Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2533
Revised: February 8, 2012
Accepted: February 26, 2012
Published online: May 28, 2012
AIM: To investigate the clinical features and prognostic factors of advanced hepatocellular carcinoma (HCC) patients presenting with lung metastasis at initial diagnosis.
METHODS: Between 2001 and 2010, we recruited 76 consecutive HCC patients initially presenting with lung metastasis, without co-existing metastasis from other sites. These patients were divided into three groups: untreated group (n = 22), single treatment group (n = 19), and combined treatment group (n = 35).
RESULTS: Metastasis of bilateral lung lobes was common and noted in 35 patients (46.1%), and most of patients (59/76, 77.6%) presented with multiple lung metastatic nodules. Nineteen patients (25.0%) received single-method treatment, including hepatectomy in 4, transcatheter arterial chemoembolization in 6, radiotherapy in 5, and oral sorafenib in 4. Thirty-five patients (46.1%) received combined treatment modalities. The overall median survival of the all patients was 8.7 ± 0.6 mo; 4.1 ± 0.3, 6.3 ± 2.5 and 18.6 ± 3.9 mo, respectively in the untreated group, single treatment group and combined treatment group, respectively, with a significant difference (log-rank test, P < 0.001). Multivariate analysis revealed that Child-Pugh score, the absence or presence of portal vein tumor thrombus, and treatment modality were three independent prognostic factors affecting survival of patients with advanced HCC and concomitant lung metastasis.
CONCLUSION: Combined treatment modalities tend to result in a better survival as compared with the conservative treatment or single treatment modality for HCC patients initially presenting with lung metastasis.
- Citation: Yang T, Lu JH, Lin C, Shi S, Chen TH, Zhao RH, Wang Y, Wu MC. Concomitant lung metastasis in patients with advanced hepatocellular carcinoma. World J Gastroenterol 2012; 18(20): 2533-2539
- URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2533
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world. The number of new cases is estimated to be 500 000-1 000 000 per year[1]. Overall, 80% of HCC are attributed to chronic hepatitis B and C infection. Surgical resection with complete extirpation of tumor gives the best chance of a cure for patients with HCC[2]. However, a majority of HCC patients are considered as advanced or even at end-stage at their first hospital visit, with extensive tumor status, for example, macroscopic vascular invasion, extrahepatic metastasis, etc. Of various metastatic sites, the most common site is lung, followed by lymph node, bone and brain[3].
As one type of advanced HCC, HCC presenting with lung metastasis is not unusual, and its prognosis is very poor[4]. Nowadays, there are various treatment modalities for both intrahepatic tumor and extrahepatic metastatic foci of HCC, including surgical resection, transcatheter arterial chemoembolization (TACE), radiotherapy, chemotherapeutics, and recent molecular targeted therapeutic drugs[2]. However, the prognosis and treatment outcomes of advanced HCC presenting with lung metastasis remain poorly evaluated. We investigated the prognosis, treatment outcomes, and independent prognostic factors affecting the survival of a series of HCC patients presenting with lung metastasis at initial diagnosis.
From January 2001 to December 2010, 103 consecutive patients were diagnosed as having lung metastasis at the first time of HCC diagnosis in the 5th Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China. To evaluate the efficacy of anti-tumor treatment modality for patients with HCC and concomitant isolated lung metastasis, we excluded those patients who showed evidence of severe organ failure (renal, respiratory, or cardiologic problems), poor liver function (Child-Pugh class C) that could affect survival, or co-existing metastasis from other sites. Therefore, 76 of the 103 patients (59 males and 17 females) were recruited for the study. The last follow-up date was January 30, 2011. This study protocol was approved by the Institutional Review Board of the Eastern Hepatobiliary Hospital.
We used a prospectively maintained database and conducted a retrospective study among these patients. We focused on the prognosis and prognostic factors affecting the survival of HCC patients presenting with lung metastasis. To investigate whether local regional or systemic therapy could affect survival, we stratified these 76 patients into three groups: untreated group (n = 22), single treatment group (n = 19), and combined treatment group (n = 35). All of the patients in the untreated group refused any anti-tumor invasive treatment other than conservative treatment, including the support of liver function, after being informed of the expenses and the possible side effects of any anti-tumor invasive treatment. The single treatment method for these patients included hepatectomy, TACE, radiotherapy, chemotherapeutics, and oral sorafenib, while combined treatment modalities were defined as more than one of the above treatment methods or pulmonary metastasectomy.
