Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2674
Revised: December 8, 2011
Accepted: April 28, 2012
Published online: June 7, 2012
AIM: To determine whether Helicobacter pylori (H. pylori)-infected children have reduced body weight (BW) and height (BH) growth, and if H. pylori eradication may restore growth while improving serum acylated ghrelin.
METHODS: This longitudinal cohort study with one-year follow-up enrolled 1222 children aged 4 to 12 years old into an observation cohort (18 with and 318 without H. pylori) and intervention cohort (75 with and 811 without). The 7-d triple therapy was used for eradication in the intervention cohort. The net increases of BW and BH as well serum acylated ghrelin after one-year follow-up were compared between successful eradicated H. pylori-infected children and controls.
RESULTS: In the observation cohort, the H. pylori-infected children had lower z score of BW (-1.11 ± 0.47 vs 0.35 ± 0.69, P = 0.01) and body mass index (BMI) (0.06 ± 0.45 vs 0.44 ± 0.73, P = 0.02) at enrollment and lower net BW gain after one-year follow-up (3.3 ± 2.1 kg vs 4.5 ± 2.4 kg, P = 0.04) than the non-infected controls. In the intervention cohort, the H. pylori-infected children had lower z score of BMI (0.25 ± 1.09 vs 0.68 ± 0.87, P = 0.009) and serum acylated ghrelin levels (41.8 ± 35.6 pg/mL vs 83.6 ± 24.2 pg/mL, P < 0.001) than the non-infected controls. In addition to restoring decreased serum ghrelin levels (87.7 ± 38.0 pg/mL vs 44.2 ± 39.0 pg/mL, P < 0.001), the H. pylori-infected children with successful eradication had higher net gains (P < 0.05) and increase of z scores (P < 0.05) of both BW and BH as compared with non-infected controls after one-year follow-up.
CONCLUSION: H. pylori-infected children are associated with low serum acylated ghrelin and growth retardation. Successful eradication of H. pylori restores ghrelin levels and increases growth in children.
-
Citation: Yang YJ, Sheu BS, Yang HB, Lu CC, Chuang CC. Eradication of
Helicobacter pylori increases childhood growth and serum acylated ghrelin levels. World J Gastroenterol 2012; 18(21): 2674-2681 - URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2674
Primary infection with Helicobacter pylori (H. pylori) usually occurs during childhood[1]. This organism has been proven to cause chronic gastritis, peptic ulcer diseases, and had a high correlation with gastric cancer in humans[2,3]. In children, the H. pylori prevalence rate was relatively lower than adults[4,5]. Besides the link with gastric diseases, the association between H. pylori infection and growth retardation in children has raised clinical attention to this issue and caused some debate recently. Some cross-sectional analyses have indicated that H. pylori-infected children had subnormal growth retardation as compared with non-infected children[6-8], but some others did not support such findings[9,10]. Long-term observational studies have reported that children with persistent H. pylori infection have reduced body weight (BW) and height (BH) growth than the non-infected peers[11-13]. Therefore, to further support the causal relationship between H. pylori infection and growth retardation in children, interventional trials involving H. pylori eradication may provide new insights using a rigorous study design.
Ghrelin, a growth-hormone-releasing peptide biosynthesized mainly in the fundic mucosa, regulates appetite and body composition and is affected by inflammatory and atrophic events associated with H. pylori infection[14,15]. Previous studies showed conflicting results regarding the correlation between plasma ghrelin levels and H. pylori infection after eradication of bacteria[16-18]. This controversy may be caused by the measurement of total plasma ghrelin, which contains both acylated and desacylated forms. Acylated ghrelin is a more potent agonist on the growth-hormone-stimulating receptor than the desacylated form and undergoes a compensatory elevation in patients with chronic atrophic gastritis[19-21]. This study seeks to examine active ghrelin levels and its relationship with growth in patients before and after H. pylori eradication.
Although eradication of H. pylori can restore body mass index (BMI) and serum albumin in adult patients with infection[22,23], such improvement has not yet been documented in H. pylori-infected children. Moreover, it is unclear whether the improving growth parameters after H. pylori eradication are subsequently linked to increase serum acylated ghrelin levels. Therefore, this study sought to examine whether H. pylori eradication improves BW and BH growth in children in parallel with increases in serum acylated ghrelin levels.
This study enrolled 1292 students, aged 4 to 12 years old from three elementary schools and their associated preschool kindergartens in Tainan City, Taiwan. The participants were consecutively enrolled into two study cohorts. Each participant provided informed consent documentation that was signed by her/his parents.
