Published online Jun 21, 2012. doi: 10.3748/wjg.v18.i23.2938
Revised: March 26, 2012
Accepted: April 2, 2012
Published online: June 21, 2012
AIM: To investigated the interaction between toll-like receptor 4 (TLR4)-activated hepatoma cells and macrophages in the induction of tumor-immune suppression mediated by CD4+CD25high family of transcription factor P3 (FOXP3) regulatory T cells (Tregs).
METHODS: The proportion of FOXP3+ Tregs was identified in peripheral blood and tumor tissues of 60 hepatocellular carcinoma (HCC) patients. TLR4 expression was examined in tumor tissues and cell lines. The correlation was examined between FOXP3+ Tregs in peripheral blood and TLR4 expression of HCC tissues. Following activation of TLR4 in H22 murine hepatoma cells pre-incubated with lipopolysaccharide (LPS) and co-cultured with macrophage cell line RAW246.7, the synthesis of cytokines tumor necrosis factor-α, CCL22, and interleukin (IL)-10 by the two cell lines was detected and analyzed.
RESULTS: FOXP3+ Tregs were enriched in tumor sites, and circulating FOXP3+ Tregs were increased in HCC patients in correlation with multiple tumor foci and up-regulated TLR4 expression in HCC tissues. Semi-quantitative analysis indicated that TLR4 was over-expressed in HCC compared with the matched normal tissues. Cell cultivation experiments indicated that the mRNAs of IL-10 and CCL22 were significantly up-regulated in the RAW246.7 cell line when co-cultured with LPS pre-incubated H22 cells.
CONCLUSION: In hepatoma cell lines, TLR4 may indirectly facilitate the recruitment of Tregs to the tumor site and promote intrahepatic metastasis through its interaction with macrophages.
-
Citation: Yang J, Zhang JX, Wang H, Wang GL, Hu QG, Zheng QC. Hepatocellular carcinoma and macrophage interaction induced tumor immunosuppression
via Treg requires TLR4 signaling. World J Gastroenterol 2012; 18(23): 2938-2947 - URL: https://www.wjgnet.com/1007-9327/full/v18/i23/2938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i23.2938
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and is the third most common cause of cancer related deaths[1]. Previous studies have demonstrated that the majority of HCC patients develop tumor-specific immune responses; however, in most patients, tumors progress despite tumor-specific humoral and cellular immune responses. These findings imply that HCC escapes the anti-tumor immune response through various strategies.
Recently, many studies have suggested that the tumor microenvironment plays an important role in the establishment and progression of tumors. Lymphocytes contribute to the tumor microenvironment through immunity and inflammation. Through diverse strategies, tumor cells play an important role in the suppression of anti-tumor immunity in the surrounding microenvironment via interactions with infiltrated immune cells or macrophages, which in turn potentially facilitates growth of the tumor itself. Moreover, induction of the differentiation and/or recruitment of regulatory T cells (Tregs), a unique population of CD4+ T cells, potentially comprises one of the key mechanisms. Tregs are defined based on their expression of CD4, CD25 and forkhead, or winged helix family of transcription factor P3 (FOXP3), which is critical for the development and function of Tregs in mice and humans[2]. Tregs play a critical role in immunologic self-tolerance and suppression in the tumor immune response[3,4]. Early evidence indicates that Tregs (not FOXP3+ T cells but CD4+CD25+ T cells) are increased in patients with various types of cancer[5-7]. It is hypothesized that their systemic and/or local accumulation promotes tumor growth through suppression of the host anti-tumor response[8]. However, considerable uncertainty remains regarding the characteristics, functions, and regulation of Tregs. Therefore, we investigated the clinicopathologic significance of CD4+CD25highFOXP3+ Tregs in HCC patients, and analyzed their ability to suppress the immune response. Elucidation of the mechanisms underlying Treg elevation is essential for the development of new approaches that aim to modulate the frequency and function of Tregs in order to enhance the efficacy of cancer immune-based therapies.
Toll-like receptors (TLRs) recognize specific structural regions of invading pathogens and initiate innate and adaptive immune responses; their expression has been detected in immune cells and also in many cancer cells[9]. Lipopolysaccharide (LPS), a ligand for TLR4, stimulates immune cells and triggers the production of inflammatory cytokines and other mediators via TLR4, which in turn regulates the host immune defense system and eliminates pathogens[10,11]. It has been reported that inflammatory cytokines induced by inflammatory stimuli counteract immune surveillance and facilitate tumor growth[12,13]. However, various other reports have suggested that diverse TLRs potentially exhibit different effects on Tregs due to differences in pathogens and immune environment, resulting in either increased suppression or abrogation of suppression[14-17]. The suppressive function of Tregs is tightly regulated to respond to the different requirements of immunity. The mechanism underlying the selective control of Treg function remains obscure. Precise modulation of the suppressive function of Tregs is crucial for the development of effective cancer immunotherapy. Thus, in this study we investigated whether TLR4 is expressed in HCC, and whether tumor TLR4 is functionally active in inducing cytokines, and we also examined the clinicopathological correlation between tumor TLR4 signaling and CD4+CD25highFOXP3+ Tregs in tumor immune escape.
Currently, the roles of CD4+CD25highFOXP3+ Tregs and TLR4 in HCC and their regulatory activity in the tumor microenvironment remain unclear. In the present study, we described the clinicopathological significance of Tregs in 60 HCC patients, and the expression of TLR4 in hepatic cancer cells. Our findings indicated that TLR4 ligation promotes the secretion of inhibitory cytokine interleukin (IL)-10 and chemokine CCL22 from co-cultured macrophages, but not from the tumor cells themselves. Furthermore, the prevalence of Tregs significantly correlated with the presence of multifocal tumor. Our results suggest a mechanistic path for the indirect modulation of CD4+CD25highFOXP3+ Tregs via tumor TLR4 signaling, and demonstrate that interactions between hepatoma cells and macrophages induce anti-tumor immune suppression via Tregs.
