Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2704
Revised: February 2, 2012
Accepted: March 9, 2012
Published online: June 7, 2012
AIM: To investigate the function of gamma-aminobutyric acid (GABA) and gamma-aminobutyric acid A receptor θ subunit (GABRQ) in hepatocellular carcinoma (HCC).
METHODS: Semiquantitative polymerase chain reaction was used for detecting the expression of GABRQ receptor among HCC cell line HepG2, normal liver cell line L-02, non-malignant Chang’s liver cells, 8 samples of HCC tissues and paired non-cancerous tissues. HepG2 cells were treated with GABA at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L), and their proliferating abilities were analyzed with the methyl thiazolyl tetrazolium assay, cell cycle analysis and tumor implanted in nude mice. Small interfering RNA was used for knocking down the endogenous GABRQ in HepG2. Proliferating abilities of these cells treated with or without GABA were analyzed.
RESULTS: We identified the overexpression of GABRQ in HCC cell lines and half of the tested HCC tissues. Knockdown of endogenous GABRQ expression in HepG2 attenuated HCC cell growth, suggesting its role in HCC cell viability. We studied the effect of GABA in the proliferation of GABRQ-positive cell lines in vitro and in vivo, and found that GABA increased HCC growth in a dose-dependent manner. Notably, the addition of GABA into the cell culture medium promoted the proliferation of GABRQ-expressing HepG2 cells, but not GABRQ-knockdown HepG2 cells, which means that GABA stimulates HepG2 cell growth through GABRQ.
CONCLUSION: GABRQ play important roles in HCC development and progression and could be a promising molecular target for the development of new diagnostic and therapeutic strategies of HCC.
- Citation: Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q, Xie PL, Li GC. GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World J Gastroenterol 2012; 18(21): 2704-2711
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2704
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and one of the most common malignancies in the world, accounting for approximately one million deaths per year[1,2]. Although liver resection and local ablation are regarded as potentially curative treatment[3], its prognosis is poor. Most of the patients are diagnosed with advanced disease at presentation for which palliative therapy forms the mainstay of treatment[4].
To improve this situation, the development of novel molecular therapies against effective targets is an urgent issue. Toward this direction, we previously used a method combining an in silico screen and experimental verification to identify genes that are differentially expressed in cancers compared with their corresponding normal tissues[5]. Among genes that are overexpressed in HCC cells, we focused on the gamma-aminobutyric acid (GABA) gene. Gamma-aminobutyric acid A receptor θ subunit (GABRQ) is a subunit of gamma-aminobutyric acid A (GABAA) receptors that may associate with other GABAA receptor subunits to form a functional chloride channel which mediates inhibitory synaptic transmission in the mature central nervous system (CNS). GABA primarily functions as an inhibitory neurotransmitter in the mature CNS by activating the GABA receptor, but it can also modulate the proliferation, migration and differentiation of neuronal cells during CNS development[6-9] and the proliferation of peripheral non-neuronal cells[10,11]. GABA and GABAA receptors are also present in peripheral tissues, including cancerous cells, but their precise functions are poorly defined.
This study demonstrates that GABRQ is overexpressed in HCC and that GABA promotes the proliferation of cancer cells through GABRQ.
HCC cell line HepG2 and normal liver cell lines Chang’s liver and L-02 were maintained by our lab and cultured in Dulbecco-modified Eagle medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco). Cells were maintained at 37 °C atmosphere of humidified air with 5% CO2.
All samples of HCC tissues and paired non-cancerous tissues (5 cm away from tumor) were obtained during surgical resection from the Xiangya Hospital of Central South University. Written consent was obtained from the patients, who agreed to the collection of tissue samples. The resected tissue samples were immediately cut into small pieces and snapfrozen in liquid nitrogen until use. All tumor tissue and paired non-cancerous tissue samples were pathologically confirmed.