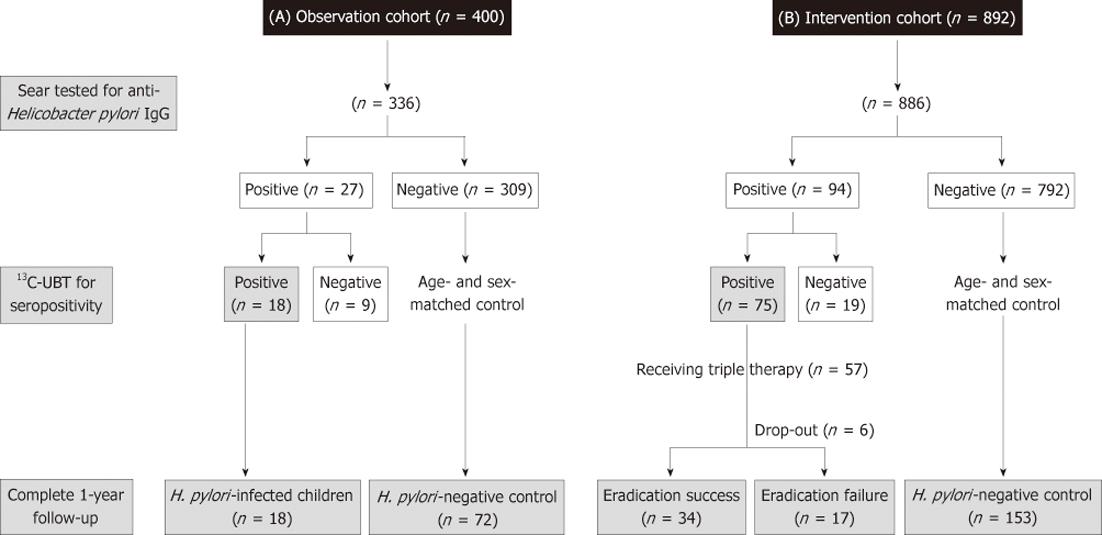
The first cohort (observation cohort) enrolled 400 children in 2005 to screen for the H. pylori infection, and they were then scheduled to return for follow-up growth status by a half-year interval of up to one year. The second group was an interventional cohort which enrolled 892 children in 2006 to screen for the H. pylori infection. Moreover, the H. pylori-infected subjects were invited to receive one-week of triple therapy for H. pylori eradication. As well, the children in the 2nd cohort were scheduled to return for follow-up growth status by a half-year interval of up to one year.
In each cohort, both the enrolled children and their parents were reviewed with a questionnaire to record data on underling medical diseases, H. pylori infection status, and a range of demographic variables, including socioeconomic status, such as number of family members[8], and annual household income (low income indicated less than $15 000 US/year). The same nursing assistant provided the introduction of questionnaire to the enrolled subjects. Children with pre-established and severe medical/organic conditions predisposing to the failure of thrive, such as genetic/metabolic disorders and cyanotic congenital heart diseases, were not included. The study also excluded children with a known past history to receive anti-H. pylori therapy and children underwent eradication therapy or acid suppressors, during the follow-up period in the observation group. In both groups, the control cases were randomly selected (1:4 in the observation and 1:3 in the interventional cohorts) and were matched by age and gender to children with 13C-labeled urea breath test (13C-UBT)-confirmed H. pylori infection. Moreover, for the H. pylori-infected (confirmed by a positive 13C-UBT) children at entry, the H. pylori status was assessed with a 13C-UBT after 6 mo (intervention cohort) and one year follow-up (both cohort).
For each participant, the overnight fasting BW and BH were serially measured at enrollment and at the follow-up period on the 6th mo and the 12th mo, respectively. The BMI was defined as BW in kilograms/squared of body length in meter (kg/m2). The z scores (SD scores) of BW, BH and BMI were calculated using the reference population of 2003 Taiwanese boys and girls based on health-related physical fitness and based on 2006 World Health Organization standards[24]. The net changes of BW, BH and BMI were calculated by the value of each parameter at follow-up minus the corresponding value at enrollment. We also defined the increase of z score means that z scores of BW, BH and BMI were upgrade at the one-year follow-up than at the enrollment (the net change > 0).
In each enrolled child, the serum was tested for anti-H. pylori IgG antibodies (HEL-p TEST™ II, AMRAD Biotech, Australia) by enzyme-linked immunosorbent assay (ELISA) methods. The serologic kit has been validated with a favorable sensitivity and specificity (> 90%) in detecting H. pylori infection in our previous studies[25]. The seropositive children further confirmed by 13C-UBT to diagnose ongoing H. pylori infection[8]. The cut-off value of positive 13C-UBT was defined as excess 13CO2/12CO2 ratio more than 3.5‰[8,26].