This study was conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Ethics Committee of Tongji Medical College (Wuhan, China) approved the study protocol. All patients provided informed written consent before blood and tumor sampling.
Blood samples were collected from 60 HCC patients who underwent hepatic resection in the Center of Hepatobiliary Surgery, Union Hospital, Wuhan, China from March 2008 to October 2008. Control blood samples were obtained from 20 healthy volunteers. HCC patients were pathologically diagnosed following surgical resection. Among the 60 HCC patients, there were 51 males and nine females aged 17-77 years (mean, 51.3 years). According to the International Union against Cancer tumor-node-metastasis classification[18], there were 19 (31.7%), 24 (40%) and 17 (28.3%) cases at stage I, II and III, respectively. No patient was treated with local ablative therapy, chemotherapy, or immunotherapy prior to surgery. Clinical and laboratory characteristics of the HCC patients are shown in Table 1. Blood samples (3 mL) were collected 2-3 d before operation from each patient in the early morning. Samples were placed in ethylenediaminetetraacetic acid anticoagulant tubes for flow cytometric detection in our hospital. Next, 1 cm × 1 cm × 1 cm tumor and normal tissues were obtained from each patient intraoperatively, avoiding areas of necrosis, hemorrhage, and/or adipose tissues. One portion of each specimen was snap frozen in liquid nitrogen and the other part was fixed in 10% polyformaldehyde solution and embedded in paraffin.
| Items | Results |
| Age (yr) median (range) | 53.5 (17-77) |
| Gender (male/female) | 51/9 |
| Virus (HBV/HCV) | 48/4 |
| TNM stage (I/II/III/IV) | 19/24/17/0 |
| AFP (μg/L), median (range) | 350 (1.8-127 278) |
| Blood neutrophil (%) | 62.29 ± 10.96 |
| Blood monocyte (%) | 7.21 ± 1.69 |
| Hemoglobin concentration (g/L) | 124.47 ± 17.04 |
Peripheral blood mononuclear cells from each patient (100 μL) was separated into a heparinized container. Twenty microliters anti-human CD25-fluorescein isothiocyanate and anti-human CD4-PerCP was added to each tube, and incubated at room temperature in the dark for 15 min. After washing in 1 × phosphate-buffered saline, fixed broken membrane buffer 1 mL (1% paraformaldehyde and 70% ice-alcohol; pH 7.4) was added. The remaining lymphocytes were incubated in 20 μL FOXP3-PE at 4 °C for 30 min. Subsequently, cells were analyzed by flow cytometry (FACS Calibur, BD) with Cellquest software (Version 3.3, BD Biosciences-Pharmingen, United States). All conjugated antibodies described above and their isotype-matched monoclonal antibodies were purchased from BD, United States.
Lymphocytes were gated on forward and side scatter profiles followed by gating on CD4+ T cells, and these cells were then analyzed for CD25 expression. For FOXP3 expression analysis, cells inside the CD4+CD25high gate were analyzed.
Nuclear protein extracts were prepared in sodium dodecyl sulfate (SDS) lysis buffer containing protease inhibitors, pre-stained molecular weight markers were denatured in laemmli buffer (10% glycerol, 2% SDS, 0.1 mol/L dithiothreitol, 50 mmol/L Tris, 0.01 mg/mL bromphenol blue; pH 6.8) at 90 °C, and were separated by SDS-polyacrylamide gel electrophoresis. Resolved proteins were transferred onto polyvinylidene fluoride membrane in Trans-blot wet buffer (Bio-Rad Laboratories, United States). The membranes were blocked with 5% nonfat dry milk in 1 × tris-buffered saline (TBS), then incubated with 2 μg/mL mouse monoclonal anti-FoxP3 antibody (clone 22510; Abcam, United States) overnight at 4 °C followed by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) for 1 h at room temperature, and washed with 1 × TBS. Membranes were treated with enhanced chemiluminescence plus Western blotting detection kit (Transgen, Beijing, China), and bands were detected using STORM 840v2005 with ImageQuant software (GE, United States).
If not indicated otherwise, all substances were purchased from Gibco, United States. The H22 (murine) and HepG2 (human) hepatic cancer cell lines were a gift from Dr. Huang Bo (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China). The immortalized murine macrophage line RAW246.7 was preserved in our laboratory. The cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. All experiments were performed under endotoxin-free conditions.
The various cellular components were grown in an artificial basement membrane in a modified Polyster-Transwell (Costar, United States) plate without direct cell-to-cell contact. 1 × 105 RAW246.7 cells/mL were seeded into the upper well of the Transwell plate (0.4 μm pore diameter), which consisted of a membrane permeable to liquids but not cells, whereas the lower well was filled to the top with RPMI + 10% fetal calf serum. H22 cells (1 × 106 cells/mL RPMI) were seeded into a 12-well plate. To activate TLR4 in H22 cells, LPS (Gibco, United States) was used at 1 μg/mL. The culture medium was removed after LPS-stimulating the H22 cells for 12 h. Next, the Transwells were inserted into the 12-well plate. Gene expression was compared between control and macrophages co-cultured with H22 cells after 24 h, and macrophages with or without conditioned tumor medium were analyzed using reverse transcriptase polymerase chain reaction (RT-PCR). All experiments were performed at least in triplicate.