RNA isolated from cells was reverse-transcribed and amplified using the One-Step reverse transcription polymerase chain reaction (RT-PCR) System (Fermentas, Vilnius, Lithuania). The sets of primers for GABRQ receptor subunit are Sense 5’-TCGAGTTCTCCTCTGCTGTG-3’, Antisense 5’-TATGCAGATCCAGGGACAA-3’ (465 bp); Sense 5’-AATCCCATCACCATCTTCCA-3’ and antisense 5’-CCTGCTTCACCACCTTCTTG-3’ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 580 bp). After heating at 95 °C for 1 min, samples were exposed to 30 cycles (GAPDH, 25 cycles) of 95 °C for 30 s, 60 °C for 30 s and 68 °C for 1 min 30 s with a final extension at 68 °C for 10 min. Reaction products were separated on 1.5% agarose gels containing ethidium bromide and the level of amplification was analyzed using a Phosphor Imager.
To knockdown GABRQ expression, we used pGCsi-U6/Neo/GFP vector encoding a small hairpin RNA directed against the target gene in HepG2.The target sequences for GABRQ were: 5’-taGCAAGGAGGTGTATTTCTA-3’ (Si-1), 5’-caGCTATGGTGTTCGCTTTAA-3’(Si-2), 5’-
caGGCTGATGACAGTA TTATT-3’(Si-3), 5’-aaGGATGCTTTCGTGCATGAT-3’(Si-3). As a negative control, we used shRNA vector without hairpin oligonucleotides (Si-Mock).
Human HCC cell line HepG2 was plated onto 6-well plates, and transfected with these small interfering RNA (siRNA) expression vectors using FuGENE6 (Roche) according to the instructions of the manufacturer, followed by 800 μg/mL of neomycin selection. The cells were harvested 10 d later to analyze the knockdown effect on GABRQ by RT-PCR using the primers shown above and by flow cytometry using rabbit anti-human polyclonal antibody against GABRQ (Chemicon).
HepG2/Si-1, HepG2/Si-Mock cells were seeded with serum-free medium at a density of 103 cells/well in 96-well plates (n = 6), grown overnight, washed in phosphate-buffered saline (PBS), and incubated with 10% FBS with or without 40 μmol/L GABA DMEM at 37 °C, 5% CO2, for varying periods and exposed to fresh media every other day. During the last 4 h of each day’s culture, the cells were treated with methyl thiazolyl tetrazolium (MTT, 50 μg per well, Sigma, United States). The generated formazan was dissolved in dimethyl sulfoxide (DMSO) and the ODs at 490 nm were measured for detecting the cell viability.
The effect of GABRQ silencing on the colony formation of HepG2 cells was analyzed by colony formation assay. HepG2/Si-1, HepG2/Si-Mock cells at 100 cells per well in 6-cm plates were incubated with serum-fee medium for 24 h, and then cultured in 10% FBS with or without 40 μmol/L GABA DMEM at 37 °C, 5% CO2, for 3 wk. The cell colonies were washed twice with PBS, fixed by 4% paraformaldehyde for 15 min and stained with Giemsa for 30 min. Individual clones with more than 50 cells were counted. Clone forming efficiency for individual type of cells was calculated, according to the number of colonies/number of inoculated cells × 100%.
To evaluate the impact of GABRQ silencing on the HepG2 cells and the effect of GABA stimulation on the HepG2 cells, cell cycle was examined by flow cytometry analysis. HepG2/Si-1, HepG2/Si-Mock cells were incubated with serum-fee medium for 24 h, and then cultured in DMEM with 10% FBS with or without 40 μmol/L GABA, then harvested at 70%-80% confluence and resuspended in fixation fluid at a density of 106/mL; 1500 μL propidium iodide (PI) solution was added, and the cell cycle was detected by FACS Caliber (Becton-Dickinson).
To study the effect of GABA on the proliferation of GABRQ-expressing HCC cells, cell proliferation was tested in vitro. In the MTT assay, HepG2 cells were seeded with serum-free medium at a density of 103 cells/well in 96-well plates (n = 6), grown overnight, washed in PBS, and incubated with GABA (Sigma-Aldrich) at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L) in appropriate medium supplemented with 1% FBS. The samples were tested every 24 h for 6 d. MTT was added (50 μg/well) for 4 h. Formazan products were solubilized with DMSO, and the optical density was measured at 490 nm.