For the H. pylori-infected children in the intervention cohort, lansoprazole (1 mg/kg per day, max. 30 mg bid), amoxicillin (50 mg/kg per day, max. 1 g bid), and clarithromycin (15 mg/kg per day, max. 500 mg bid) were prescribed for one week[26]. We have educated the participants and their parents for the compliance and report of complications. Successful eradication therapy was defined by a negative result of 13C-UBT on both the 6th and the 12th mo follow-up, respectively[27].
The serum acylated ghrelin levels of the interventional cohorts at enrollment were compared between children with and without H. pylori infection. In addition, the serial serum acylated ghrelin levels of the children with H. pylori eradication collected at enrollment, the 6th mo, and the 12th mo follow-up were compared. Each blood sample of child was collected in the morning before breakfast and was incubated in the ice-bath container immediately. The sera were separated by centrifugation within 2-3 h and were stored in a -80 °C refrigerator until use. These samples’ serum acylated ghrelin levels were analyzed in duplicate by a commercial kit (LINCO Research, St. Charles, Missouri, United States), using ELISA methods.
The χ2 test with the odds ratio (OR) and 95% conference interval (CI) and logistic regression test were applied as an estimate of the possibly related factors between H. pylori-infected and non-infected children. The Student’s t test and one-way analysis of variance with least significant difference test correction were used as appropriate to compare the differences of ghrelin, BW, BH, BMI and their net changes during one-year follow-up periods among different study groups. The paired t test was used to analyze the difference of the serial serum acylated ghrelin levels before and after eradication therapy within the same study group. A P value less than 0.05 was considered statistically significant.
There were 84% (336/400) children in the observation and 99% (886/892) children in the intervention cohorts who completed the questionnaires and provided their sera for the anti-H. pylori IgG antibodies tested, respectively. In Figure 1, the case numbers of each cohort were serially summarized during the one-year follow-up. One hundred and twenty-one (27 in the observation cohort, 94 in the intervention cohort) were defined with seropositivity of H. pylori infection. Among them, 113 children received 13C-UBT, of which only 93 (82%) children were positive (18 in the observation cohort and 75 in the intervention cohort). Accordingly, the overall H. pylori prevalence was 7.6% in these two cohorts.
For the 18 H. pylori-positive children in the observation cohort, the infection was persisted with a positive 13C-UBT until the end of follow-up on the 1st year. Among the 75 H. pylori-infected children in the intervention cohort, 57 children were enrolled to receive the 7-d eradication therapy. A total of 6 subjects withdrew or were lost to follow-up. All of the finally eligible subjects had good drugs compliance (taking drugs at least 6 d) and none had major adverse complications. The intention-to-treat and per-protocol eradication rate of H. pylori-infected children were 60% (34/57) and 67% (34/51), respectively.
In the observation cohort, there were 72 age- and gender-matched subjects selected to serve as H. pylori-negative controls. In Table 1, the H. pylori-infected children had lower BW (29.9 ± 7.0 kg vs 35 ± 10.1 kg, P = 0.02), z score of BW (-1.11 ± 0.47 vs 0.35 ± 0.69, P = 0.01), BMI (16.8 ± 1.6 kg/m2vs 18.4 ± 2.8 kg/m2, P = 0.03) and z score of BMI (0.06 ± 0.45 vs 0.44 ± 0.73, P = 0.02) than those non-infected controls. Moreover, after one-year follow-up, the H. pylori-infected children had a significantly lower BW (33.2 ± 7.5 kg vs 39.5 ± 11.3 kg, P = 0.007), z score of BW (-0.17 ± 0.45 vs 0.38 ± 0.67, P = 0.002), BMI (17.2 ± 1.7 kg/m2vs 19 ± 3.0 kg/m2, P = 0.01), and z score of BMI (0 ± 0.38 vs 0.45 ± 0.75, P = 0.01) than the non-infected ones. Also in Table 1, there was a significantly lower net BW gain in the H. pylori-infected children than that in the non-infected controls (3.3 ± 2.1 kg vs 4.5 ± 2.4 kg, P = 0.04) after one-year follow-up. However, the net changes of BH, and BMI were not different between the children with and without H. pylori infections (P > 0.05).