Total RNA was extracted from patient samples, H22, HepG2, and RAW246.7 cells, using Trizol reagent (Invitrogen, United States). Using a first strand cDNA synthesis kit (ToYoBo, Shanghai, China), cDNA was generated with Oligo dT primer. Primers for TLR4, tumor necrosis factor-α (TNF-α), IL-10, CCL22 and β-actin were designed using Premier 5.0 software (Table 2). Primers were synthesized by Sangon Inc. Shanghai, China.
| Gene | Sequence | Product size (bp) | Annealing temperature (°C) |
| mTLR4 | 5′-GCTTTCACCTCTGCCTTCAC-3′ | 259 | 57 |
| 3′-AGGCGATACAATTCCACCTG-5′ | |||
| HuTLR4 | 5′-GAAATGGAGGCACCCCTTC-3′ | 506 | 52 |
| 3′-GAATATTCCTTTGCATAGGT-5′ | |||
| CCL22 | 5′-AAGACAGTATCTGCTGCCAGG-3′ | 141 | 57 |
| 3′-GATCGGCACAGATATCTCGG-5′ | |||
| IL-10 | 5′-GGTTGCCAAGCCTTATCGGA-3′ | 190 | 60 |
| 3′-ACCTGCTCCACTGCCTTGCT-5′ | |||
| TNF-α | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | 175 | 58 |
| 3′-TGGGAGTAGACAAGGTACAACCC-5′ | |||
| β-actin | 5′-TCACCCACACTGTGCCCATCTACGA-3′ | 300 | 50 |
| 3′-GATAACCGTTGCTCGCCAAGGCTAC-5′ |
Each reaction mixture contained 2.5 μL 10 × buffer, 2.5 μL 2.5 mmol MgCl2, 0.5 μL 10 mmol of dNTP, 0.2 μL 5 U/μL Taq DNA polymerase, 1 μL each of sense and antisense primer, and 1 μL cDNA in a final volume of 25 μL (Fermentas, United States). Reaction mixtures were incubated at 94 °C for 5 min to activate the Taq DNA polymerase, and then amplified using 40 cycles of 30 s at 94 °C (denaturation) and 40 s at annealing temperature for TLR4, TNF-α, IL-10, CCL22, and β-actin, respectively. PCR was performed using the Agarose Gel Electrophoresis Imaging Analysis System (Beijing, China).
To assess TLR4 protein expression in HCC and normal tissues, a polyclonal rabbit anti-human TLR4 (ab47093; Abcam, United States) was used. Paraffin-embedded sections (5-μm thick) were fixed in freshly prepared 10% paraformaldehyde for 5 min. After blocking the endogenous peroxidase activity with 0.3% hydrogen peroxide in TBS for 15 min, the sections were immersed in horse serum diluted 1:10 in TBS for 30 min to reduce nonspecific binding, and then incubated with the primary antibody overnight at 4 °C after washing in TBS. Next, the sections were incubated in biotinylated horse anti-mouse or goat anti-rabbit IgG for 30 min, and avidin-biotin-peroxidase complex for 30 min. After each step of the staining procedure, the sections were given three 5-min washes in TBS. Immunoreactivity (IR) was visualized using 1 mg/mL diaminobenzidine as chromogen and 0.01% hydrogen peroxide as substrate. The peroxidase reaction was stopped after 5 min with distilled water, and the sections were counter-stained with Toluidine blue, dehydrated, and then mounted with Entellan.
Slides were evaluated under a light microscope (× 400 magnification). For digital image analysis, the software Adobe Photoshop version 7.0 was used. Results were scored by two independent investigators as positive, heterogeneous, or negative. The two scores were averaged.
