Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2695
Revised: July 6, 2011
Accepted: July 13, 2011
Published online: June 7, 2012
AIM: To investigate the effect of intensive vs conventional insulin therapy on perioperative nutritional substrates metabolism in patients undergoing radical distal gastrectomy.
METHODS: Within 24 h of intensive care unit management, patients with gastric cancer were enrolled after written informed consent and randomized to the intensive insulin therapy (IIT) group to keep glucose levels from 4.4 to 6.1 mmol/L or the conventional insulin therapy (CIT) group to keep levels less than 10 mmol/L. Resting energy expenditure (REE), respiratory quotient (RQ), resting energy expenditure per kilogram (REE/kg), and the lipid oxidation rate were monitored by the indirect calorimeter of calcium citrate malate nutrition metabolism investigation system. The changes in body composition were analyzed by multi-frequency bioimpedance analysis. Blood fasting glucose and insulin concentration were measured for assessment of Homeostasis model assessment of insulin resistance.
RESULTS: Sixty patients were enrolled. Compared with preoperative baseline, postoperative REE increased by over 22.15% and 11.07%; REE/kg rose up to 27.22 ± 1.33 kcal/kg and 24.72 ± 1.43 kcal/kg; RQ decreased to 0.759 ± 0.034 and 0.791 ± 0.037; the lipid oxidation ratio was up to 78.25% ± 17.74% and 67.13% ± 12.76% supported by parenteral nutrition solutions from 37.56% ± 11.64% at the baseline; the level of Ln-HOMA-IR went up dramatically (P < 0.05, respectively) on postoperative days 1 and 3 in the IIT group. Meanwhile the concentration of total protein, albumin and triglyceride declined significantly on postoperative days 1 and 3 compared with pre-operative levels (P < 0.05, respectively). Compared with the CIT group, IIT reduced the REE/kg level (27.22 ± 1.33 kcal/kg vs 29.97 ± 1.47 kcal/kg, P = 0.008; 24.72 ± 1.43 kcal/kg vs 25.66 ± 1.63 kcal/kg, P = 0.013); and decreased the Ln-HOMA-IR score (P = 0.019, 0.028) on postoperative days 1 and 3; IIT decreased the level of CRP on postoperative days 1 and 3 (P = 0.017, 0.006); the total protein and albumin concentrations in the IIT group were greater than those in the CIT group (P = 0.023, 0.009). Postoperative values of internal cell fluid (ICF), fat mass, protein mass (PM), muscle mass, free fat mass and body weight decreased obviously on postoperative 7th day compared with the preoperative baseline in the CIT group (P < 0.05, respectively). IIT reduced markedly consumption of fat mass, PM and ICF compared with CIT (P = 0.009 to 0.026).
CONCLUSION: There were some benefits of IIT in decreasing the perioperative insulin resistance state, reducing energy expenditure and consumption of proteins and lipids tissue in patients undergoing gastrectomy.
-
Citation: Liu HC, Zhou YB, Chen D, Niu ZJ, Yu Y. Effect of intensive
vs conventional insulin therapy on perioperative nutritional substrates metabolism in patients undergoing gastrectomy. World J Gastroenterol 2012; 18(21): 2695-2703 - URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2695
Hyperglycemia associated with insulin resistance (IR) is a common occurrence in diabetic patients and critically ill patients undergoing severe trauma or operative procedures, including cardiopulmonary bypass surgery, and major abdominal procedures resulting in derangement of glucose metabolism[1,2]. Previous studies have shown a strong association between hyperglycemia, particularly severe hyperglycemia, and poor clinical outcomes, regardless of diabetic status[1-3]. Postoperative hyperglycemia status caused by surgical stress may increase the risk of infectious complications such as surgical site infections[4], urinary tract infections[5], sepsis[6] and diabetic cardiovascular complications[4-7] which may result in death or increased length of hospital stay and poor outcomes. In 2001, a randomized controlled trial (RCT) involving 1548 patients admitted to a surgical intensive care unit (SICU) showed that intensive insulin therapy (IIT), targeting a blood glucose concentration of 4.4-6.1 mmol/L, significantly reduced in-hospital mortality and morbidity among critically ill patients in the SICU and ameliorated the clinical outcome[8]. Some subsequent trials confirm the safety and benefit of IIT[9,10].
However, some RCTs cannot replicate these mortality benefits[11,12], what is more, a multicenter RCT-Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial - of over 6000 patients comes to almost completely the opposite conclusion that IIT increased mortality: a blood glucose target of 10 mmol/L or less resulted in lower mortality than did a target of 4.4-6.1 mmol/L[13]. Hence the effect of IIT is still controversial. But many professional organizations still recommend IIT for patients treated in ICUs due to some beneficial biological effect of insulin, despite the conflicting data on how to manage blood glucose in medical and surgical patients in the ICUs. Our previous study showed that IIT may reduce the inflammatory reaction and stress response by decreasing the level of interleukin (IL)-1, IL-6 and tumor necrosis factor-alpha (TNF-α) in postoperative patients undergoing radical gastrectomy[14]. Surgical stress results in a barrier to glucose oxidation and metabolic dysfunction of other nutritional substrates, however, the effect of IIT on perioperative substrate metabolism is still unclear.