Laboratory blood tests including hepatitis B virus (HBV) markers, anti-hepatitis C virus, serum α-fetoprotein (AFP), carcinoembryonic antigen, platelet, serum albumin, serum total bilirubin, alanine transaminase, aspartate aminotransferase, and prothrombin time were performed.
The diagnosis of HCC was based on concordance between two imaging examinations [ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI)] showing arterial hypervascularity in a focal lesion ≥ 2 cm or with the combined criteria of an imaging examination and a serum AFP level greater than 400 ng/mL, according to the criteria of the Conference of the European Association for Study of the Liver[5]. In the present study, the diagnosis of HCC was also confirmed by pathohistology in 20 patients.
Lung metastases were diagnosed in all the 76 patients using imaging techniques, including chest CT (n = 62), positron emission tomography-CT (n = 11), and chest MRI (n = 3). In addition, one patient was further confirmed by pathohistology after a biopsy, and four patients after pulmonary metastasectomy.
Continuous data were expressed as mean ± SD or median (range). Categorical variables were compared by the χ2 test or Fisher exact test, and continuous variables were compared by the Student t test or one-way analysis of variance. The survival rate was calculated using the Kaplan-Meier method, and the log-rank test was used to compare survival rates among three groups. Cox’s proportional hazards model was used for multivariate analysis. All statistical analysis in this study were done using software package SPSS11.0 (SPSS Inc., Chicago, IL). A P value < 0.05 was defined to be statistically significant.
The baseline characteristics of these 76 patients with advanced HCC initially presenting with lung metastasis are summarized in Table 1. The mean age of patients was 52.4 ± 10.7 years. The most common etiology of the liver disease was HBV infection, which occurred in 63 patients (82.9%). There was no statistical difference in the baseline characteristics among the untreated, single treatment and combined treatment groups, including age, etiology of the liver disease, the presence of cirrhosis or ascites, AFP level, Eastern Cooperative Oncology Group (ECOG) scale, Child-Pugh score, and liver function.
| Total (n = 76) | Untreated group (n = 22) | Single treated group (n = 19) | Combined treated group (n = 35) | P value | |