SPSS version 10.0 (SPSS Inc., United States) was used for statistical analysis. All data were expressed as mean ± SE. Statistical analyses were performed using the Student’s t test. If there was evidence of non-normality, Kruskal-Wallis one-way analysis of variance was used to test the difference in median among the groups. To analyze the correlation between Tregs and TLR4, Spearman’s rank correlation coefficients were performed. Difference was considered statistically significant at P < 0.05.
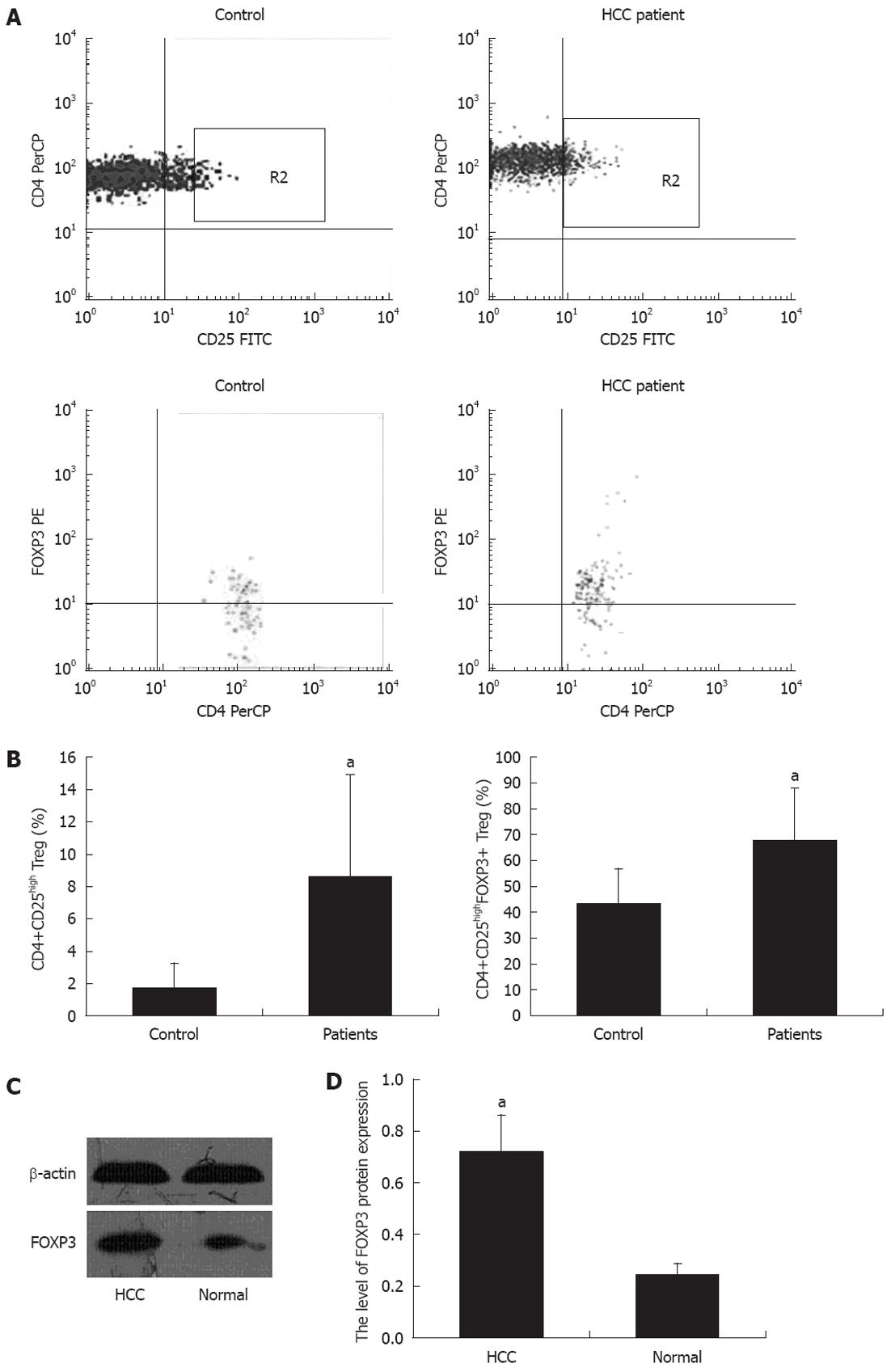
The prevalence of CD4+CD25high and CD4+CD25high FOXP3+ Tregs was analyzed in the peripheral blood of 60 patients with HCC. The population of CD4+CD25high and FOXP3+ Tregs as a percentage of total CD4+ T and CD4+CD25high T cells, respectively, was identified by flow cytometry. Representative dot plots of HCC patients and controls are shown in Figure 1A. The frequency of CD4+CD25high and FOXP3+ Tregs in HCC patients was significantly higher than in the healthy controls (Figure 1B). The expression of FOXP3 in HCC patients was detected using Western blotting analysis. As shown in Figure 1C and D, both tumor and normal tissues exhibited FOXP3 protein expression, although the expression was weaker in normal tissues.
We analyzed the correlation between the proportion of CD4+CD25highFOXP3+ Tregs in the peripheral blood and the clinicopathological characteristics of the subjects (Table 3). The proportion of CD4+CD25highFOXP3+ Tregs was significantly higher in patients with high serum AFP levels and multiple tumor foci (P = 0.009). The proportion of CD4+CD25highFOXP3+ Tregs did not correlate with other clinicopathological characteristics of HCC patients (P > 0.05).
| Items | n | Treg (%) | P values |
| Sex | 0.201 | ||
| Male | 51 | 68.95 ± 20.71 | |
| Female | 9 | 59.37 ± 18.97 | |
| UICC/TNM stage | 0.781 | ||
| I | 19 | 66.41 ± 20.28 | |
| II-III | 41 | 68.02 ± 20.97 | |
| AFP (μg/L) | < 0.001 | ||
| ≤ 20 | 23 | 53.13 ± 17.96 | |
| > 20 | 37 | 76.45 ± 16.84 | |
| HBsAg | 0.611 | ||
| Positive | 48 | 68.20 ± 19.69 | |
| Negative | 12 | 64.78 ± 24.66 | |
| Margin | 0.981 | ||
| Clear | 29 | 67.45 ± 19.74 | |
| Unclear | 31 | 67.57 ± 21.69 | |
| Capsule | 0.058 | ||
| Complete | 28 | 62.14 ± 21.92 | |
| Incomplete | 32 | 72.22 ± 18.43 | |
| Tumor diameter (cm) | 0.245 | ||
| ≤ 5 | 23 | 59.84 ± 19.06 | |
| 6-9 | 18 | 75.94 ± 18.43 | |
| ≥ 10 | 19 | 68.81 ± 21.84 | |
| Tumor number | 0.009 | ||
| Single | 52 | 63.83 ± 20.55 | |
| ≥ 2 | 8 | 86.41 ± 6.18 | |
| Cirrhosis | 0.881 | ||
| Presence | 23 | 68.02 ± 20.42 | |
| Absence | 37 | 67.19 ± 20.98 | |
| Tumor differentiation | 0.152 | ||
| WD | 11 | 56.61 ± 24.98 | |
| MD | 7 | 69.60 ± 20.45 | |
| PD | 42 | 70.02 ± 18.59 |
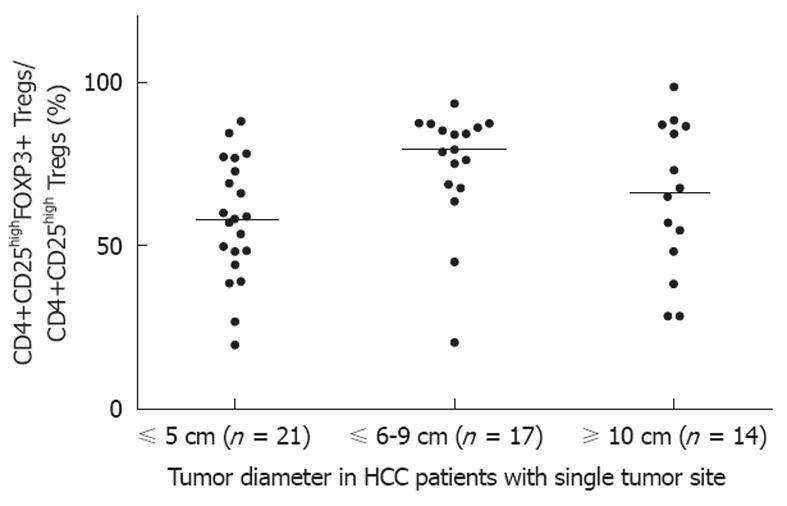
Considering the potential impact of multifocal tumor on tumor size, the correlation of the proportion of CD4+CD25highFOXP3+ Tregs with different tumor diameters was analyzed in 52 patients with a single lesion. Our findings indicated that the proportion of Tregs was low in patients with a small tumor (< 5 cm in diameter) (Figure 2).
