333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 27. | Fang X, Yu S, Eder A, Mao M, Bast RC, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635-6640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2297] [Cited by in RCA: 2291] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 30. | Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568-16575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 280] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 513] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001;276:48997-49002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041-37051. [PubMed] |