| Sex | |||||
| Male | 59 (77.6) | 16 (72.7) | 15 (78.9) | 28 (80.0) | 0.804 |
| Female | 17 (22.4) | 6 (27.3) | 4 (21.1) | 7 (20.0) | |
| Age (yr) | 52.4 ± 10.7 | 52.8 ± 9.3 | 52.5 ± 11.6 | 52.1 ± 11.4 | 0.971 |
| Etiology | |||||
| HBV | 63 (82.9) | 18 (82.9) | 16 (84.2) | 29 (82.9) | 0.989 |
| HCV | 3 (3.9) | 1 (4.5) | 1 (5.3) | 1 (2.9) | |
| Alcohol | 5 (6.6) | 1 (4.5) | 1 (5.3) | 3 (8.6) | |
| Non-B and non-C | 5 (6.6) | 2 (9.1) | 1 (5.3) | 2 (5.7) | |
| Cirrhosis | 65 (85.5) | 20 (90.9) | 17 (89.5) | 28 (80.0) | 0.443 |
| Platelet (-3) | 185.8 ± 75.5 | 180.9 ± 95.1 | 174.3 ± 74.0 | 195.1 ± 62.6 | 0.593 |
| Albumin (g/L) | 36.5 ± 4.3 | 36.3 ± 5.4 | 35.3 ± 3.5 | 37.3 ± 3.7 | 0.276 |
| ALT (IU/L), median (range) | 35 (10-352) | 35 (15-352) | 37 (15-124) | 34 (10-210) | 0.456 |
| AST (IU/L), median (range) | 35 (16-453) | 41 (16-453) | 44 (20-173) | 33 (17-223) | 0.364 |
| Total bilirubin (μmol/L) | 23.7 ± 13.3 | 28.1 ± 17.4 | 23.3 ± 8.1 | 21.2 ± 12.2 | 0.162 |
| Prothrombin time (s) | 12.9 ± 1.7 | 13.3 ± 2.3 | 13.0 ± 1.3 | 12.5 ± 1.3 | 0.241 |
| AFP (ng/mL) | |||||
| < 400 | 36 (47.4) | 10 (45.5) | 7 (36.8) | 19 (54.3) | 0.461 |
| ≥ 400 | 40 (52.6) | 12 (54.5) | 12 (63.2) | 16 (52.6) | |
| ECOG score | |||||
| 0 | 17 (22.4) | 4 (18.2) | 4 (21.1) | 9 (25.7) | 0.672 |
| 1 | 54 (71.1) | 16 (72.7) | 15 (78.9) | 23 (65.7) | |
| 2 | 5 (6.6) | 2 (9.1) | 0 (0) | 3 (8.6) | |
| Ascites | |||||
| None | 67 (88.2) | 18 (81.8) | 16 (84.2) | 33 (94.3) | 0.303 |
| Mild–moderate | 9 (11.8) | 4 (18.2) | 3 (15.8) | 2 (5.7) | |
| Child-Pugh score | |||||
| A | 61 (80.3) | 16 (72.7) | 14 (73.7) | 31 (88.6) | 0.243 |
| B | 15 (19.7) | 6 (27.3) | 5 (26.3) | 4 (11.4) |
For intrahepatic tumor status, there was no significant difference in tumor number, tumor location, and the probability of portal vein tumor thrombus or hepatic vein tumor thrombus among the three treatment groups, except for maximum tumor diameter (P = 0.007) (Table 2). As such, there is also no significant difference in number, location, and maximum diameter of lung metastatic nodules among these three groups (Table 2).
| Total (n = 76) | Untreated group (n = 22) | Single treated group (n = 19) | Combined treated group (n = 35) | P value | |
| Intrahepatic tumor | |||||
| Maximum tumor diameter (cm) | 9.3 ± 3.0 | 10.7 ± 3.2 | 9.7 ± 2.8 | 8.2 ± 2.5 | 0.007 |
| Tumor number | |||||
| Solitary | 29 (38.2) | 9 (40.9) | 6 (31.6) | 14 (40.0) | 0.791 |