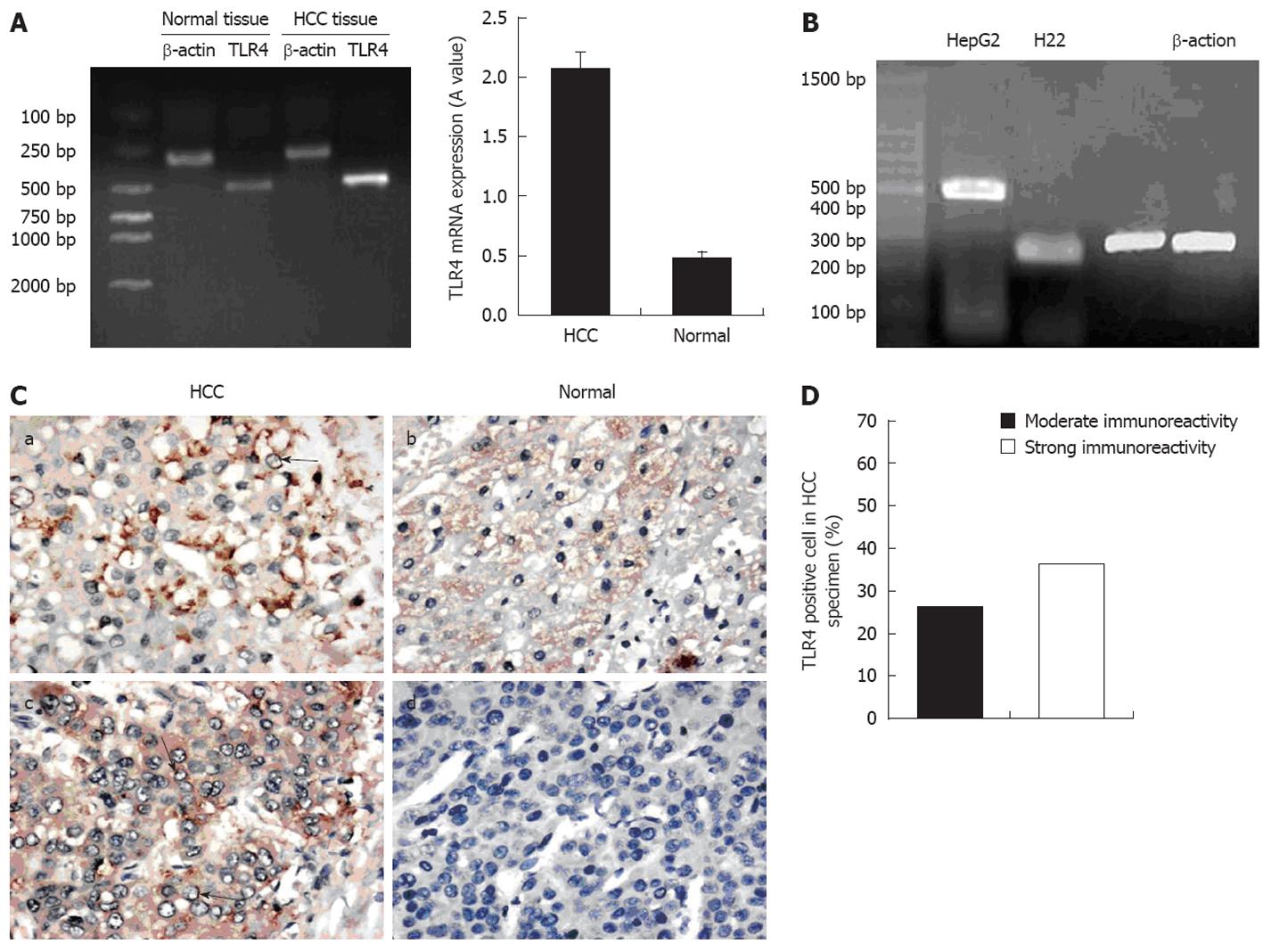
The expression of TLR4 in patient tumor samples and hepatoma cell lines was examined using RT-PCR. Tumor and normal tissues, as well as HepG2 and H22 cells, expressed TLR4 mRNA (Figure 3). We determined the relative expression of TLR4 mRNA (TLR4 mRNA level/β-actin mRNA level × 100%) and found that TLR4 mRNA expression level in HCC tissues was higher than in the normal tissues (P = 0.01) (Figure 3A). Additionally, immunohistochemical analysis confirmed the expression of TLR4 protein. TLR4 positive hepatocytes were present in paraffin-embedded sections. Moderate and strong IR for TLR4 was detected in 63.4% (38/60) of HCC specimens, and normal tissues displayed positive staining in 10% (6/60). TLR4 in cancer cells was stained more intensely than in the normal cells (Figure 3C). The distribution of TLR4 in a given HCC specimen was uneven, and the majority of positive hepatocytes exhibited expression on the membrane and in the cytoplasm. Scattered expression of TLR4 protein was also observed in a small number of liver sections derived from normal tissues.