| Groups | H. pylori-positive subjects | H. pylori-negative controls | P value |
| n | 18 | 18 | |
| Age (yr) | 9.1 ± 1.6 | 9.4 ± 1.7 | 0.57 |
| Sex (female:male) | 8:10 | 27:45:00 | 0.59 |
| Body weight (kg) | |||
| At enrollment | 29.9 ± 7.0 | 35.0 ± 10.1 | 0.02 |
| z score at enrollment | -1.11 ± 0.47 | 0.35 ± 0.69 | 0.01 |
| The 1st year | 33.2 ± 7.5 | 39.5 ± 11.3 | 0.007 |
| z score at the 1st year | -0.17 ± 0.45 | 0.38 ± 0.67 | 0.002 |
| Net change | 3.3 ± 2.1 | 4.5 ± 2.4 | 0.04 |
| Body height (cm) | |||
| At enrollment | 132.3 ± 11.4 | 136.8 ± 12.3 | 0.15 |
| z score at enrollment | -0.17 ± 0.49 | 0.15 ± 0.73 | 0.07 |
| The 1st year | 138.0 ± 11.4 | 142.5 ± 12.4 | 0.15 |
| z score at the 1st year | -0.14 ± 0.48 | 0.13 ± 0.71 | 0.1 |
| Net change | 5.7 ± 0.9 | 5.8 ± 1.9 | 0.81 |
| BMI (kg/m2) | |||
| At enrollment | 16.8 ± 1.6 | 18.4 ± 2.8 | 0.03 |
| z score at enrollment | 0.06 ± 0.45 | 0.44 ± 0.73 | 0.02 |
| The 1st year | 17.2 ± 1.7 | 19.0 ± 3.0 | 0.01 |
| z score at the 1st year | 0 ± 0.38 | 0.45 ± 0.75 | 0.01 |
| Net change | 0.35 ± 0.8 | 0.69 ± 1.0 | 0.14 |
In the intervention cohort (57 H. pylori-infected and 153 controls), children with H. pylori infection had significantly lower BMI (17.7 ± 3.8 kg/m2vs 19.0 ± 3.7 kg/m2, P = 0.02) and z score of BMI (0.25 ± 1.09 vs 0.68 ± 0.87, P = 0.009) than controls at the enrollment. In Table 2, there was no difference with regards to patients’ demographic background among the eradication failure, eradication success, and control groups at enrollment. In comparison to the observation cohort, the z score of BMI at enrollment was significantly lower in successful eradication group (0.21 ± 1.14 vs 0.68 ± 0.87, P = 0.007) than in the non-infected controls. The baseline BW and BH were still lower in the H. pylori-infected children (either with eradication success or failure) than in controls, although it is not statistically significant.
| Groups | H. pylori eradication failure | H. pylori eradication success | H. pylori-negative controls |
| n | 17 | 34 | 153 |
| Age (yr) | 8.4 ± 1.9 | 8.7 ± 2.0 | 9.0 ± 1.7 |
| Sex (female:male) | 10:07 | 19:15 | 81 : 72 |
| Family peptic ulcer history (%) | 44.4 | 29.4 | 21 |
| Intra-familial members ≥ 5 (%) | 33.3 | 29.4 | 32.1 |
| Low income (%) | 28.6 | 53.3 | 44.1 |
| Baseline serum acylated ghrelin (pg/mL)ac | 37.2 ± 31.4 | 44.2 ± 37.9 | 83.6 ± 24.2 |
| Body weight (kg) | |||
| At enrollment | 32.2 ± 10.6 | 32.9 ± 11.4 | 36.1 ± 11.4 |
| z score at enrollment | 0.44 ± 1.00 | 0.35 ± 1.04 | 0.60 ± 0.87 |
| The 1st year | 37.2 ± 11.7 | 38.7 ± 13.8 | 41.0 ± 12.6 |
| z score at the 1st year | 0.56 ± 0.88 | 0.49 ± 1.03 | 0.61 ± 0.88 |
| Net changec | 5.03 ± 2.77 | 5.84 ± 3.37 | 4.84 ± 2.35 |
| Increase of z score (%)c | 23.5 | 38.2 | 17.6 |
| Body height (cm) | |||
| At enrollment | 134.6 ± 12.7 | 134.3 ± 11.7 | 135.7 ± 12.5 |
| z score at enrollment | 0.62 ± 0.99 | 0.44 ± 0.88 | 0.33 ± 0.77 |
| The 1st year | 141.8 ± 11.8 | 142.3 ± 12.8 | 141.5 ± 13.0 |
| z score at the 1st yearac | 0.77 ± 0.94 | 0.63 ± 0.75 | 0.32 ± 0.79 |
| Net changec,e | 7.20 ± 2.85 | 8.00 ± 2.78 | 5.85 ± 1.81 |
| Increase of z score (%)c | 35.3 | 35.3 | 15.7 |
| BMI (kg/m2) | |||
| At enrollmenta | 17.1 ± 2.6 | 17.7 ± 4.1 | 19.0 ± 3.7 |
| z score at enrollmentc | 0.24 ± 0.75 | 0.21 ± 1.14 | 0.68 ± 0.87 |
| The 1st year | 18.1 ± 3.1 | 18.8 ± 4.7 | 20.0 ± 3.8 |
| z score at the 1st yearc | 0.35 ± 0.82 | 0.27 ± 1.12 | 0.67 ± 0.87 |
| Net change | 0.98 ± 1.57 | 1.10 ± 1.56 | 0.99 ± 1.03 |
| Increase of z score (%) | 35.3 | 32.4 | 17 |
There were 34 children with successful H. pylori eradication and 17 children with failure of who completed the one-year follow-up study. Moreover, we completed the one-year follow-up to the 153 age- and sex-matched non-infected controls. One-year after eradication therapy, the H. pylori-infected children with successful eradication had significantly higher net increases of BW (5.84 ± 3.37 kg vs 4.84 ± 2.85 kg, P = 0.04) and BH (8.00 ± 2.78 cm vs 5.85 ± 1.81 cm, P < 0.001) than the H. pylori-negative controls. Moreover, the rates of increase of z scores of BW (38.2% vs 17.6%, P = 0.02) and BH (35.3% vs 15.7%, P = 0.02) were significantly higher in children with successful eradication than controls. We further analyzed the enrolled age as a confounder for the increase of z scores of BW and BH by multiple logistic regression analysis. The results confirmed the successful eradication of H. pylori was an independent factor to predict the increase of BW (P = 0.01) and BH (P = 0.01) in the intervention cohort. Although triple therapy failed to achieve successful eradication, these H. pylori-infected children still had a higher net increase of BH (7.20 ± 2.85 cm vs 5.85 ± 1.81 cm, P = 0.01) than the non-infected controls. However, these H. pylori-infected children and non-infected controls had no difference in the net increase of BW (5.03 ± 2.77 kg vs 4.84 ± 2.35 kg, P = 0.78) and increase of z scores of BW (23.5% vs 17.6%, P = 0.79) during one-year follow-up.
In the intervention cohort, the H. pylori-infected children had a significantly lower serum acylated ghrelin level (41.8 ± 35.6 pg/mL vs 83.6 ± 24.2 pg/mL, P < 0.001) than the controls. In Table 2, at enrollment, both the H. pylori-infected children with and without eradication success during follow-up had lower serum acylated ghrelin levels than the non-infected controls (both P < 0.001). As the H. pylori-infected children exhibited lower BW and lower serum acylated ghrelin, the study further tested whether the children with lower BW, within the age ranges, could have a lower serum acylated ghrelin. We compared the serum acylated ghrelin levels of the children between those with BW and z score of BW above and below the selected cut-off point in the different age ranges (Table 3). Only in the H. pylori-infected children with age ranges as 8-12 years, the serum acylated ghrelin level was lower in the children with BW below the cut-off point than that with BW above (23.8 ± 22.1 pg/mL vs 51.8 ± 40.9 pg/mL, P = 0.02).
| H. pylori infection | Non-H. pylori infection | |||
| Age ranges (yr) | 4-7 | 8-12 | 4-7 | 8-12 |
| BW cut-off point, kg (n) | 26 (21) | 36 (32) | 26 (47) | 36 (87) |
| Baseline serum acylated ghrelin (pg/mL) | ||||
| Above or equal to the BW cut-off point | 51.3 ± 38.6 | 51.8 ± 40.9 | 78.2 ± 12.0 | 85.9 ± 26.9 |
| Below the BW cut-off point | 47.8 ± 36.5 | 23.8 ± 22.1 | 82.5 ± 17.2 | 83.8 ± 31.2 |
| 1P value | 0.93 | 0.02 | 0.31 | 0.78 |
| z score of BW cut-off point (n) | 0.5 (18) | 0.5 (35) | 0.5 (28) | 0.5 (106) |
| Baseline serum acylated ghrelin (pg/mL) | ||||
| Above or equal to the z score of BW cut-off point | 53.4 ± 40.6 | 46.8 ± 38.9 | 81.3 ± 14.1 | 81.7 ± 19.7 |
| Below the z score of BW cut-off point | 45.9 ± 33.6 | 27.3 ± 26.5 | 81.9 ± 16.6 | 89.6 ± 36.4 |
| 1P value | 0.68 | 0.09 | 0.91 | 0.15 |
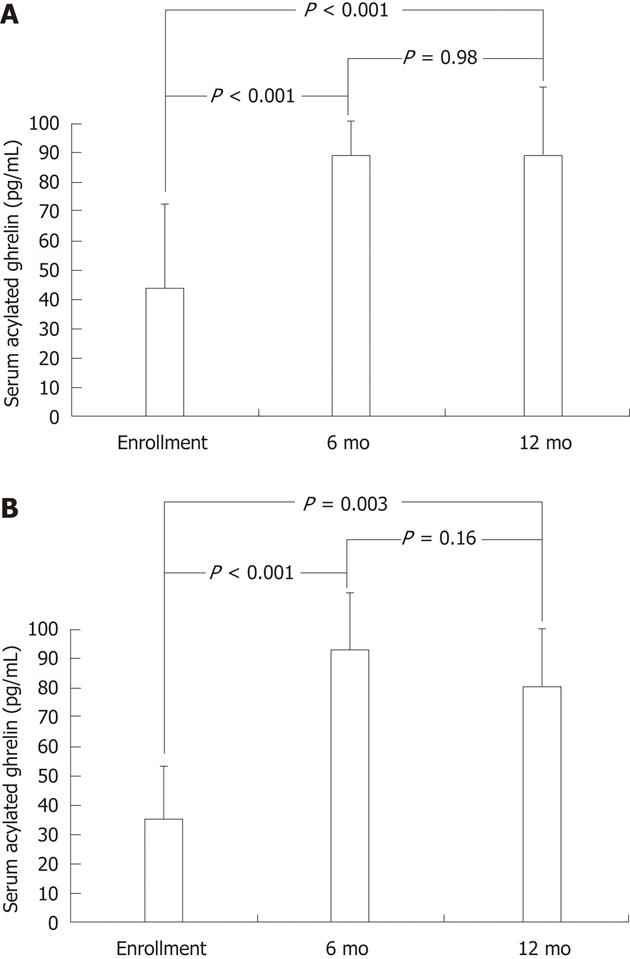
In addition to the baseline serum acylated ghrelin levels, the study subjects of the intervention cohort underwent testing for serial serum acylated ghrelin levels after triple therapy. In Figure 2A, for the children with successful H. pylori eradication, the serum acylated ghrelin levels were significantly increased after eradication therapy as early as the 6th mo (88.2 ± 17.3 pg/mL vs 44.2 ± 38.1 pg/mL, P < 0.001), until the 12th mo (87.7 ± 38.0 pg/mL vs 44.2 ± 38.1 pg/mL, P < 0.001). In Figure 2B, for the children with failure of H. pylori eradication, the serum acylated ghrelin levels could be also significantly increased by eradication therapy at the 6th mo (93.2 ± 31.6 pg/mL vs 37.2 ± 30.9 pg/mL, P < 0.001) and at the 12th mo (80.6 ± 28.8 pg/mL vs 37.2 ± 30.9 pg/mL, P = 0.003).
Extra-gastric diseases related to H. pylori infection are emerging in importance, such as iron deficiency anemia and growth retardation in children[28]. Other studies have argued that lower socioeconomic status is conjunction with the presence of H. pylori accounts for poor growth in children[29]. For overcoming the influencing bias of poor socioeconomic status, indicated by low income, to child growth, multiple logistic regression confirmed that H. pylori infection was closely related to both z scores of BW and BMI independent to socioeconomic status. Accordingly, the current study should have not encountered significant bias of social backgrounds on growth limitation in children.
Based on the data of the observation cohort, the H. pylori-infected children had significantly lower BW and BMI than gender- and age-matched controls at enrollment. In addition, the BH of H. pylori-infected children was 4.5 cm less than that of the non-infected children. After one-year follow-up, the H. pylori-infected children had profoundly lower BW, BMI, and net BW gain than the non-infected children. These findings comparing infected and matched disease-free subjects suggest that H. pylori infection exerted a negative effect on childhood growth. One possibility is that the presence of H. pylori leads to chronic gastric inflammation and thereby decreases food intake[30]. Alternatively, H. pylori infection may modify hormones related with the appetite, such as ghrelin[19-21,31,32].
Besides having a lower BW, the H. pylori-infected children had a significantly lower baseline serum acylated ghrelin level than that of the gender- and age-matched controls. These data not only suggest a relationship between H. pylori infection and lower BW, but that infection is linked to reduced ghrelin levels. Accordingly, it is important to determine whether children with a lower BW had lower serum acylated ghrelin. Supported by the findings in Table 3, only in the H. pylori-infected children with age ranging from 8-12 years, the serum acylated ghrelin levels were lower in children with BW below the cut-off point than that with BW above. This finding indicates a lower serum acylated ghrelin, which is induced by the H. pylori infection, could be related to induce a lower BW in children aged 8-12 years old.
Eradication of H. pylori in symptomatic adult patients has been reported to increase BMI[22,23], and to restore decreased ghrelin levels induced by H. pylori infection[16,17]. However, there is still limited evidence to prove such effects in children. Recently, a trial demonstrated H. pylori eradication may result in a significant increase in BMI, but with a decrease in the circulating ghrelin levels in a small group of children[33]. Consistent with these findings, our study supported H. pylori eradication to address positive impact on the improvement of childhood growth concomitant with an increase of serum acylated ghrelin level during the one-year long-term follow-up. Therefore, this study is the first to support that H. pylori eradication can restore decreased serum acylated ghrelin in children with lower BW. In the future, a longer follow-up study may help to determine whether ongoing elevations in BMI will ultimately lower serum ghrelin via a feedback loop. Our study was nonetheless sufficient to show that within one-year of H. pylori eradication, the children could achieve normal growth stature with concomitant restoration of the decreased serum acylated ghrelin induced by the H. pylori infection.
Even though some H. pylori-infected children had a failure of triple therapy, there was still existed an increase of BW, BH, and serum acylated ghrelin levels at the 6th and the 12th mo. Triple therapy can decrease bacterial loads or gastric inflammation[15,33]. We have analyzed the 51 pairs of 13C-UBT and ghrelin levels (at enrollment, the 6th and 12th mo follow-up) in 17 children with a failure of triple therapy. The result shows the bacterial loads, indicated by the values of 13C-UBT are not correlated well to the ghrelin levels (r2 = 0.03, P = 0.25). Therefore, it is possibly due to transient improvement of gastric inflammation to restore serum acylated ghrelin levels. Lack of endoscopic evidence in children with failure of therapy is the limitation in this study. A longer follow-up period is thus needed to clarify this transient improving effect in children with failure of therapy.
In summary, H. pylori infection can be associated with decreased serum acylated ghrelin levels, BW and BH in children. Successful H. pylori eradication can restore ghrelin levels and the growth of BW and BH in the infected children with growth retardation.
Helicobacter pylori (H. pylori) infection in children causes not only gastric inflammation and peptic ulcer diseases but also extragastric disorder. Longitudinal observational have found that children with persistent H. pylori infection have reduced body weight (BW) and height (BH) growth than the non-infected ones. In addition, previous studies showed conflicting results regarding the correlation between plasma ghrelin levels and H. pylori infection after eradication of bacteria. Therefore, long-term follow up the childhood growth as well ghrelin levels in H. pylori-infected children after eradication therapy can illustrate the causal relationship between H. pylori infection and growth retardation in children.
Growth retardation in H. pylori-infected children without any organic diseases remains controversial for eradication therapy. The authors aimed to establish a new indication for treating H. pylori infection in children with growth retardation and to explore the serum acylated ghrelin levels correlated to eradication therapy.
This study demonstrated that H. pylori infection can be associated with decreased serum acylated ghrelin levels, BW and BH in children. In the interventional study, successful H. pylori eradication can restore serum acylated ghrelin levels and the growth of BW and BH in the infected children with growth retardation at the 1-year follow-up.
This study confirmed the causal relationship of H. pylori infection and childhood growth retardation. Therefore, we supposed that eradication therapy should be considered as a treatment strategy in H. pylori-infected children with growth retardation, which was not related to other organic diseases.
Growth retardation is indicated by poor BW and BH growth as compared to the age- and gender-matched normal population. Eradication therapy means that a treatment strategy to eradicate H. pylori from stomach. The first-line regiment consists of one proton pump inhibitor and two antibiotics.
This is an interesting study aimed at determining whether H. pylori-infected children have reduced growth rates and lower levels of ghrelin compared to uninfected and if H. pylori eradication may reverse those changes. The study is well written and well designed.
Peer reviewer: Francesco Franceschi, MD, PhD, Assistant Professor, Internal Medicine, Catholic University of Rome, Gemelli Hospital, 00168 Rome, Italy
S- Editor Shi ZF L- Editor A E- Editor Xiong L
| 1. | Malaty HM, Kumagai T, Tanaka E, Ota H, Kiyosawa K, Graham DY, Katsuyama T. Evidence from a nine-year birth cohort study in Japan of transmission pathways of Helicobacter pylori infection. J Clin Microbiol. 2000;38:1971-1973. [PubMed] |
| 2. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] |
| 3. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] |
| 4. | Yang YJ, Wang SM, Chen CT, Huang MC, Chang CJ, Liu CC. Lack of evidence for fecal-oral transmission of Helicobacter pylori infection in Taiwanese. J Formos Med Assoc. 2003;102:375-378. [PubMed] |
| 5. | Lin DB, Lin JB, Chen CY, Chen SC, Chen WK. Seroprevalence of Helicobacter pylori infection among schoolchildren and teachers in Taiwan. Helicobacter. 2007;12:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Büyükgebiz A, Dündar B, Böber E, Büyükgebiz B. Helicobacter pylori infection in children with constitutional delay of growth and puberty. J Pediatr Endocrinol Metab. 2001;14:549-551. [PubMed] |
| 7. | Choe YH, Kim SK, Hong YC. Helicobacter pylori infection with iron deficiency anaemia and subnormal growth at puberty. Arch Dis Child. 2000;82:136-140. [PubMed] |
| 8. | Yang YJ, Sheu BS, Lee SC, Yang HB, Wu JJ. Children of Helicobacter pylori-infected dyspeptic mothers are predisposed to H. pylori acquisition with subsequent iron deficiency and growth retardation. Helicobacter. 2005;10:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Chimonas MA, Baggett HC, Parkinson AJ, Muth PT, Dunaway E, Gessner BD. Asymptomatic Helicobacter pylori infection and iron deficiency are not associated with decreased growth among Alaska Native children aged 7-11 years. Helicobacter. 2006;11:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sauvé-Martin H, Kalach N, Raymond J, Senouci L, Benhamou PH, Martin JC, Briet F, Maurel M, Flourié B, Dupont C. The rate of Helicobacter pylori infection in children with growth retardation. J Pediatr Gastroenterol Nutr. 1999;28:354-355. [PubMed] |
| 11. | Patel P, Mendall MA, Khulusi S, Northfield TC, Strachan DP. Helicobacter pylori infection in childhood: risk factors and effect on growth. BMJ. 1994;309:1119-1123. [PubMed] |
| 12. | Passaro DJ, Taylor DN, Gilman RH, Cabrera L, Parsonnet J. Growth slowing after acute Helicobacter pylori infection is age-dependent. J Pediatr Gastroenterol Nutr. 2002;35:522-526. [PubMed] |
| 13. | Bravo LE, Mera R, Reina JC, Pradilla A, Alzate A, Fontham E, Correa P. Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2003;37:614-619. [PubMed] |
| 14. | Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 15. | Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [PubMed] |
| 18. | Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423-426. [PubMed] |
| 19. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5889] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 20. | Campana D, Nori F, Pagotto U, De Iasio R, Morselli-Labate AM, Pasquali R, Corinaldesi R, Tomassetti P. Plasma acylated ghrelin levels are higher in patients with chronic atrophic gastritis. Clin Endocrinol (Oxf). 2007;67:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327-6333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 22. | Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002;16:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Yang YJ, Sheu BS, Chang WL, Cheng HC, Yang HB. Increased body mass index after H. pylori eradication for duodenal ulcer predisposes to erosive reflux esophagitis. J Clin Gastroenterol. 2009;43:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Chen W, Chang MH. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr Neonatol. 2010;51:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Sheu BS, Lin CY, Lin XZ, Shiesh SC, Yang HB, Chen CY. Long-term outcome of triple therapy in Helicobacter pylori-related nonulcer dyspepsia: a prospective controlled assessment. Am J Gastroenterol. 1996;91:441-447. [PubMed] |
| 26. | Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, Macarthur C, Snyder J, Sherman PM. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:490-497. [PubMed] |
| 27. | Sheu BS, Lee SC, Yang HB, Wu HW, Wu CS, Lin XZ, Wu JJ. Lower-dose (13)C-urea breath test to detect Helicobacter pylori infection-comparison between infrared spectrometer and mass spectrometry analysis. Aliment Pharmacol Ther. 2000;14:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 29. | Sood MR, Joshi S, Akobeng AK, Mitchell J, Thomas AG. Growth in children with Helicobacter pylori infection and dyspepsia. Arch Dis Child. 2005;90:1025-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Weigt J, Malfertheiner P. Influence of Helicobacter pylori on gastric regulation of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Ozçay F, Demir H, Ozen H, Gürakan F, Saltik IN, Yüce A, Koçak N. Normal growth in young children with Helicobacter pylori infection. J Pediatr Gastroenterol Nutr. 2002;35:102. [PubMed] |
| 32. | Perri F, Pastore M, Leandro G, Clemente R, Ghoos Y, Peeters M, Annese V, Quitadamo M, Latiano A, Rutgeerts P. Helicobacter pylori infection and growth delay in older children. Arch Dis Child. 1997;77:46-49. [PubMed] |
| 33. | Pacifico L, Anania C, Osborn JF, Ferrara E, Schiavo E, Bonamico M, Chiesa C. Long-term effects of Helicobacter pylori eradication on circulating ghrelin and leptin concentrations and body composition in prepubertal children. Eur J Endocrinol. 2008;158:323-332. [PubMed] [DOI] [Full Text] |