The purpose of our study is to investigate perioperative characteristics of metabolic nutritional substrates and the effect of IIT keeping blood glucose levels between 4.4 and 6.1 mmol/L on the perioperative nutritional substrates metabolism as compared to conventional insulin therapy (CIT) keeping levels less than 10 mmol/L in patients undergoing radical distal gastrectomy as a surgical trauma.
The study was a prospective, randomized, controlled trial and was approved by the ethics committees of QingDao University Medical College Hospital. Written informed consent was obtained before randomization. Patients enrolled in this study had to be at least 18 years of age, non-diabetic patients, who had obtained a clear pathological diagnosis of stomach carcinoma at the middle or lower part, undergoing radical distal gastrectomy for gastric cancer. The exclusion criteria included current enrollment in another research study; pregnancy or nursing; severe malnutrition [body mass index (BMI) < 15 kg/m2 or > 30 kg/m2]; major organ failure such as liver failure, heart failure and pulmonary failure; palliative resection or unresectable gastric carcinoma; diabetes and other factors. Patients were enrolled between December 2009 and December 2010.
Patients underwent radical distal gastrectomy with D2 or D2+ lymphadenectomy under epidural anesthesia. The guidelines of the Japanese Research Society for Gastric Cancer were used in this study for standard surgical treatment and pathological assessment[15]. All the operations were performed by the same team of surgeons and were admitted into the general surgical intensive care unit (GSICU) after surgery. At the time of entry into the GSICU, patients were randomly assigned to either an IIT group keeping blood glucose between 4.4 and 6.1 mmol/L or a CIT group keeping blood glucose between 4.4 and 10 mmol/L. Blood glucose management in the two groups was performed according to the NICE-SUGAR study treatment algorithm (https://studies.thegeorgeinstitute.org/nice/).Treatment assignments were kept in a sealed envelope to be opened after enrollment at which time clinical staff were aware of assigned treatment groups.
In the IIT group, continuous insulin infusion which was a solution (50 units regular insulin in 50 milliliters of 0.9% sodium chloride solution) was pumped via a central or peripheral venous catheter if the blood glucose level exceeded 6.1 mmol/L and the velocity of infusion was adjusted to maintain normoglycemia. If the patient was randomized to the CIT group, insulin pump infusion was started when the blood glucose level exceeded 10 mmol/L. For the details of management please see the algorithm. Whenever the blood glucose level was lower than 6.1 mmol/L, the insulin infusion was stopped. Blood glucose levels were measured and monitored by GSICU nurses with a bedside point-of-care glucometers (OneTouch Ultra 2, LifeScan) or laboratory analyzers in routine use. The glucometers were calibrated regularly by the manufacturer to ensure the accuracy and reliability.
All patients were fed with exogenous glucose (120-150 g) and 30-40 mL/kg liquid including sodium, potassium, chlorine and insulin on the operation day. The next day, patients were fed with total balanced parenteral nutrient solution[16,17], or combined parenteral and enteral nutrition, including 20-25 non-protein kcal/kg body weight (BW) per 24 h and a balanced composition with 1 g nitrogen every 120 kcal per 24 h, 50%-60% of non-protein calories in the form of lipids and optimal doses of glutamine, magnesium, calcium and other specific vitamins and minerals. Enteral feeding with nasointestinal tube or intestinal feeding tube was attempted as early as possible and these tubes were removed as soon as the patients could take a semi-liquid diet.
The patient’s gender, age, personal identification number, pathological diagnosis of gastric carcinoma, concurrent diagnoses, medical history, and GSICU admission were recorded. To quantify preoperative function of organs and to evaluate the risk of operation, the Child-Pugh class, New York Heart Association, American Society of Anesthesiologists (ASA) physical status and sequential organ failure assessment also needed be recorded. To evaluate the malnutrition of the patients, nutritional risk screening (NRS 2002)[14] and the mini-nutrition assessment had to be written down.
The gold standard method for assessing insulin sensitivity was the technology of euglycemic insulin clamp in diabetic patients[18], but it was time-consuming, laborious, and costly. The present study used homeostasis model assessment (HOMA) to assess the insulin resistance state because multicenter RCTs show the HOMA-IR score is reliable and has good reproducibility and consistency[19]. The HOMA-IR score is calculated using the following mathematic formula: HOMA-IR = FBI*FBG/22.5, where FBI was fasting blood insulin (IU/mL), FBG was fasting blood glucose (mmol/L), and 22.5 was a constant. Blood samples for FBI and FBG analysis were drawn from peripheral veins at 6’clock on the preoperative day and postoperative days 1, 3 and 7 after overnight fasting, when fluid infusions were discontinued for at least 6 h. Insulin was tested using insulin reaction kits (Elecsys Automatic Electrochemiluminescence Immunoassay Instrument, Roche, Germany). Blood glucose was detected using an automatic chemistry analyzer (Hitachi, Japan). These blood samples were also used to measure the concentration of plasma total protein (TP), albumin (ALB), triglyceride (TG), free fatty acids (FFA); these were tested using an automatic chemistry analyzer (Hitachi, Japan) and Elisa kit of FFA.