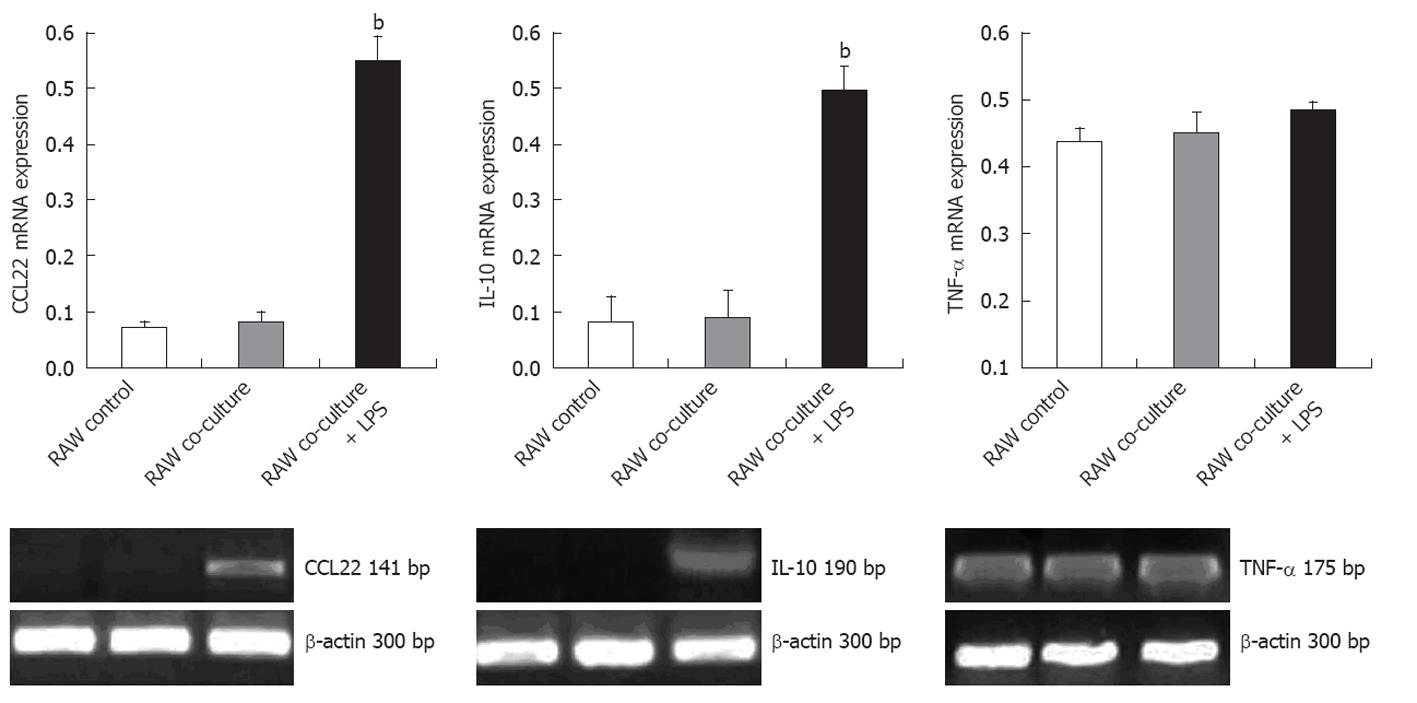
Hepatoma cell line H22 cells were pre-incubated with LPS. The RAW246.7 cells and the H22 cells were co-cultured in a transwell system for 24 h. Semiquantitative RT-PCR was performed to detect the expression of TNF-α, IL-10, and CCL22 in different cell lines. RAW246.7 cells co-cultured with LPS pre-treated H22 cells exhibited significant up-regulation of mRNA for genes IL-10 and CCL22 (P < 0.001), whereas expression of TNF-α remained unchanged (Figure 4). Tumor cells without LPS pre-incubation induced negligible gene expression changes in co-cultured RAW246.7 cells. H22 cells alone did not produce IL-10 and CCL22 under conditions of TLR4 activation following LPS stimuli or not (data not shown). These findings, taken together, suggested that activated TLR4 on H22 cells facilitated the induction of cytokine secretion of IL-10 and CCL22 by RAW246.7 cells.