| Multiple/diffuse | 47 (61.8) | 13 (59.1) | 13 (68.4) | 21 (60.0) | |
| Tumor location | |||||
| Right | 39 (51.3) | 13 (59.1) | 8 (42.1) | 18 (51.4) | 0.834 |
| Left | 15 (19.7) | 4 (18.2) | 4 (21.1) | 7 (20.0) | |
| Both | 22 (28.9) | 5 (22.7) | 7 (36.8) | 10 (28.6) | |
| Portal vein tumor thrombus | |||||
| Absence | 54 (71.1) | 14 (63.6) | 12 (63.2) | 28 (80.0) | 0.283 |
| Presence | 22 (28.9) | 8 (36.4) | 7 (36.8) | 7 (20.0) | |
| Hepatic vein tumor thrombus | |||||
| Absence | 67 (88.2) | 19 (86.4) | 15 (78.9) | 33 (94.2) | 0.238 |
| Presence | 9 (11.8) | 3 (13.6) | 4 (21.1) | 2 (5.7) | |
| Lung metastasis | |||||
| Number of metastasis | |||||
| Solitary | 17 (22.4) | 4 (18.2) | 3 (15.8) | 10 (28.6) | 0.479 |
| Multiple | 59 (77.6) | 18 (81.8) | 16 (84.2) | 25 (71.4) | |
| Location of metastasis | |||||
| Right lung lobe | 28 (36.8) | 6 (27.3) | 8 (42.1) | 14 (40.0) | 0.721 |
| Left lung lobe | 13 (17.1) | 4 (18.2) | 2 (10.5) | 7 (20.0) | |
| Bialteral lung lobes | 35 (46.1) | 12 (54.5) | 9 (47.4) | 14 (40.0) | |
| Maximum metastasis diameter (cm) | 2.5 ± 0.9 | 2.6 ± 0.9 | 2.2 ± 1.2 | 2.5 ± 0.8 | 0.445 |
Among all the 76 patients, 22 patients did not receive any anti-tumor treatment but conservative treatment. We divided the remaining 54 patients into two groups according to their treatment schemes. Nineteen patients (25.0%) received single treatment modality (locoregional or systemic therapy), including hepatectomy in 4 patients, TACE in 6, radiotherapy for intrahepatic tumor and lung metastatic nodules in 5 (including γ knife radiosurgery in 3 and X-ray radiotherapy in 2), and oral sorafenib in 4. Thirty-five patients (46.1%) received combined treatment modalities (Table 3). The common treatment modality in the combined treatment group was radiotherapy (30/35, 85.7%), followed by hepatectomy (23/35, 65.7%), TACE (18/35, 51.4%), and oral sorafenib therapy (11/35, 31.4%). In the combined treatment group, radiotherapy (γ knife radiosurgery in 24 and X-ray radiotherapy in 4) was mainly used for lung metastatic nodules (27/28) rather than intrahepatic tumor nodules (8/28). In addition, pulmonary metastasectomies were carried out in 4 patients who underwent hepatectomy during the same operation.
| Treatment modalities | n (%) |
| Single treatment modality in treated patients (n = 19) | |
| Hepatectomy | 4 (21.1) |
| Pulmonary metastasectomy | 0 (0) |
| Transcatheter arterial chemoembolization | 6 (31.6) |
| Radiotherapy | 5 (26.3) |
| Oral sorafenib | 4 (21.1) |
| Combined treatment modalities in treated patients (n = 35) | |
| Hepatectomy + pulmonary metastasectomy | 3 (8.6) |
| Hepatectomy + pulmonary metastasectomy + oral sorafenib | 1 (2.9) |
| Hepatectomy + transcatheter arterial chemoembolization + radiotherapy | 6 (17.1) |
| Hepatectomy + radiotherapy | 9 (25.7) |
| Hepatectomy + transcatheter arterial chemoembolization + radiotherapy + oral sorafenib | 2 (5.7) |
| Hepatectomy + radiotherapy + oral sorafenib | 2 (5.7) |
| Transcatheter arterial chemoembolization + radiotherapy | 6 (17.1) |
| Transcatheter arterial chemoembolization + radiotherapy + oral sorafenib | 3 (8.6) |
| Transcatheter arterial chemoembolization + oral sorafenib | 1 (2.9) |
| Radiotherapy + oral sorafenib | 2 (5.7) |
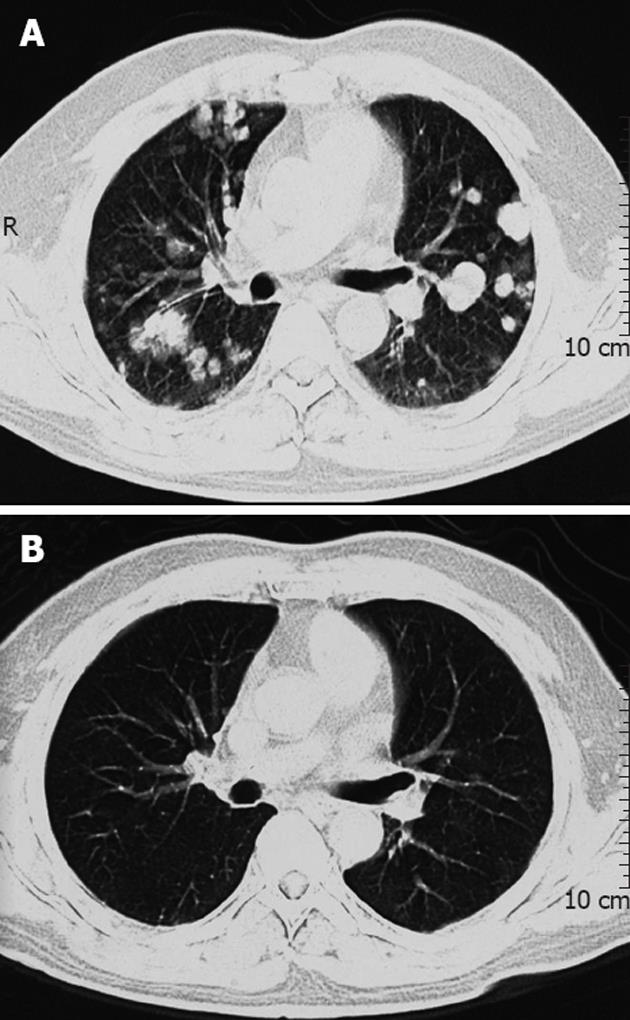
After a median follow-up of 8.7 ± 0.6 mo (range, 1.1-68.7 mo), 59 patients died and 17 patients remained alive. The causes of mortality were disease progression, including hepatic failure in 31 patients (52.5%); cachexia in 12 (20.3%); upper gastrointestinal bleeding in four (6.8%); pneumonia in one (1.7%); and pulmonary thromboembolism in one (1.7%). The cause of mortality of 10 patients was not confirmed. Among 17 surviving patients, only 4 patients were disease-free, including 3 patients who underwent hepatectomy and pulmonary metastasectomy, and one patient who received hepatectomy and subsequent γ-knife radiosurgery. Among 13 patients who were not disease-free but alive, 6 patients still took medicine of sorafenib, and achieved complete or partial response on radiographic examination (Figure 1A and B). The 6-mo, 1-year and 2-year cumulative survival rates of all these patients were 64.5%, 40.1% and 18.5%, respectively.
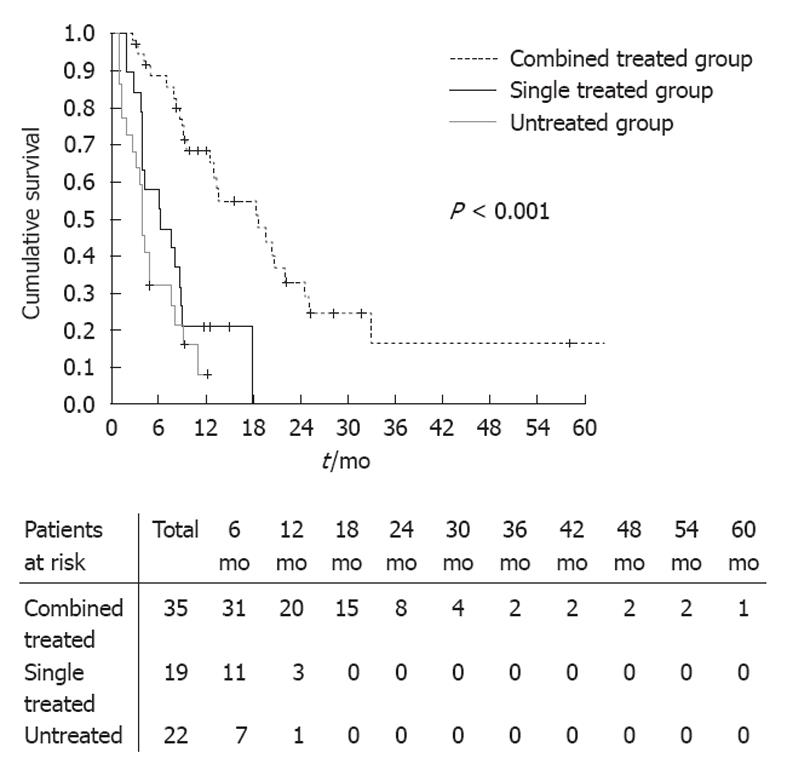
The median survival time of combined treatment group was 18.6 ± 3.9 mo, which was longer than that of single treatment group (6.3 ± 2.5 mo) and untreatment group (4.1 ± 0.3 mo), and there was significant statistical differences among these groups (log-rank test, P < 0.001) (Figure 2). The 6-mo cumulative survival rate of combined treatment, single treatment and untreated group was 88.6%, 57.9% and 31.8%, respectively; and the 1-year survival rate of the three groups was 68.5%, 21.1% and 8.0%, respectively.
In investigation of prognostic factors of survival of HCC presenting with lung metastasis at initial diagnosis, univariate analysis showed that ECOG score, Child-Pugh score, ascites, AFP level, maximum intrahepatic tumor diameter, the absence or presence of portal vein tumor thrombus, the absence or presence of hepatic vein tumor thrombus, lung metastatic tumor number, and treatment modality had prognostic significance (Table 4).
| Variables | P value | |
| Univariate analysis | Multivariate analysis | |
| Sex | ||
| Male/female | 0.988 | – |
| Age (yr) | ||
| < 50/≥ 50 | 0.423 | – |
| Etiology | ||
| Non-hepatitis/hepatitis | 0.111 | – |
| Cirrhosis | ||
| Absence/presence | 0.36 | – |
| ECOG score | ||
| 2000/1/2 | 0.012 | 0.3 |
| Child-Pugh score | ||
| A/B | < 0.001 | < 0.001 |
| Ascites | ||
| No/yes | 0.031 | 0.879 |
| Platelet (-3) | ||
| < 100/≥ 100 | 0.198 | – |
| Albumin (g/L) | ||
| < 37.7/≥ 37.7 | 0.134 | – |
| ALT (IU/L) | ||
| < 40/≥ 40 | 0.734 | – |
| AST (IU/L) | ||
| < 40/≥ 40 | 0.356 | – |
| Total bilirubin (μmol/L) | ||
| < 17.1/≥ 17.1 | 0.319 | – |
| Prothrombin time (s) | ||
| < 14.0/≥ 14.0 | 0.251 | – |
| AFP (ng/mL) | ||
| < 400/≥ 400 | 0.03 | 0.384 |
| Maximum intrahepatic tumor diameter (cm) | ||
| < 8.0/≥ 8.0 | 0.039 | 0.214 |
| Intrahepatic tumor number | ||
| Solitary/multiple or diffuse | 0.279 | – |
| Intrahepatic tumor location | ||
| Single lobe/both lobes | 0.435 | – |
| Portal vein tumor thrombus | ||
| No/yes | < 0.001 | < 0.001 |
| Hepatic vein tumor thrombus | ||
| No/yes | 0.031 | 0.194 |
| Lung metastatic tumor number | ||
| Solitary/multiple | 0.018 | 0.321 |
| Lung metastatic tumor location | ||
| Single lobe/both lobes | 0.156 | – |
| Maximum metastasis diameter (cm) | ||
| < 2.5/≥ 2.5 | 0.129 | – |
| Treatment modality | ||
| No/yes | < 0.001 | < 0.001 |
In the Cox proportional hazard model, Child-Pugh score, the absence or presence of portal vein tumor thrombus at the initial presentation, and treatment modality were three independent prognostic factors that affected the survival of HCC patients that presented with lung metastasis at initial diagnosis (Table 4).
To the best of our knowledge, this is the first study that investigates in detail the prognosis and prognostic factors of advanced HCC presenting with lung metastasis at initial diagnosis. In our study, the variables of tumor status and liver function status at initial diagnosis were almost comparable. However, the survival times from date of diagnosis until death or last visit among the three groups were significantly different, and patients underwent specific combined treatment modalities had longer survival than those treated with single method or those without anti-tumor treatment. However, it must be noted that there are many therapeutic modalities for tumor nodules, whether regional or systemic, which have their own indications and contraindications, and could not be applied altogether on a specific individual.
There are many case reports and clinical studies of patients with advanced HCC and lung metastasis who underwent radical hepatectomy and pulmonary metastasectomy[6-12]. In the present study, four patients also underwent combined surgical resection, three of whom were still alive at the end of follow-up. We think that the combined radical surgery should be positively considered if intrahepatic tumor and lung metastatic lesion were completely resectable, if the remaining volume of the liver was adequate, and the lung metastatic lesion was single[13]. In theory, removing all existing tumor lesions, including primary and second lesions, is the only possible therapeutic approach till now.
Recently, with development in radiotherapy techniques, radiotherapy has been shown to play a potential role in a wide spectrum of HCC, therefore it is necessary to evaluate the effect of radiotherapy[14-16]. In our center, stereotactic radiotherapy, particularly γ knife radiosurgery, has become the major therapeutic mordality for lung and brain metastases of HCC. In the present study, there were 35 patients who underwent radiotherapy for intrahepatic HCC tumors and/or lung metastatic nodules, accounting for more than 50%, whether or not they are combined with other anti-tumor treatment modalities. Despite advances in radiotherapy delivery, liver toxicity following radiotherapy remains a dose-limiting factor, and investigations to better understand the pathophysiology of radiotherapy-induced liver toxicity are warranted. There is a particular interest in combining radiotherapy with anti-vascular endothelial growth factor targeting agents for their independent activity in HCC as well as their radiation sensitization properties[17].
Sorafenib is a multikinase inhibitor with effects against tumor proliferation and angiogenesis, and was recently approved for the treatment of advanced HCC[18,19]. Maintenance sorafenib would probably prevent or delay the intrahepatic and extrahepatic spread of HCC after radiotherapy, which provides the rationale for the combination of these treatment modalities[20]. In the present study, 15 patients received oral sorafenib. Although we did not find its significant efficacy in HCC presenting with lung metastasis due to few cases and less strict design, we believed that sorafenib could be used as one part of combined treatment modalities for HCC patients with lung metastasis. However, a large-scale randomized controlled trial is needed to confirm it. Combining surgical resection with sorafenib would be considered as the most optimal treatment modality for HCC patients with lung metastasis.. However, it remains to be confirmed by a randomized controlled clinical trial in the future.
Till now, we have not obtained enough evidences to confirm the role of locoregional hepatectomy in HCC patients with lung metastasis. In our opinion, hepatectomy can be performed when primary intrahepatic HCCs are completely resected, the number of lung metastases is less than 3, and the diameter of individual lung metastasis is less than 3 cm. In addition, any of treatment modalities, such as radiotherapy or TACE, should be used together for lung metastasis.
In the univariate and subsequent multivariate analyses, Child-Pugh score was an independent prognostic factor for HCC patients with lung metastasis. This indicates that although invasive treatment other than conservative treatment can prolong the survival in well-selected patients, one must be careful before applying invasive treatment to all patients.
This study has several limitations. First, it was designed retrospectively. Although the patients’ status, including ECOG scale, Child-Pugh score, and liver function, was reviewed in the medical records and did not differ statistically among the three treatment groups, the clinical circumstances at the initial presentation might differ. Therefore, the decision for the treatment mordalities might be biased, and the subsequent patient stratification into various treatment groups might also be biased. Second, treatment modalities, i.e., radiotherapy, TACE, varied, and the number of patients treated was too small to confirm the effectiveness of each treatment modality. Third, the indications of specific treatment modality varied, and selective modalities were given to selective patients with advanced HCC and lung metastasis. It is very hard to build up a standard therapeutic regime for all patients, regardless of primary and secondary tumor site, size and number, liver functional reserve, and patients’ general condition.
In conclusion, the present study showed that the prognosis of advanced HCC with concomitant lung metastasis at initial diagnosis is very poor, and combined comprehensive treatment modalities tended to significantly prolong the survival of the patients compared with conservative treatment or single treatment modality. Furthermore, further randomized trials might be required to investigate the optimal treatment modality in the near future.
The prognosis and treatment outcomes of advanced hepatocellular carcinoma (HCC) presenting with lung metastasis remain poorly evaluated.
There are various treatment modalities for advanced HCC, including surgical resection, transcatheter arterial chemoembolization, radiotherapy, chemotherapeutics, and administration of molecular targeted therapeutic drugs. This study investigated the prognosis, treatment outcomes, and independent prognostic factors affecting the survival of a series of HCC patients presenting with lung metastasis at initial diagnosis.
In this study, the survival times from date of diagnosis until death or last visit between untreated group, single treatment group, and combined treatment group were significantly different, and patients underwent specific combined treatment modalities had longer survival than those treated with single method or those without anti-tumor treatment. Multivariate analysis revealed that Child-Pugh score, the absence or presence of portal vein tumor thrombus, and treatment modality were three independent prognostic factors affecting survival of patients with advanced HCC and concomitant lung metastasis.
As one type of advanced HCC, HCC presenting with lung metastasis is not unusual, and its prognosis is very poor. The findings in this study may contribute to its prognosis, and combined treatment modalities tend to result in a better survival for patients with advanced HCC initially presenting with lung metastasis.
The authors firstly investigated that combined treatment modalities tended to yield better survival prolongation compared with conservative treatment or single treatment modality for HCC patients initially presenting with lung metastasis, which may be contribute to its prognosis.
Peer reviewer: Dr. Alessandro Ferrero, Surgery, Ospedale Mauriziano, Largo Turati 62, 10128 Torino, Italy
S- Editor Gou SX L- Editor Ma JY E- Editor Zheng XM
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [PubMed] |
| 2. | Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453-469. [PubMed] |
| 3. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [PubMed] |
| 4. | Zhang SM, Zeng ZC, Tang ZY, Sun J, Cheng JM, Liu R, Wang P, Zhang BH. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int. 2008;2:237-243. [PubMed] |
| 5. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 6. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. [PubMed] |
| 7. | Nakagawa T, Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Matsushita M, Todo S. Pulmonary resection for metastases from hepatocellular carcinoma: factors influencing prognosis. J Thorac Cardiovasc Surg. 2006;131:1248-1254. [PubMed] |
| 8. | Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, Higashiyama M, Ishikawa O, Kodama K, Imaoka S. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192:46-51. [PubMed] |
| 9. | Kuo SW, Chang YL, Huang PM, Hsu HH, Chen JS, Lee JM, Lee PH, Lee YC. Prognostic factors for pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2007;14:992-997. [PubMed] |
| 10. | Chen F, Sato K, Fujinaga T, Sonobe M, Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T. Pulmonary resection for metastases from hepatocellular carcinoma. World J Surg. 2008;32:2213-2217. [PubMed] |
| 11. | Kawamura M, Nakajima J, Matsuguma H, Horio H, Miyoshi S, Nakagawa K, Fujisawa T, Kobayashi K. Surgical outcomes for pulmonary metastases from hepatocellular carcinoma. Eur J Cardiothorac Surg. 2008;34:196-199. [PubMed] |
| 12. | Kwon JB, Park K, Kim YD, Seo JH, Moon SW, Cho DG, Kim YW, Kim DG, Yoon SK, Lim HW. Clinical outcome after pulmonary metastasectomy from primary hepatocellular carcinoma: analysis of prognostic factors. World J Gastroenterol. 2008;14:5717-5722. [PubMed] |
| 13. | Yang T, Zhang J, Lu JH, Yang LQ, Yang GS, Wu MC, Yu WF. A new staging system for resectable hepatocellular carcinoma: comparison with six existing staging systems in a large Chinese cohort. J Cancer Res Clin Oncol. 2011;137:739-750. [PubMed] |
| 14. | Dawson LA. The evolving role of radiation therapy in hepatocellular carcinoma. Cancer Radiother. 2008;12:96-101. [PubMed] |
| 15. | Cárdenes HR. Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clin Transl Oncol. 2009;11:276-283. [PubMed] |
| 16. | Ma S, Jiao B, Liu X, Yi H, Kong D, Gao L, Zhao G, Yang Y, Liu X. Approach to radiation therapy in hepatocellular carcinoma. Cancer Treat Rev. 2010;36:157-163. [PubMed] |
| 17. | Tse RV, Guha C, Dawson LA. Conformal radiotherapy for hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;67:113-123. [PubMed] |
| 18. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] |
| 19. | Colombo M. Sorafenib in advanced hepatocellular carcinoma: a further step toward personalized therapy of liver cancer. Gastroenterology. 2009;136:1832-1835. [PubMed] |
| 20. | Zhao JD, Liu J, Ren ZG, Gu K, Zhou ZH, Li WT, Chen Z, Xu ZY, Liu LM, Jiang GL. Maintenance of Sorafenib following combined therapy of three-dimensional conformal radiation therapy/intensity-modulated radiation therapy and transcatheter arterial chemoembolization in patients with locally advanced hepatocellular carcinoma: a phase I/II study. Radiat Oncol. 2010;5:12. [PubMed] |