In the flow cytometry assay, HepG2 cells were incubated with serum-fee medium for 24 h, and then cultured in DMEM with 10% FBS and serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L) GABA for 48 h. Cells were harvested and resuspended in fixation fluid at a density of 106/mL, 1500 μL PI solution was added, and the cell cycle was detected by FACS Caliber (Becton Dickinson).
The influence of GABRQ silencing and GABA stimulation on the tumor development of HCC in vivo was examined. Briefly, HepG2, HepG2/Si-Mock and HepG2/Si-1 cells were treated with or without GABA (40 μmol/L) for 24 h first, and then the cells (3 × 106) were suspended in 0.2 mL of extracellular matrix gel and injected subcutaneously in the left back flank of the animals. The 8-wk-old BALB/c nude (nu/nu) mice (Slac Laboratory Animal Center, Shanghai, China) were divided into six groups: (1) the mice were injected with HepG2 and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2, n = 4); (2) the mice were injected with HepG2 and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the implanted tumor (HepG2 + GABA, n = 4); (3) the mice were injected with HepG2/ Si-Mock and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2/Si-Mock, n = 4); (4) the mice were injected with HepG2/Si-Mock and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the implanted tumor (HepG2/Si-Mock + GABA, n = 4); (5) the mice were injected with HepG2/Si-1 and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2/Si-1, n = 4); and (6) the mice were injected with HepG2/Si-1 and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the implanted tumor (HepG2/Si-1 + GABA, n = 4). The same operator carried out the injections every other day starting from “day 0” when the tumors were implanted. Tumor variables were measured every 3 d by an electronic caliper, and tumor volume was calculated using a standard formula[12,13]: tumor volume = width2× length × 0.5. At the end of the experiment, all mice were sacrificed and individual tumor weights were measured.
All data were expressed as mean ± SD. Differences among groups were determined by analysis of variance analysis and comparison between two groups was analyzed by the Student’s t test using the GraphPad Prism soft-ware version 4.0 (GraphPad Software, Inc, San Diego, CA). A value of P < 0.05 was used to indicate statistical significance.
We documented GABRQ mRNA expression in HepG2, Chang’s liver and L-02 cell lines as well as in 8 pairs of HCC and adjacent non-tumor tissues. The results of semiquantitative RT-PCR show GABRQ receptor subunit was detected in HepG2 and in Chang’s liver cells, but not in normal cell line L-02 (Figure 1A). GABRQ receptor subunit was also detected in HCC tissues (6/8), but not in adjacent non-tumor tissues (Figure 1B).
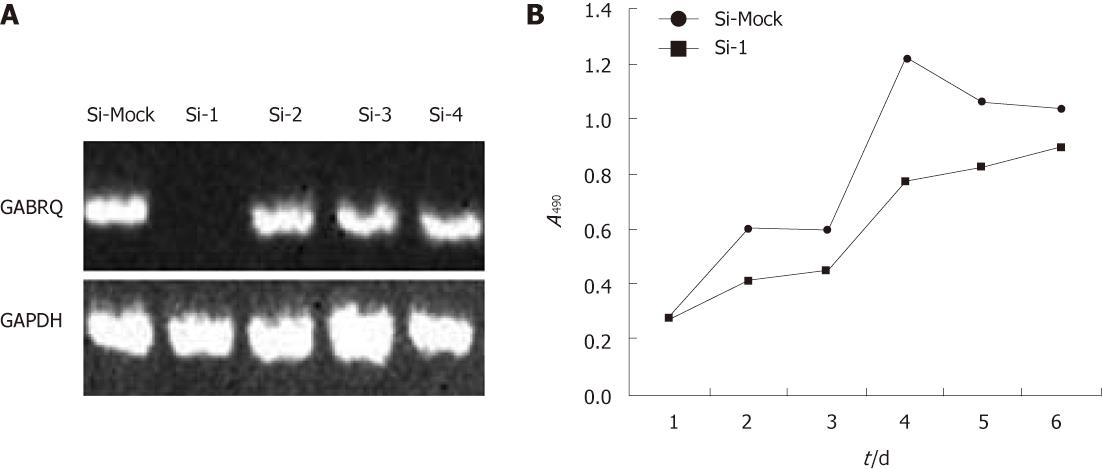
To investigate the biological significance of GABRQ overexpression in HCC cells, we constructed four siRNA expression vectors (Si-1, Si-2, Si-3 and Si-4) specific to GABRQ transcripts and transfected them into HepG2 cells that endogenously expressed high levels of GABRQ, as shown in Figure 1. A knockdown effect was observed by RT-PCR when we transfected Si-1, but not Si-2, Si-3, Si-4 or a negative control Si-Mock (Figure 2A). MTT assay (Figure 2B) revealed a drastic reduction in the number of cells transfected with Si-1 compared with Si-Mock for which no knockdown effect was observed. Cell proliferation was detected by flow cytometry; results showed HepG2 cells with GABRQ siRNA blocked the cell cycle in G1 phase, which may inhibit the growth of HepG2 cells (Table 1). This result was consistent with the MTT analysis.
| Si-1 | Si-Mock | |
| G0/G1 (%) | 53.95 ± 3.22 | 49.95 ± 3.56 |
| G2/M (%) | 22.38 ± 2.79 | 21.61 ± 3.83 |
| S (%) | 24.29 ± 3.32 | 28.74 ± 3.85a |
A RT-PCR verified the knockdown effect on GABRQ expression by Si-1, but not by Si-2, Si-3, Si-4 and a negative control Si-Mock in HepG2 cells. GPDH was used to quantify RNAs; Figure 2B illustrates MTT assay of HepG2 cells transfected with Si-1 vectors to GABRQ and a negative control vector (Si-Mock). Y-axis: Average value of absorbance at 490 nm, measured with a microplate reader (n = 6, P < 0.05).
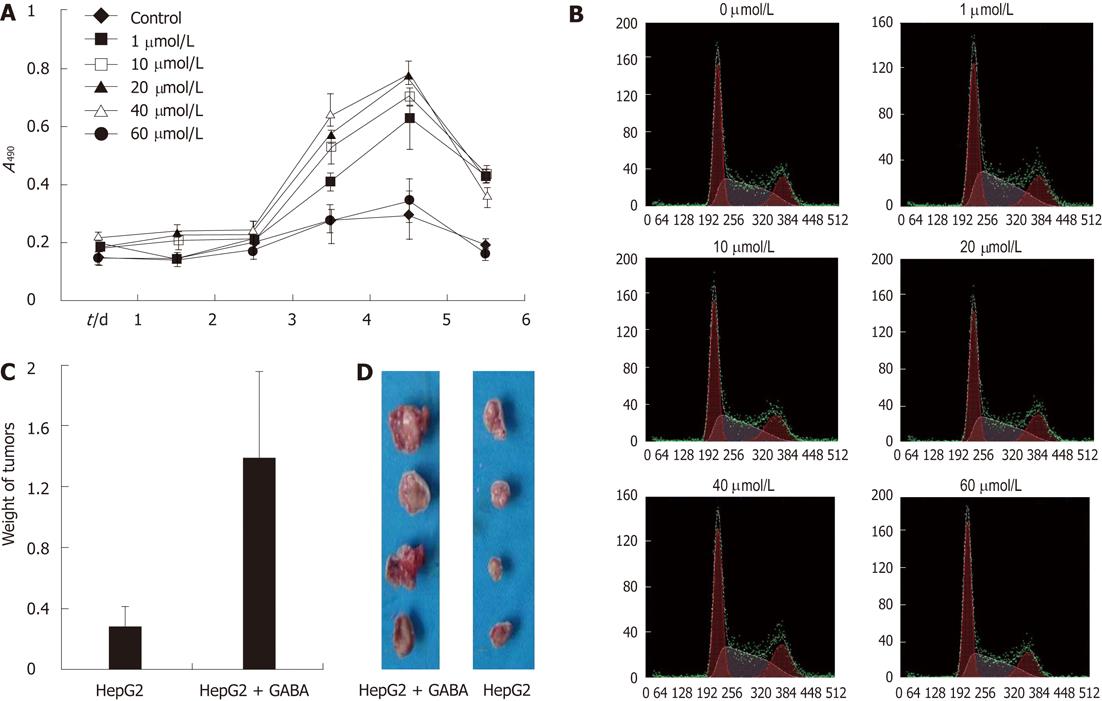
Results displayed in Figure 3A show the addition of GABA in the culture media enhanced the proliferation of HepG2 cells in a dose-dependent manner. The promoting effect on HCC cell proliferation was more evident with the GABA concentration ranging from 1 μmol/L to 40 μmol/L. When the GABA concentration was increased to 60 μmol/L, the promoting effect became insignificant.
The promoting effect on HCC cell proliferation was also detected by flow cytometry analysis. After treating with GABA at serial concentrations, the G0/G1-phase fraction of HepG2 cells significantly decreased; on the contrary, S-phase cells significantly increased, especially at the concentration of 20 μmol/L and 40 μmol/L (Figure 3B, Table 2); this result was consistent with the results above.
| 0 | 1 | 10 | 20 | 40 | 60 | |
| G0/G1 (%) | 40.9 ± 2.92 | 39.6 ± 2.73 | 33.8 ± 2.79 | 31.7 ± 2.56 | 30.2 ± 2.17 | 47.2 ± 2.93 |
| G2/M (%) | 18.1 ± 0.84 | 19.8 ± 0.97 | 20.7 ± 0.85 | 21.3 ± 0.79 | 20.3 ± 1.18 | 18.5 ± 1.23 |
| S (%)a | 31.0 ± 1.89 | 30.6 ± 1.94 | 35.5 ± 2.28 | 37.0 ± 2.17 | 39.5 ± 2.34 | 34.3 ± 2.02 |
In the nude mice implanted with tumors (injected with HepG2 cells), the development of solid HCC tumors was monitored for 40 d. As a result, a significant difference in tumor weight was found in GABA-treated (at the concentration of 40 μmol/L) mice compared with mice injected with 0.9% NaCl only (Figure 3C and D).
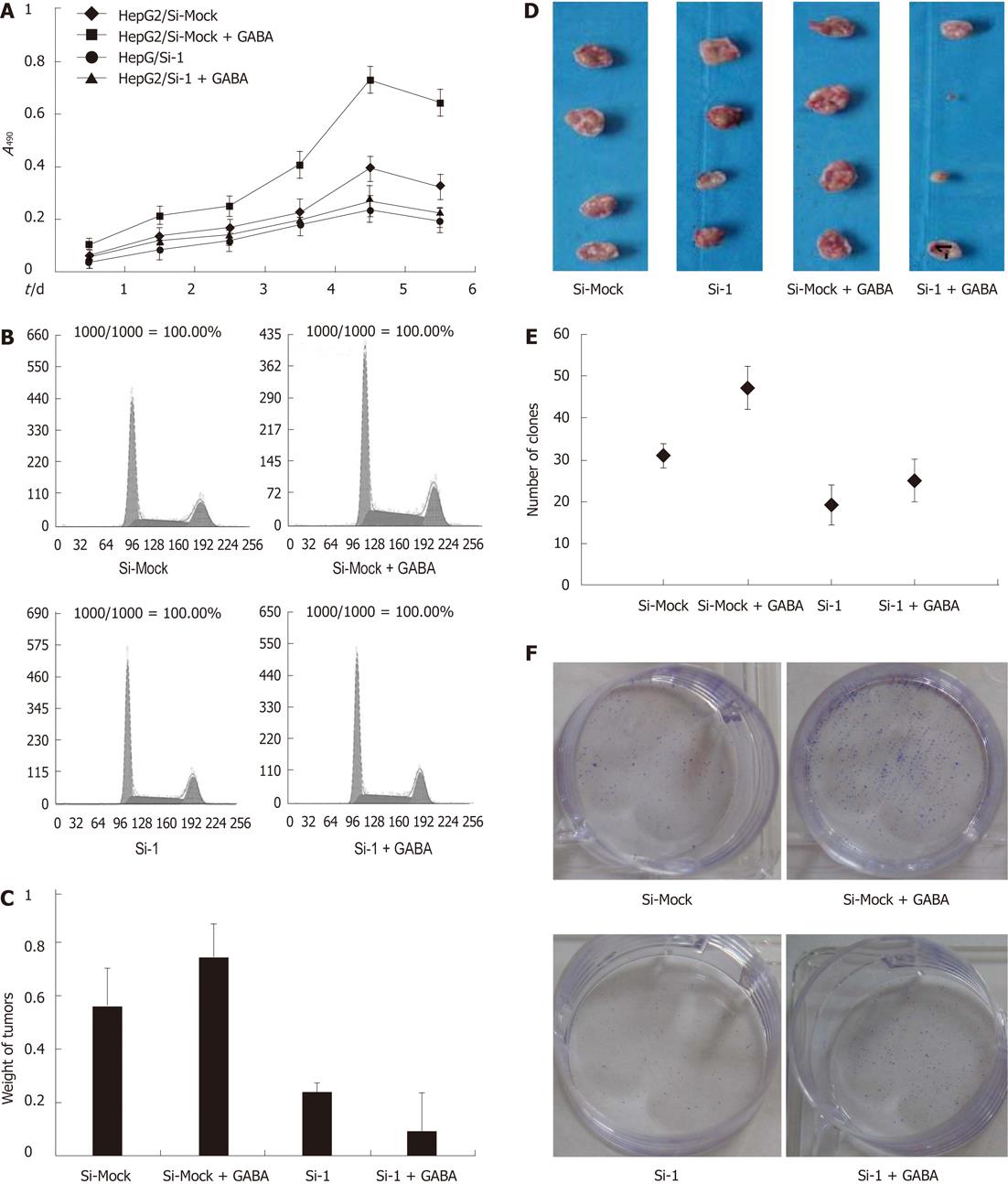
To examine the function of GABRQ as a GABA receptor on the growth of GABRQ-expressing HCC cells, we treated HepG2/Si-1 and HepG2/Si-Mock cells with or without GABA (40 μmol/L). The results are shown in Figure 4A: GABA enhanced the growth of HepG2/Si-Mock compared with the HepG2/Si-Mock without GABA.
On the other hand, the proliferating ability of HepG2/Si-1, which did not express GABRQ, was not enhanced by GABA. In the nude mice injected with HepG2/Si-Mock, the tumor weight of the mice treated with GABA was much larger than that of the mice treated without GABA, while the mice injected with HepG2/Si-1 did not present such differences (Figure 4C and D).
To further explore GABA stimulation of HepG2 cell growth through GABRQ, we examined the effects of Si-1 and Si-Mock on cell cycle. After treatment with 40 μmol/L GABA, the G0/G1-phase fraction of HepG2/Si-Mock cells significantly decreased; in contrast, S-phase cells significantly increased, but this event did not occur in HepG2/Si-1 cells (Table 3, Figure 4B).
The other illustration of growth effect of reduced GABRQ expression in HepG2 cells was achieved in a colony formation assay (Figure 4E and F). As a result, the average colony number of Si-1 cells was decreased compared with Si-Mock cells. After treatment with 40 μmol/L
GABA, the numbers of cell colonies of HepG2/Si-Mock cells significantly increased, but this did not occur in HepG2/Si-1 cells. These data indicate that GABA stimulates HepG2 cell growth through GABRQ.
In this study, we validated the overexpression of GABRQ in more than half of the tested HCC tissues compared with the adjacent non-tumor liver tissues; GABRQ was expressed in malignant liver cell lines HepG2 and moderately expressed in normal cell line Chang’s liver, but not in normal cell line L-02, implicating that GABRQ may be a good molecular target for the diagnosis of HCC. Functional analysis using siRNA of GABRQ strongly supported its involvement in the development and progression of HCC. In our study, the proliferation rate of HepG2 cells after GABRQ knockdown was significantly reduced, whereas proliferation of Si-Mock cells was not inhibited. This result indicated that GABRQ may increase the proliferation ability of hepatocytes. Primarily, GABA and GABA receptors function as an inhibitory neurotransmitter in the mature CNS, but their precise functions in nonneuronal cells or tumor cells are unknown. Joseph et al[14] reported that GABA could inhibit colon cancer migration associated with the norepinephrine-induced pathway. On the other hand, another report showed that GABA and GABAB receptor pathways could be involved in prostate cancer metastasis or invasion through the regulation of metalloproteinase production[15]. Therefore, it is controversial whether GABA-associated pathways could act positively or negatively in the regulation of cancer cell behavior. However, our findings in this study can clearly indicate evidence supporting the theory that GABA and GABAA receptor with GABRQ promote HCC cell proliferation.
By comparing the proliferative activity of the GABRQ-knockdown HepG2 cells treated with GABA, we found that GABA stimulated HepG2 cell growth through GABRQ. The proliferating ability of the cells treated with GABA was not enhanced compared with the cells without GABA treatment. Previous studies suggest that GABA stimulates collagen synthesis and proliferation of human fibroblasts[16]. Biju et al[17] reported that, in N-nitrosodiethylamine-induced neoplasia in the rat liver, GABAB receptors were increased and that the GABAB receptor agonist baclofen increased epidermal growth factor-mediated DNA synthesis in hepatocytes. Thus, GABA-associated pathways also could act positively in the regulation of cancer cell behavior. Our findings in this study also support the theory that GABA and GABRQ promote HepG2 cell proliferation in vivo and in vitro. Interestingly, GABAA receptor antagonist bicuculline methiodide could also promote the proliferation of HepG2 cells (data not shown), indicating that it might activate some other signal pathways[18].
Although GABA usually induces hyperpolarization in adult neurons, GABA has been shown to exert depolarizing responses in the immature CNS structures and CNS tumors[19,20]. In particular, GABA increased the proliferation of immature cerebellar granule cells through the activation of GABAA receptors and voltage-dependent calcium channels[21,22]. Takehara et al[23] reported that GABA stimulated pancreatic cancer growth through GABRP by increasing intracellular Ca2+ levels and activating the mitogen-activated protein kinase/extracellular signal-regulated kinase cascade. Also, Minuk et al[24] reported that human HCC tissues were depolarized compared with adjacent non-tumor tissues. From the results above, we deduce that GABA may promote the HepG2 cell proliferation through GABRQ by voltage-dependent calcium channels. Interestingly, GABA inhibited the growth of the GABRQ-knockdown HepG2 cells. This indicates that GABA activates some other receptors to inhibit the proliferation without GABRQ, which is identical to some previous reports[25-27].
In conclusion, compared with adjacent non-tumor tissues, HCC tissues have increased GABRQ receptor expression. Knockdown of GABRQ expression in receptor-expressing malignant hepatocytes results in attenuated in vitro and in vivo tumor growth. Moreover, GABA promotes hepatocyte proliferation through GABRQ. These findings highlight the importance of elucidating the role of GABAergic activity in the pathogenesis of HCC. They also raise the potential for new therapeutic and diagnostic approaches to human HCC.
Gamma-aminobutyric acid A receptor θ subunit (GABRQ) is a subunit of the gamma-aminobutyric acid A (GABAA) receptors that may associate with other GABAA receptor subunits to form a functional chloride channel which mediates inhibitory synaptic transmission in the mature central nervous system (CNS). gamma-aminobutyric acid (GABA) functions as an inhibitory neurotransmitter for activating GABA receptors.
Recently, abnormal levels of gene and protein expression of some GABA receptor subunits have been detected in many malignant tumors. This research indicates that GABAergic system may play an important role in the pathogenesis and development of malignant tumors.
This study demonstrated the overexpression of GABRQ in hepatocellular carcinoma (HCC), which has not been previously described, and illustrated that GABA stimulates HCC cell proliferation through GABRQ.
Further characterization of GABRQ will provide new insights into the role of GABRQ in the molecular pathogenesis and therapy of HCC.
GABA stands for gamma-aminobutyric acid, which is an inhibitory neurotransmitter. GABRQ stands for gamma-aminobutyric acid A receptor θ subunit.
The authors have analyzed the expression and the role of GABRQ in hepatocellular carcinoma. The manuscript is well-written and the study is conducted appropriately in order to understand the molecular mechanisms that control hepatocarcinogenesis, and also raise the potential for new therapeutic and diagnostic approaches to human HCC.
Peer reviewer: Fernando J Corrales, Associate Professor of Biochemistry, Division of Hepatology and Gene Therapy, CIMA, University of Navarra, Center for Applied Medical Research, Proteomics Laboratory, Av. Pío VII 55, 31008 Pamplona, Spain
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Hao K, Luk JM, Lee NP, Mao M, Zhang C, Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;9:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Huang J, Li Y, Guo F, Tong Y, Wang J, Hu J, Li G. Expression of scFv SA3 against hepatoma fused with enhanced green fluorescent protein and its targeted ability in vivo. Zhongnan Daxue Xuebao Yixueban. 2011;36:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248-S260. [PubMed] |
| 4. | Paul SB, Gamanagatti SR, Mukund A, Abbas SZ, Acharya SK. Transarterial chemoembolization for hepatocellular carcinoma: significance of extrahepatic collateral supply. Indian J Cancer. 2011;48:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Li YH, Guo FJ, Wang JJ, Sun RL, Hu JY, Li GC. Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid A receptor alpha 3 subunit. World J Gastroenterol. 2008;14:7175-7182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764-5774. [PubMed] |
| 7. | Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899-909. [PubMed] |
| 8. | Neelands TR, Zhang J, Macdonald RL. GABA(A) receptors expressed in undifferentiated human teratocarcinoma NT2 cells differ from those expressed by differentiated NT2-N cells. J Neurosci. 1999;19:7057-7065. [PubMed] |
| 9. | Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(A) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol Cell Neurosci. 2003;23:600-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tamayama T, Maemura K, Kanbara K, Hayasaki H, Yabumoto Y, Yuasa M, Watanabe M. Expression of GABA(A) and GABA(B) receptors in rat growth plate chondrocytes: activation of the GABA receptors promotes proliferation of mouse chondrogenic ATDC5 cells. Mol Cell Biochem. 2005;273:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 343] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437-11446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Guo F, Li Y, Liu Y, Wang J, Li Y, Li G. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai). 2010;42:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467-6469. [PubMed] |
| 15. | Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003;63:8090-8096. [PubMed] |
| 16. | Scutt A, Meghji S, Harvey W. Stimulation of human fibroblast collagen synthesis in vitro by gamma-aminobutyric acid. Biochem Pharmacol. 1987;36:1333-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Biju MP, Pyroja S, Rajeshkumar NV, Paulose CS. Enhanced GABA(B) receptor in neoplastic rat liver: induction of DNA synthesis by baclofen in hepatocyte cultures. J Biochem Mol Biol Biophys. 2002;6:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Mares P, Chino M, Kubová H, Mathern P, Veliký M. Convulsant action of systemically administered glutamate and bicuculline methiodide in immature rats. Epilepsy Res. 2000;42:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Labrakakis C, Patt S, Hartmann J, Kettenmann H. Functional GABA(A) receptors on human glioma cells. Eur J Neurosci. 1998;10:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res. 1999;115:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Fiszman ML, Schousboe A. Role of calcium and kinases on the neurotrophic effect induced by gamma-aminobutyric acid. J Neurosci Res. 2004;76:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704-9712. [PubMed] |
| 24. | Minuk GY, Zhang M, Gong Y, Minuk L, Dienes H, Pettigrew N, Kew M, Lipschitz J, Sun D. Decreased hepatocyte membrane potential differences and GABAA-beta3 expression in human hepatocellular carcinoma. Hepatology. 2007;45:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Tatsuta M, Iishi H, Baba M, Nakaizumi A, Ichii M, Taniguchi H. Inhibition by gamma-amino-n-butyric acid and baclofen of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1990;50:4931-4934. [PubMed] |
| 26. | Zhang M, Gong Y, Assy N, Minuk GY. Increased GABAergic activity inhibits alpha-fetoprotein mRNA expression and the proliferative activity of the HepG2 human hepatocellular carcinoma cell line. J Hepatol. 2000;32:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 27. | Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A. Effect of selective and non-selective muscarinic blockade on baclofen inhibition of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Carcinogenesis. 1996;17:293-296. [PubMed] |