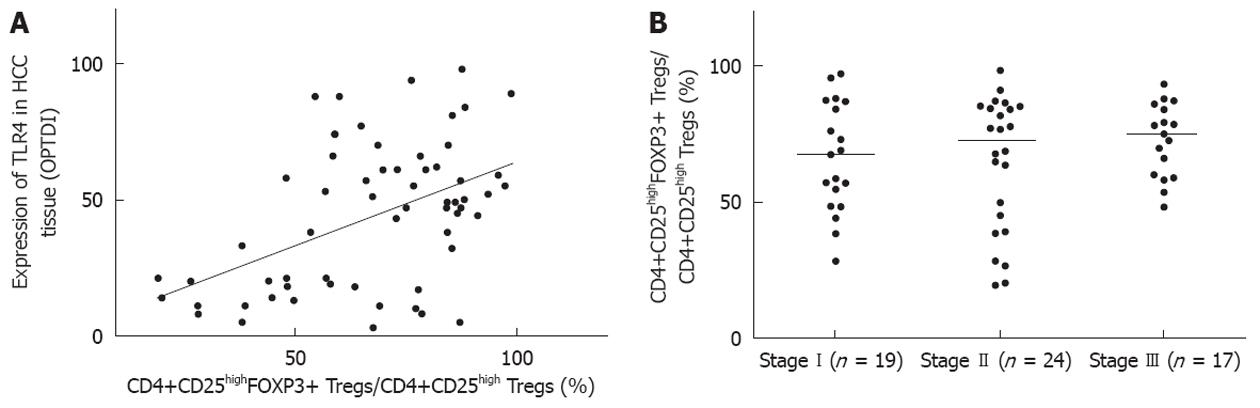
We investigated the associations between tumor Union for International Cancer Control (UICC) stage, circulating CD4+CD25highFOXP3+ Tregs, and TLR4 in the HCC tissues. The expression level of TLR4 protein in 60 HCC specimens was positively correlated with the frequency of CD4+CD25highFOXP3+ Tregs in peripheral blood (Figure 5A). There was an increased frequency of Tregs in the peripheral blood of HCC patients, which exhibited a high level of expression of TLR4 protein. However, there was no significant correlation between the number of CD4+CD25highFOXP3+ Tregs and tumor UICC stage (Figure 5B).
T cells play an essential role in the immunosurveillance and destruction of cancer cells. Among CD4+ T lymphocytes, CD4+CD25+ Tregs are thought to comprise a functionally unique subpopulation of T cells that act to maintain immune homeostasis. The lack of CD4+CD25+ Tregs results in various autoimmune syndromes. Alternatively, Tregs-mediated suppression potentially hinders an effective immune response, which is crucial for elimination of tumors and infection[19]. Humans with cancer exhibit increased numbers of peripherally circulating and tumor Tregs[5,20,21]. Ormandy et al[22] found that in HCC patients the number of Tregs in the peripheral blood was significantly increased. However, Yang et al[23] found that the proportion of CD4+CD25+ Tregs in the peripheral blood of HCC patients was significantly decreased. Too few samples and different patient inclusion criteria has potentially led to conflicting results in previous studies. This study presents evidence of an accumulation of FOXP3+ Tregs in HCC tumor tissues and peripheral blood. A few previous studies have also investigated the clinicopathologic significance of Tregs, but conclusions regarding correlations with various clinicopathologic features were contradictory. Our results demonstrated that the proportion of Tregs was not correlated with hepatitis and cirrhosis, which was in concordance with other studies[22,24]. Shen et al[25] indicated that the increased prevalence and expanded function of Tregs in the tumor microenvironment of HCC correlated with cancer stage. However, no significant differences between number of Tregs and tumor UICC stage were observed in our HCC patients; other research groups also confirmed our findings[26,27]. The discrepancy among different research groups potentially results from differences in patient profiles, e.g., Shen et al[25] selected 31 patients who had undergone hepatectomy for their study. Moreover, we demonstrated a positive correlation between the number of Tregs and serum AFP levels and multifocal tumor. In order to exclude the influence of multifocal tumor, we separately analyzed 52 HCC patients with a single lesion, and found that the proportion of Tregs was higher in patients with a larger tumor size (Figure 3, P = 0.0252). Our results suggested that the number of Tregs in HCC potentially contributes to the inhibition of effective anti-tumor immune responses and promotes intrahepatic tumor metastasis.
Despite the important role of Tregs in controlling immune responses to self-antigen and non-self-antigens and their natural ligands, the molecular mechanisms underlying the regulation and recruitment of Tregs in the tumor microenvironment remain poorly understood. One family of receptors involved in immune regulation is TLRs, a class of receptors that recognizes pathogen-associated molecular patterns or endogenous inflammation-associated molecules[28]. Recently, TLRs were identified on cancer cells and T cells, including Tregs[9,11]. Thus, TLR-signaling directly or indirectly regulates the immunosuppressive function of Tregs in the immune response[29-32]. A few studies concerning indirect regulatory function have been conducted to clarify the complex cross-talk between TLRs and Tregs in tumors. Our data provide some important clues regarding the interaction of TLR4 expression in tumor tissues and the number of Tregs in hepatocellular cancer. We demonstrated that TLR4 was expressed in diverse hepatoma cells and enriched in HCC tumor tissues, and that the expression of TLR4 in HCC positively correlated with the frequency of FOXP3+ Tregs in both circulation and tumor tissues (P < 0.001). These results suggest that the recruitment and proliferation of Tregs are indirectly regulated via TLR4 signaling.
Although some evidence implicates Tregs in the immunopathogenesis of cancer, suppressive mechanisms in the tumor microenvironment and the underlying mechanisms of regulation remain poorly understood[33]. Early evidence suggests that CCL22 preferentially attracts activated antigen-specific T cells to dendritic cells[34]. Curiel recently demonstrated that tumor cells and microenvironmental macrophages in ovarian carcinoma produce the chemokine CCL22, which mediates Treg trafficking to the tumor[35]. Therefore, tumor cells potentially utilize this effect to attract Tregs to the microenvironment. Our data suggested that co-culture of macrophages with a hepotoma cell line leads to a significant increase in the expression of IL-10 and CCL22 (P < 0.001). The source of this CCL22 is co-cultured macrophages and hepatoma cells preincubated with LPS. IL-10 is a pleiotropic cytokine produced by myeloid cells and lymphocytes that displays immunoregulatory effects[36]. IL-10 inhibits the production of other cytokines such as IL-2, IL-12 and TNF-α and plays an important role in suppression of T cell anti-tumor responses[37-39]. In this study, we demonstrated that co-cultured macrophages and LPS pre-treated hepatoma cells significantly up-regulate IL-10 expression. Our findings indicated that the activation of TLR4 on hepatoma cells indirectly modulates the suppressive function of Tregs and enhances Tregs recruitment by inducing cytokines, resulting in the immune escape of HCC.