Resting energy expenditure (REE) was determined by the Indirect calorimeter of calcium citrate malate (CCM) nutrition metabolism investigation system (Medical Graphics Corporation, United States). The O2 consumption and the CO2 production per minute were used to calculate the actual REE using the Weir formula[20]; respiratory quotient (RQ) was the ratio of CO2 production and O2 consumption (VCO2/VO2). The normal range of RQ in humans was 0.67-1.2[21]. RQ was 1 representing carbohydrate as the major energy substrate and 0.71 representing lipids as the major substrate. Carbohydrate and lipid oxidation rates were calculated from the collected data. In order to avoid muscle activation patients stayed in bed in the morning for at least 30 minutes. Room temperature was maintained at 24 °C to 26 °C, with a humidity of 45%-60%. The volume and gas were calibrated for the CCM nutrition metabolism investigation system; each subject’s height, weight, gender, and age were recorded. The value of energy metabolism may be related to variation of BW. Therefore, the REE/kg of all patients was also evaluated.
A multi-frequency bioimpedance analysis (BIA) with eight-point tactile electrodes (InBody 3.0, Biospace Co., Seoul, South Korea) was used. This analyzer uses an AC of 250 mA with multi-frequency of 5, 50, 250 and 500 kHz. Each grip and plate had two electrodes built in. This analyzer measures segmental impedances at the right arm, left arm, right leg, left leg and trunk for all frequencies, and can automatically display measurements of free fat mass (FFM), fat mass (FM), muscle mass (MM), bone and mineral mass (BMM), total body water (TBW), internal cell fluid (ICF), external cell fluid (ECF) and TBW. The prediction equations of TBW and FFM were developed for Asians, including Chinese patients.
Differences in baseline characteristics were analyzed using the student’s t test and Pearson’s χ2 test for continuous and categorical variables, respectively. Differences between the two intervention groups in the REE, RQ, TP, ALP and FFA and other values were monitored on the pre-operative day and on postoperative days 1, 3 and 7 and were analyzed using repeated measures experimental design and methods. The difference at the same time was analyzed by one-way analysis of variance between two groups. The level of significance was set at P < 0.05. Data analysis was performed using SPSS version 12.0.
Sixty patients were enrolled and randomly assigned into the IIT group (32 patients) and the CIT group (32 patients). 48 patients (75%) were male. There were no statistically significant differences in age, gender, waistline, height, weight, BMI, medical history, carcinoembryonic antigen and CA199, surgery time, Child-Pugh, ASA grading, sequential organ failure assessment and HYHA, respectively (P > 0.05). All patients underwent radical distal gastrectomy inducing a similar stress response under epidural anesthesia, and all patients had some comparability (Table 1).
| Variable | IIT | CIT | Statistics | Sig |
| Age (yr) | 59.13 ± 10.06 | 58.94 ± 10.99 | t-tests | 0.943 |
| Male-no/total (no %) | 24/32 (75%) | 24/32 (75%) | χ2 tests | 1 |
| Weight (kg) | 61.18 ± 6.82 | 60.47 ± 5.49 | t-tests | 0.411 |
| BMI (kg/m2) | 23.83 ± 1.37 | 24.15 ± 1.12 | t-tests | 0.309 |
| Waistline (cm) | 84.38 ± 7.76 | 87.94 ± 8.71 | t-tests | 0.089 |
| CEA (ng/mL) | 4.52 ± 5.61 | 3.37 ± 4.88 | t-tests | 0.384 |
| CA-199φ (U/mL) | 23.94 ± 23.20 | 10.69 ± 13.64 | t-tests | 0.07 |
| Surgery time (min) | 145.81 ± 19.57 | 141.25 ± 26.24 | t-tests | 0.387 |
| Ln-HOMA-IR | 0.586 ± 0.104 | 0.633 ± 0.116 | t-tests | 0.769 |
| Hypertension | 10/32 | 10/32 | χ2 tests | 1 |
| SOFA (organs dysfunction) | ||||
| Blood/lung/renal/hepatic | 2/0/4/0 | 2/2/2/0 | χ2 tests | 0.446 |
| Child-Pugh (A/B/C) | 28/4/0 (32) | 30/2/0 (32) | χ2 tests | 0.518 |
| ASA grading (I/II/III/> III) | 2/22/8/0 (32) | 2/22/8/0 (32) | χ2 tests | 1 |
| NYHA (I/II/III/IV) | 8/18/6/0 (32) | 10/16/6/0 (32) | χ2 tests | 0.496 |
| NRS 1-2/3-4/5-6 scores | 10/20/2 (32) | 18/14/0 (32) | χ2 tests | 0.1 |
| MNA 26-24/24-17/17-14 | 6/24/2 (32) | 8/24/0 (32) | χ2 tests | 0.09 |
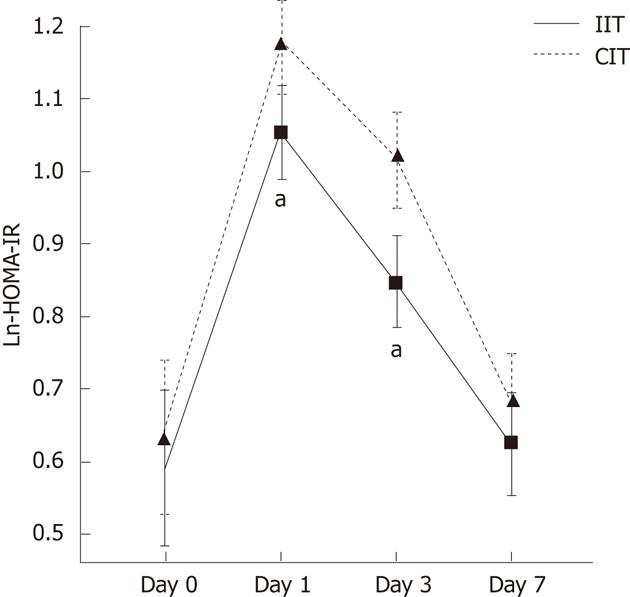
The data from HOMA-IR formed skewed distribution measurement variables and shifted into normal distribution with Napierian Logarithm (Ln-HOMA-IR). There was no difference in the two groups at baseline. The baseline of Ln-HOMA-IR was mean 0.61 ± 0.077 and 95% confidence interval was 0.46 to 0.76 in two groups. The postoperative level of Ln-HOMA-IR score went beyond the upper confidence limit on the postoperative 1st and 3rd day by surgical stress. Figure 1 presented the dynamic changes of the HOMA-IR score reflecting insulin resistance state over time. IIT decreased marginally Ln-HOMA-IR score on postoperative days 1 and 3 compared with the CIT group (1.052 ± 0.112 vs 1.175 ± 0.092, P = 0.042; 0.846 ± 0.064 vs 1.113 ± 0.074, P = 0.038).
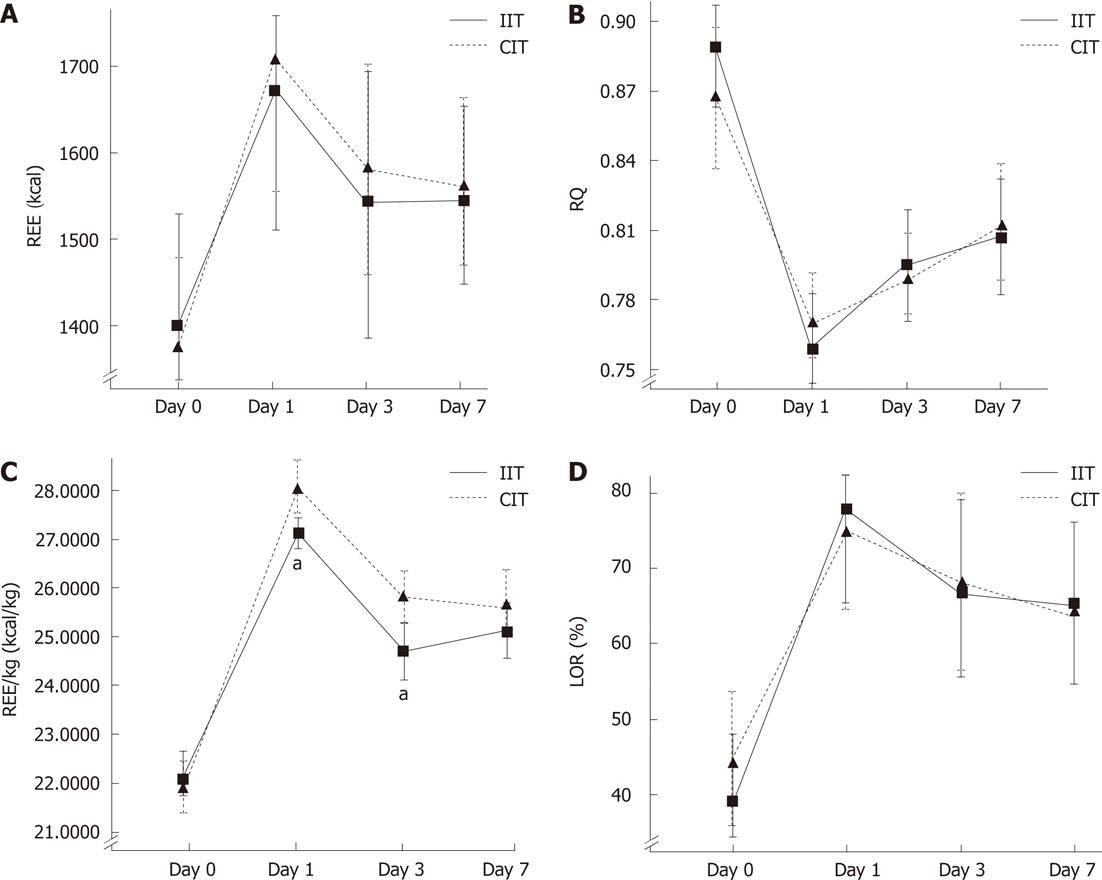
All patients in the IIT group were supported with 567.12 ± 11.55 kcal by glucose oxidation on the operation day and with 1187 ± 59 kcal during postoperative days 1 and 3 by parenteral nutrition as substrate oxidation, and was not different from the CIT group (P = 0.624). There was no significant difference between two groups in REE, RQ and lipid oxidation rate (P = 0.357-0.887, respectively), but IIT decreased the level of REE/kg on the postoperative 1st and 3rd days compared with CIT. For dynamic changes in energy metabolism please see Figure 2. Figure 2A showed that postoperative REE values increased by 22.15%, 11.07% and 11.08% on postoperative days 1, 3 and 7 compared with the pre-operative value of 1390.00 ± 172.34 kcal in the ITT group (P < 0.05, respectively); REE in the CIT group increased by 26.28%, 17.79% and 15.30% compared with preoperative baseline. Figure 2C presented the dynamic changes of REE/kg over time. The values of REE/kg in the IIT group were significantly lower than the values in the CIT group on postoperative days 1 and 3 (27.22 ± 1.33 kcal/kg vs 29.97 ± 1.47 kcal/kg, P = 0.008; 24.72 ± 1.43 kcal/kg vs 25.66 ± 1.63 kcal/kg, P = 0.013). Figure 2B displayed the fact that postoperative RQ declined dramatically and then rose gradually; the RQ of 0.809 ± 0.044 in the IIT group on postoperative day 7 was lower than the preoperative baseline value of 0.878 ± 0.066 and was lower than 0.82 when supported by parenteral nutrition solutions (P < 0.05). The variation trend in the postoperative lipid oxidation ratio (LOR) was opposite to the changes of RQ in two groups; LOR was exceeded beyond that of the preoperative baseline and by more than 50% when supported by fat emulsion in parenteral nutrition solutions after surgical trauma in the two groups.
The dynamic changes of body composition values are shown in Table 2. Postoperative values of ICF, FM, protein mass (PM), MM and BW were reduced significantly compared with the preoperative baseline in the CIT group (P < 0.05, respectively). There was no difference in BMM, ECF and TBW between preoperative and postoperative time in the two groups. BW was decreased by 5.68 kg, especially as the components of weight, FM and FFM dropped by 2.34 kg and 3.34 kg respectively in the CIT group. The PM declined by 1.74 kg during the loss of FFM. The table proved that the PM reduced dramatically on the postoperative 1st day compared with FM which decreased gradually. From table 2, the values of ICF, FM and PM in the IIT group were lower than in the CIT group on postoperative day 7 (P = 0.009 to 0.026), indicating there were some benefit of IIT in markedly reducing consumption of FM, PM and ICF. There were no significant differences in ECF, BMM, TBW, MM, FFM and BW between two groups (P = 0.094 to 0.418).
| Values | Group | n | Day 0 | 1st | 3rd | 7th | P value |
| ICF | IIT | 32 | 22.42 ± 2.55 | 21.90 ± 2.57 | 21.48 ± 2.64 | 21.09 ± 2.19c,e | 0.026 |
| CIT | 32 | 22.66 ± 2.69 | 21.97 ± 2.69 | 21.34 ± 2.54 | 20.17 ± 2.02c | ||
| ECF | IIT | 32 | 11.31 ± 1.64 | 11.57 ± 1.75 | 11.34 ± 1.62 | 11.06 ± 1.55c | 0.381 |
| CIT | 32 | 11.35 ± 1.71 | 11.63 ± 1.63 | 11.40 ± 1.58 | 11.11 ± 1.63c | ||
| FM | IIT | 32 | 11.76 ± 3.43 | 11.18 ± 3.55 | 10.65 ± 2.87 | 10.17 ± 2.40c,e | 0.042 |
| CIT | 32 | 11.83 ± 4.15 | 10.93 ± 3.88 | 10.02 ± 2.84 | 9.18 ± 2.24c | ||
| BM | IIT | 32 | 2.70 ± 0.34 | 2.68 ± 0.33 | 2.63 ± 0.35 | 2.64 ± 0.39 | 0.418 |
| CIT | 32 | 2.69 ± 0.36 | 2.61 ± 0.34 | 2.60 ± 0.34 | 2.62 ± 0.36 | ||
| PM | IIT | 32 | 12.13 ± 1.68 | 11.41 ± 1.53a | 11.08 ± 1.62 | 11.01 ± 1.41e | 0.009 |
| CIT | 32 | 12.19 ± 1.52 | 11.38 ± 1.53 | 10.89 ± 1.52 | 10.45 ± 1.24c | ||
| TBW | IIT | 32 | 33.73 ± 5.09 | 33.47 ± 4.94 | 32.83 ± 4.49 | 32.15 ± 4.51 | 0.263 |
| CIT | 32 | 34.01 ± 4.86 | 33.6 ± 4.84 | 32.74 ± 4.73 | 32.28 ± 4.85 | ||
| MM | IIT | 32 | 45.86 ± 6.05 | 44.88 ± 5.84 | 43.91 ± 5.70 | 43.16 ± 5.32c | 0.146 |
| CIT | 32 | 46.20 ± 5.85 | 44.98 ± 5.74 | 43.63 ± 5.42 | 42.73 ± 5.24c | ||
| FFM | IIT | 32 | 48.56 ± 7.24 | 47.56 ± 6.98 | 46.54 ± 6.85 | 45.80 ± 7.01 | 0.179 |
| CIT | 32 | 48.89 ± 7.11 | 47.59 ± 7.01 | 46.23 ± 6.94 | 45.35 ± 6.86c | ||
| BW | IIT | 32 | 60.32 ± 8.45 | 58.74 ± 8.55 | 57.19 ± 7.84 | 55.97 ± 7.55c | 0.094 |
| CIT | 32 | 60.72 ± 8.88 | 58.52 ± 8.32 | 56.25 ± 7.68 | 54.53 ± 7.12c |
Table 3 showed that there were no differences in preoperative levels of TP, ALB, TC and TG (P = 0.243 to 0.455) between the two groups. Postoperative TP and ALP levels declined dramatically on postoperative day 1 and fell to rock bottom on postoperative day 3, compared with preoperative levels (P < 0.05, respectively) in the two groups; the postoperative levels of TP and ALB in IIT group were greater than values in the CIT group (P = 0.023, 0.009).
| Values | Group | n | Day 0 | 1st | 3rd | 7th | P value |
| ALB (g/L) | IIT | 32 | 35.50 ± 2.36 | 33.72 ± 2.02a,g | 31.70 ± 2.37c,g | 34.64 ± 2.29e,g | 0.009 |
| CIT | 32 | 36.09 ± 2.47 | 32.47 ± 2.47 | 30.34 ± 3.13 | 33.45 ± 2.93 | ||
| TP (g/L) | IIT | 32 | 61.90 ± 3.97 | 57.97 ± 3.83a,g | 55.85 ± 3.53c,g | 59.81 ± 3.56e | 0.023 |
| CIT | 32 | 62.18 ± 4.26 | 56.09 ± 3.57 | 54.13 ± 2.96 | 58.89 ± 3.78 | ||
| TG (mol/L) | IIT | 32 | 1.45 ± 0.40 | 0.70 ± 0.16a | 0.83 ± 0.18c | 1.44 ± 0.21e | 0.267 |
| CIT | 32 | 1.64 ± 0.30 | 0.76 ± 0.23 | 0.94 ± 0.18 | 1.52 ± 0.19 | ||
| TC (μmol/L) | IIT | 32 | 4.36 ± 0.16 | 3.64 ± 0.12a | 3.55 ± 0.10 | 3.92 ± 0.13e | 0.207 |
| CIT | 32 | 4.74 ± 0.16 | 3.81 ± 0.12 | 3.60 ± 0.10 | 4.30 ± 0.13 | ||
| FFAn (nmol/L) | IIT | 32 | 0.43 ± 0.084 | 0.92 ± 0.114a,g | 0.86 ± 0.097c,g | 0.71 ± 0.086e,g | 0.016 |
| CIT | 32 | 0.40 ± 0.092 | 1.03 ± 0.148 | 0.93 ± 0.104 | 0.84 ± 0.106 |
Our results showed that postoperative energy expenditure was increased and high resting energy consumption persisted during the perioperative period in patients undergoing radical distal gastrectomy. The REE value on the postoperative 7th day did not return back to the original state. The value of REE was measured and calculated directly by consumption of O2 and CO2 regardless of patients’ weight changes, so mean REE/kg was more representive than REE for changes of energy expenditure during the perioperative period. IIT decreased the mean REE/kg during the perioperative period. The postoperative high energy expenditure state was associated directly with trauma stress.
Radical distal gastrectomy, as a major abdominal operation, triggered a serious stress response which excited the sympathetic nervous system (SNS) and was triggered following cataract reaction[22,23]: activation of the neuroendocrine and inflammatory systems increased the level of epinephrine, glucagon, cortisol, growth hormone (counterregulatory hormones) and secreted inflammatory mediators and then very rapidly increased to set the body in a high metabolic state of stress. These counterregulatory hormones work in concert to maintain hyperglycemia by targeting substrate supply, enhance capacity of the liver to take up gluconeogenesis precursors, and mobilize glycogen, lipids and protein stores for high energy expenditure in the postoperative fasting state. Meanwhile these enhanced counterregulatory hormones decreased the biological effect and sensitivity to any given concentration of insulin, especially in insulin-dependent tissues or cells, such as muscle, adipose and liver whose glucose transporter membrane proteins of the cells surface were regulated upon activation of the insulin receptor. So insulin-dependent tissues or cells had some obstacle of utilizing glucose oxidation for energy in the state of insulin resistance[24]. Postoperative hyperglycemia and insulin resistance were obvious in postoperative patients and were directly related to the degree of surgical trauma. Thorell et al[25] studied 10 patients undergoing elective open cholecystectomy surgery under general anesthesia and showed that insulin resistance was most noticeable on postoperative day 1, persisted for up to 5 d, and then reverted back to normal. Accumulated data showed that the open procedure involving the chest and abdomen elicited a more profound and prolonged insulin resistance response than lower risk peripheral or laparoscopic surgery[26-28]. Our previous results proved IIT decreased the concentration of plasma inflammatory mediators and improved the short-term outcome of patients undergoing gastrectomy for gastric cancer[14]. Our results showed that IIT decreased the extent of insulin resistance and hyperglycemia on postoperative days 1 and 3, but had no effect of carbohydrate oxidation compared with the CIT group.
When in the state of having insulin resistance and stress response, there was a barrier to glucose uptake and oxidation, so the tissues and cells used protein and lipid mass as the energy substrate. Our results showed that postoperative energy expenditure rose dramatically by more than the amount of calories supplied. Our results also showed that postoperative RQ, BW and FM decreased significantly which indicated directly the lipid was the major energy substrate. Lipid metabolism plays an important role in keeping a balance between energy intake and energy expenditure[29]. Lipid metabolism was regulated by the SNS[30]. Surgical trauma stress resulted in increased activities of the SNS which triggered the downstream reaction: increase of sympathetic transmitter norepinephrine release which activated and banded its receptor Adrb3 on the surface of adipocytes, and then elevated intracellular levels of cyclic AMP that activate protein kinase A and mediate phosphorylation and activation of hormone sensitive lipase. Fat mobilization and β-oxidation were increased after the surgical operation stress response, and our results reported that the FM and RQ decreased and lipid oxidation rate increased during the postoperative period. Insulin resistance and glucose oxidation dysfunction were common phenomena in the patients with diabetes mellitus[31,32] and obesity in which the concentration of FFA and TG were greater than in non-diabetic patients. As our results postoperative plasma FFA concentrations increased significantly compared with preoperative baseline. The increased amount of FFA may further enhance the inflammatory cascade, decrease insulin sensitivity that ultimately leads to failure of GLUT-4 translocation, and contribute to insulin resistance[33]. Equally, the insulin resistance state can further enhance lipolysis, increase plasma FFA concentration, and create a vicious cycle[29,30]. Our results showed that IIT reduced plasma FFA concentration and the consumption of FM compared with CIT. This may be related with the decreased insulin resistance state by IIT.
Postoperative FFM, MM and BW measured by multi-frequency BIA were decreased significantly by surgical trauma compared with preoperative baseline; in particular MM was reduced most in weight decrement. Proteins lost were associated with perioperative management, energy supply and stress response. The study of Boulétreau et al[34] showed that a big enough energy supply induced a small protein sparing effect. Our data showed that the calory supply in the two groups was lower than energy expenditure and thermogenesis which was the cause of fat and protein lost by mobilization and oxidation. The insulin resistance state resulted in a barrier to glucose uptake and oxidation, so plentiful amino acids from protein catabolites turn into the energy substrate, known as ‘cannibalism phenomenon’. Our results showed that IIT can reduce the extent of insulin resistance and the consumption of proteins.
Therefore we may deduce the beneficial experience of nutrition support in the perioperative period: (1) providing adequate calories is very important to curtail expenditure of body components; (2) avoiding significant exogenous glucose supply in the insulin resistance state because insulin-dependent tissues (muscle and lipid tissues) have glucose oxidation dysfunction. It was easy to induce secondary stress and to form a vicious cycle when exogenous glucose input is more than the body’s utilization ability; and (3) providing proper proportion of fatty acids and amino acids to reduce consumption of body components.
Our results showed that IIT can reduce the level of IR and cut down the consumption of fat and PM. The mechanism was not clear. This may be associated with enhanced signal transduction pathway of insulin[35,36].
The main limitations of our study were the small number of patients enrolled and that we could not keep the treatment arm blinded from the treating staff once treatment was started. Our study was limited to patients with gastric cancer in GSICU and cannot necessarily be extrapolated to other populations. The exact amount of exogenous glucose and a reasonable ratio of glucose, lipid and protein supported for patients were unclear. Our results need to be further proved by multicenter randomized controlled trials.
In conclusion, there were some benefits of IIT in decreasing perioperative IR level, reducing mean energy expenditure and the consumption of protein and fat tissue in this small population undergoing radical distal gastrectomy, although IIT did not reverse the status of perioperative insulin resistance, high energy expenditure and catabolic metabolism.
Previous studies have shown a strong association between hyperglycemia, particularly severe hyperglycemia and poor clinical outcomes, regardless of diabetic status. Insulin intensive therapy (IIT) was an effective method for controlling the postoperation hyperglycemia. Effect of IIT on mortality and morbidity of complication was still controverted, meanwhile, the mechanism and the effect of IIT on perioperative substrate metabolism was still unclear.
Most of clinical trials study the effect of intensive vs conventional insulin therapy on the mortality and of morbidity of complication in the patients undergoing the severe trauma or operation, although the conclusion was still controverted, few trials were related to the mechanism of IIT on improving the clinical outcome from nutrition substrates metabolism’s angle. Moreover, current nutrition support therapies were based on empiricism by physician or surgeon who estimated roughly the energy consumptions according to patient’s sex, height, weight and age. So the authors monitored real-time the changes of nutritional substrates metabolism by indirect calorimeter of CCM nutrition metabolism investigation system and changes of body composition analyzed by multi-frequency BIA, to investigate the effect and mechanism of IIT of intensive vs conventional insulin therapy on perioperative nutritional substrates metabolism in patients undergoing radical gastrectomy.
IIT can reduced in-hospital mortality and morbidity among critically ill patients in the surgery ICU and ameliorated the clinical outcome. In despite of outcome came from large sample analysis, the mechanism was still not clear and lack of theoretical basis. In terms of nutrition, the authors found energy expenditure rose dramatically and there was a barrier of glucose uptake and oxidation in the status of IR and stress response, that is to say, there is high energy expenditure and katabolic metabolism state of postoperative patients in the status of IR and stress response. The data showed that there were some benefits of IIT in decreasing perioperative IR level, reducing mean energy expenditure and the consumption of protein and fat tissue in this small population undergoing the radical distal gastrectomy.
There were some benefits of IIT in decreasing perioperative IR level, reducing mean energy expenditure and the consumption of protein and fat tissue in this small population undergoing the radical distal gastrectomy, although IIT not yet reversed the status of perioperative IR, high energy expenditure and katabolic metabolism state.
Intensive insulin therapy (IIT) was one of the methods for controlling postoperation hyperglycemia associated with insulin resistance, targeting a blood glucose concentration of 4.4 to 6.1 mmol/L by continuous insulin infusion. Insulin resistance was the status in which there was a barrier of glucose uptake and oxidation for energy.
The study investigates the effect of intensive insulin therapy on perioperative nutritional substrates metabolism in patients undergoing radical distal gastrectomy. REE, RQ, REE/kg and LOR were monitored by Indirect calorimeter of CCM nutrition metabolism investigation system and the changes of body composition were analyzed by multi-frequency BIA. It revealed that IIT decreased perioperative IR level, reduced mean energy expenditure and the consumption of protein and fat tissue in this small population undergoing the radical distal gastrectomy, although IIT not yet reversed the status of perioperative IR, high energy expenditure and katabolic metabolism state. The results are interesting and may alleviate IR and may represent a metabolism mechanism of IR in the patients after operation.
Peer reviewers: Ki-Baik Hahm, MD, PhD, Professor, Department of Gastroenterology, Gachon Graduate School of Medicine, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon 406-840, South Korea; Dr. Takaaki Arigami, Department of Surgical Oncology and Digestive, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima 890-8520, Japan
S- Editor Sun H L- Editor O’Neill M E- Editor Xiong L
| 1. | Midtvedt K, Hartmann A, Hjelmesaeth J, Lund K, Bjerkely BL. Insulin resistance is a common denominator of post-transplant diabetes mellitus and impaired glucose tolerance in renal transplant recipients. Nephrol Dial Transplant. 1998;13:427-431. [PubMed] |
| 2. | Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69-78. [PubMed] |
| 3. | Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Guvener M, Pasaoglu I, Demircin M, Oc M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Gale SC, Sicoutris C, Reilly PM, Schwab CW, Gracias VH. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73:454-460. [PubMed] |
| 6. | Hirasawa H, Oda S, Nakamura M. Blood glucose control in patients with severe sepsis and septic shock. World J Gastroenterol. 2009;15:4132-4136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7077] [Cited by in RCA: 6182] [Article Influence: 257.6] [Reference Citation Analysis (2)] |
| 9. | Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 1881] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 10. | Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Treggiari MM, Karir V, Yanez ND, Weiss NS, Daniel S, Deem SA. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008;12:R29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Inzucchi SE, Siegel MD. Glucose control in the ICU--how tight is too tight? N Engl J Med. 2009;360:1346-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3409] [Cited by in RCA: 3185] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 14. | Cao SG, Zhou YB, Zhang CK, Chen D, Yu YY, Lu LF. [Effect of intensive insulin therapy on the clinical results of postoperative patients with gastric cancer]. Zhonghua Waike Zazhi. 2008;46:918-920. [PubMed] |
| 15. | The general rules for the gastric cancer study in surgery ad pathology. Part II. Histological classification of gastric cancer. Jpn J Surg. 1981;11:140-145. [PubMed] |
| 16. | Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 986] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 18. | Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi T, Shoji T, Okuno Y, Morii H. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care. 1999;22:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 321] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1665] [Cited by in RCA: 1796] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 20. | WEIR JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1-9. [PubMed] |
| 21. | Yamanaka H, Genjida K, Yokota K, Taketani Y, Morita K, Miyamoto KI, Miyake H, Tashiro S, Takeda E. Daily pattern of energy metabolism in cirrhosis. Nutrition. 1999;15:749-754. [PubMed] [DOI] [Full Text] |
| 22. | Turina M, Miller FN, Tucker CF, Polk HC. Short-term hyperglycemia in surgical patients and a study of related cellular mechanisms. Ann Surg. 2006;243:845-51; discussion 851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110:478-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Sane S, Baba M, Kusano C, Shirao K, Yamada H, Aikou T. Relation between effective utilization of exogenous fat emulsion as energy substrate and oxygen metabolism after surgery. World J Surg. 2000;24:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Thorell A, Efendic S, Gutniak M, Häggmark T, Ljungqvist O. Development of postoperative insulin resistance is associated with the magnitude of operation. Eur J Surg. 1993;159:593-599. [PubMed] |
| 26. | Clarke RS. The hyperglycaemic response to different types of surgery and anaesthesia. Br J Anaesth. 1970;42:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Luo K, Li JS, Li LT, Wang KH, Shun JM. Operative stress response and energy metabolism after laparoscopic cholecystectomy compared to open surgery. World J Gastroenterol. 2003;9:847-850. [PubMed] |
| 28. | Thorell A, Nygren J, Essén P, Gutniak M, Loftenius A, Andersson B, Ljungqvist O. The metabolic response to cholecystectomy: insulin resistance after open compared with laparoscopic operation. Eur J Surg. 1996;162:187-191. [PubMed] |
| 29. | Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1730] [Cited by in RCA: 1594] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 30. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 994] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 31. | Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 32. | Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1609] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 33. | Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368:1-19. [PubMed] |
| 34. | Boulétreau P, Chassard D, Allaouchiche B, Dumont JC, Auboyer C, Bertin-Maghit M, Bricard H, Ecochard R, Rangaraj J, Chambrier C. Glucose-lipid ratio is a determinant of nitrogen balance during total parenteral nutrition in critically ill patients: a prospective, randomized, multicenter blind trial with an intention-to-treat analysis. Intensive Care Med. 2005;31:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105-10117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 36. | Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1181] [Article Influence: 62.2] [Reference Citation Analysis (0)] |