The accumulation of FOXP3+ Tregs in HCC suggests a trend toward intrahepatic metastasis. The modulation of TLR4 activation on tumor cells links between Tregs suppressive function and the immunopathogenesis of human cancer. The association between TLR4-signaling and functional control of CD4+CD25highFOXP3+ Tregs indicates intriguing potential opportunities to suppress anti-HCC immunity and improve therapeutic effectiveness for the patients. Our study provides the evidence which is useful for the development of an improved immunotherapeutic approach to HCC, and further studies are warranted.
We thank Ms. Yanli He for her technical assistance in flow cytometric detection.
The survival of hepatocellular carcinoma (HCC) is limited in the majority of such cases. The active suppression of immune responses against tumor is a major barrier to the likely success of cancer immunotherapy. There is now compelling evidence implicating CD4+CD25high family of transcription factor P3 (FOXP3)+ regulatory T cells (Tregs) as being key players driving immune suppression. However, precise function regulation of CD4+CD25highFOXP3+ Tregs remains obscure.
Toll-like receptors (TLRs) have recently emerged as a critical pathogen-associated molecular pattern of the innate immune system for detecting microbial infection and activation of dendritic cell maturation programs to induce adaptive immune responses. TLR4, an important member of TLRs family, plays a central role in phagocytosis, signal transduction and cell apoptosis. New evidence suggests that TLR signaling may directly or indirectly regulate the suppressive function of Treg cells. TLR4 signaling pathway activation induces production of massive cytokines, and finally intrigues the downstream nuclear factor kappa B to shift the balance between CD4+ T-helper and Treg cells, in ways that may facilitate tumor evasion of immune surveillance.
Currently, the roles of CD4+CD25highFOXP3+ Tregs and TLR4 in HCC and their regulatory activity in the tumor microenvironment remain unclear. In the present study, authors described the clinicopathological significance of Tregs in 60 HCC patients, and the expression of TLR4 in hepatic cancer cells. The findings indicated that TLR4 ligation promotes the secretion of inhibitory cytokine interleukin-10 and chemokine CCL22 from co-cultured macrophages, not from the tumor cells themselves. Furthermore, the prevalence of Tregs significantly correlated with the presence of multifocal tumor. The results suggest a mechanistic path for the indirect modulation of CD4+CD25highFOXP3+ Tregs via tumor TLR4 signaling, and demonstrate that interactions between hepatoma carcinoma cells and macrophages induce anti-tumor immune suppression via Tregs.
The authors report that activation of TLR4 on hepatoma cells followed by its interaction with macrophages may indirectly facilitate the recruitment of Tregs into tumor site and promote the intrahepatic metastasis, which suggests innate immunity mediated cellular interference maybe an effective therapeutic target in the treatment of HCC patients.
CD4+CD25highFOXP3+ Treg: Tregs are defined based on their expression of CD4, CD25 and forkhead, or winged helix FOXP3, which is critical for the development and function of Tregs in mice and humans. Tregs play a critical role in immunologic self-tolerance and suppression in the tumor immune response; TLR: To recognize specific structural regions of invading pathogens and initiate innate and adaptive immune responses; their expression has been detected in immune cells and also in many cancer cells. TLR4, an important member of TLRs family, plays a central role in phagocytosis, signal transduction and cell apoptosis.
The article shows that TLR on hepatoma cell lines may indirectly facilitate the recruitment of Treg cells within tumor site via the potential activation of macrophages. This is a good exploratory study in which authors analyze the relationship of tumor TLR4 signaling pathway and CD4+CD25highFOXP3+ Treg cells in tumor immune escape.
Peer reviewer: Dr. Fabrizio Montecucco, Internal Medicine, University of Geneva, avenue de la Roseraie, 64, 1211 Geneva, Switzerland
S- Editor Gou SX L- Editor Ma JY E- Editor Zheng XM
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 2. | Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 702] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 3. | Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18-32. [PubMed] [DOI] [Full Text] |
| 4. | Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. [PubMed] |
| 6. | Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009-5014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6034] [Cited by in RCA: 6316] [Article Influence: 300.8] [Reference Citation Analysis (0)] |
| 11. | Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403-411. [PubMed] |
| 12. | He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Yang H, Zhou H, Feng P, Zhou X, Wen H, Xie X, Shen H, Zhu X. Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J Exp Clin Cancer Res. 2010;29:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 579] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 15. | Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 584] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 16. | van Maren WW, Jacobs JF, de Vries IJ, Nierkens S, Adema GJ. Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology. 2008;124:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Signaling through TLR7 enhances the immunosuppressive activity of murine CD4+CD25+ T regulatory cells. J Leukoc Biol. 2010;87:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Greene FL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th ed. New York: Springer 2002; 131-144. |
| 19. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 692] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 20. | Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398-7405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 23. | Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 27. | Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85-95. [PubMed] [DOI] [Full Text] |
| 29. | Wang RF, Peng G, Wang HY. Regulatory T cells and Toll-like receptors in tumor immunity. Semin Immunol. 2006;18:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249-7258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048-7053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033-1036. [PubMed] [DOI] [Full Text] |
| 33. | Wang RF. Regulatory T cells and innate immune regulation in tumor immunity. Springer Semin Immunopathol. 2006;28:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Tang HL, Cyster JG. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819-822. [PubMed] |
| 35. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3880] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 36. | Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci MG, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371-2376. [PubMed] |
| 38. | Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099-2105. [PubMed] |
| 39. | Zeng L, O'Connor C, Zhang J, Kaplan AM, Cohen DA. IL-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine. 2010;49:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |