Published online Jan 14, 2012. doi: 10.3748/wjg.v18.i2.144
Revised: June 12, 2011
Accepted: June 19, 2011
Published online: January 14, 2012
AIM: To assess B1a cell expression in the rectal mucosa of ulcerative colitis (UC) patients in comparison with healthy controls.
METHODS: Rectal mucosa biopsies were collected from 15 UC patients and 17 healthy controls. CD5+ B cells were analysed by three colour flow cytometry from rectal mucosal samples after mechanical disaggregation by Medimachine®. Immunohistochemical analysis of B and T lymphocytes was also performed. Correlations between, on the one hand, rectal B1a cell concentrations and, on the other, erythrocyte sedimentation rate and C-reactive protein levels and clinical, endoscopic and histological disease activity indices were evaluated.
RESULTS: Rectal B-lymphocyte (CD19+/CD45+) rate and concentration were higher in UC patients compared with those in healthy controls (47.85% ± 3.12% vs 26.10% ± 3.40%, P = 0.001 and 501 ± 91 cells/mm2vs 117 ± 18 cells/mm2, P < 0.001); Rectal B1a cell density (CD5+CD19+) was higher in UC patients than in healthy controls (85 ± 15 cells/mm2vs 31 ± 6.7 cells/mm2, P = 0.009). Rectal B1a cell (CD5/CD19+) rate correlated inversely with endoscopic classification (Rs = -0.637, P < 0.05).
CONCLUSION: B1a lymphocytes seem to be involved in the pathogenesis of UC, however, the role they play in its early phases and in disease activity, have yet to be defined.
- Citation: Polese L, Boetto R, De Franchis G, Angriman I, Porzionato A, Norberto L, Sturniolo GC, Macchi V, De Caro R, Merigliano S. B1a lymphocytes in the rectal mucosa of ulcerative colitis patients. World J Gastroenterol 2012; 18(2): 144-149
- URL: https://www.wjgnet.com/1007-9327/full/v18/i2/144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i2.144
The aetiology of ulcerative colitis (UC) is still unknown, but several studies have demonstrated that there is an abnormal immunologic response to gut antigens[1,2]. One of the mechanisms which protects the body against intestinal luminal antigens before a specific aggressive inflammatory response is unleashed, is the production of natural antibodies, in particular immunoglobulin A (IgA)[3,4]. A large proportion of IgA is produced in a T-independent way by B cells and in particular, according to several studies, by the B1 sub-population. The majority of B1 cells, also called B1a, express CD5 on their surface. In a previous study[5] we reported that B1a cell (CD5+CD19+) blood concentrations were reduced in UC patients with respect to those in healthy controls, and that the B1a cell rate was inversely correlated with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels in UC patients. The aim of the present study was to analyse the rate and concentration of B1a cells in the rectal mucosa of UC patients, to compare these values with those found in healthy controls, and to assess any possible correlation with disease activity.
The study population consisted of 15 UC patients and 17 healthy controls. The study protocol was drafted in accordance with the Declaration of Helsinki and all of the patients and controls who participated signed informed consent statements. Patients taking immunosuppressive drugs or corticosteroids were excluded from the study.
The disease activity of the UC patients, undergoing ordinary follow-up colonoscopy, was classified in accordance with the Seo clinical score[6], the modified endoscopic Baron classification[7] and Geboes’ histological scoring[8]. Subjects who underwent colonoscopy as a cancer screening procedure and whose result was negative, were enrolled as healthy controls.
Blood samples and 6 rectal mucosal biopsy specimens, taken 10-15 cm from the anal verge during colonoscopy, were collected from each patient.
A cellular suspension, obtained by fragmentizing 4 rectal biopsies from each patient using a Medimachine (Consul TS, Rivalta di Torino, Italy), was used for flow cytometry. The method utilized was as follows: the collected rectal biopsies were placed in physiologic solution (Na 0.9%) and immediately processed. The biopsies were first washed twice with physiologic solution for 2 min each wash. Each biopsy was minced into < 1 mm3 pieces which were placed in a sterile microblade-equipped polyethylene chamber (Medicons, BD Biosciences, San Jose, CA, United States) with 1 mL phosphate buffered saline (PBS) 0.01 mol/L. The Medicons contain an immobile stainless steel screen with 100 hexagonal holes, each surrounded by six microblades. When the Medicons are inserted into the Medimachine the tissue comes into contact with the blades by means of a rotating element and is disaggregated. A micropump positioned under the screen forces the liquid to pass through the bore-holes, ensuring that the bore-holes remain clean. Medicons with 35 μm separator screens were used.
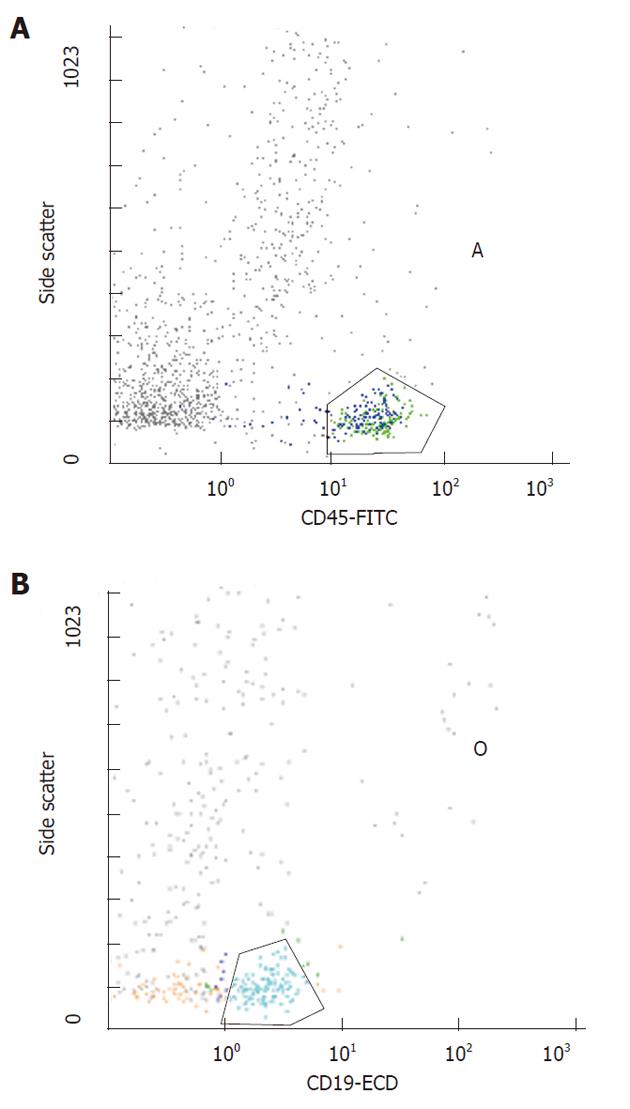
Fragments were dissociated 4 times for 20 s at a constant speed of 100 r/min. The suspension was filtered using filters with 30 μm diameter holes (Flicons, BD Bioscences, San Jose, CA, United States) and then analysed by flow-cytometry (Figures 1 and 2).
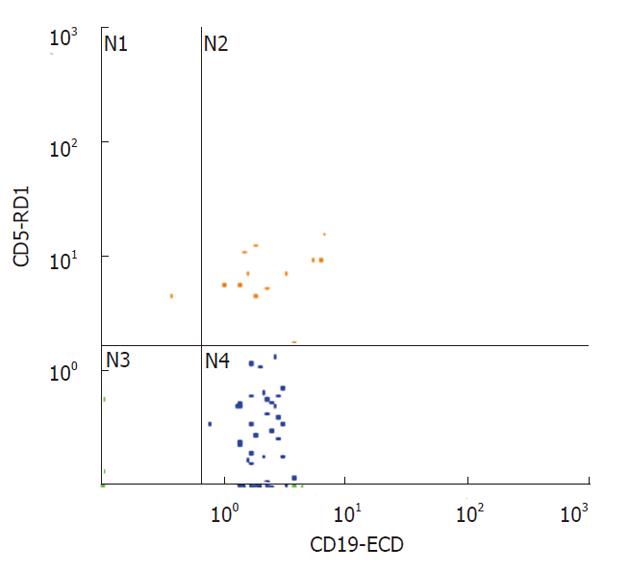
A three-colour flow-cytometric analysis was performed using the following associations: CD45 fluorescein isothiocyanate (FITC), Isotype IgG1 (clone J.33, mouse) (Beckman Coulter Inc, Fullerton, CA, United States), CD5 RD-1 (phycoerythrin), Isotype IgG2a (clone SFCI24T6G12, mouse) (Beckman Coulter Inc.), CD19 ECD (Texas red), Isotype IgG1 (clone J4.119, mouse) (Beckman Coulter Inc.). Flow cytometry was performed as previously described[5].
All mAbs were used at optimal saturating concentrations as recommended by the manufacturers. The cells were washed in a FACS buffer containing PBS/5% FCS/0.05% and sodium azide, then incubated with 10 mg of human IgG (Sigma Chemical Co., St. Louis, MO, United States) for 30 min at 4 °C-8 °C to block Fc receptors. Cells were washed to remove excess IgG and were triple-stained with either RD-1-conjugated mAb against CD5, or RD-1-control IgG mAb, ECD-conjugated mAb against CD19 or ECD-control IgG mAb and FITC-conjugated mAb against CD45 or FITC-control IgG mAb for 30 min at 4 °C-8 °C. Cells were washed twice, re-suspended in a FACS buffer and fixed with 1% paraformaldehyde. At that point, 2 mL of lysing solution (0.17 mol/L NH4Cl) were added and following 15 min of incubation, samples were analyzed by flow cytometry using a Coulter EPICS XL-MCL (Beckman Coulter Inc). Mononuclear cells were gated depending on their CD45 expression characteristics. Different subsets of cells (CD19) were then gated on the basis of fluorescence 1 and fluorescence 2 staining. An immunologic gate was performed on CD19+ cells to uncover CD5. Isotypic controls were used for all the samples. A two-colour flow cytometric analysis was similarly performed to study the T cells using anti-CD45 FITC mAb (Isotype IgG1, mouse, clone J.33, Beckman Coulter Inc.) and anti-CD3 Phycoerythrin-Cyanin 5 (Pc5) mAb (Isotype IgG1, mouse, clone UCHT 1, Beckman Coulter Inc.).
Two rectal biopsies from each patient studied underwent immunohistochemical analysis. Samples were fixed in 10% neutral buffered formalin, processed for embedding in paraffin wax, and cut into 5 μm-thick sections. Sections were dewaxed and rehydrated by routine protocols. For anti-CD3 immunohistochemistry, antigen unmasking was performed with 10 mmol/L sodium citrate buffer, pH 6.0, in a microwave oven at 96 °C for 30 min. Antigen unmasking was not necessary for anti-CD20 analysis. Sections were incubated in 0.3% hydrogen peroxide for 10 min at room temperature to remove endogenous peroxidase activity, and then in blocking serum for 30 min. Samples were incubated with primary antibodies at room temperature for 45 min. The primary antibodies used were: anti-CD3 (Polyclonal rabbit anti-Human, Dako, Milan, Italy) and anti-CD20 (Monoclonal mouse anti-human CD20cy, Dako) diluted 1:50 and 1:200, respectively. Sections were then washed three times in PBS for 5 min each wash, treated with secondary antibody (Envision System HRP, Dako) for 30 min at room temperature and developed in 3,3’-diaminobenzidine (DAB, Sigma-Aldrich, Milan, Italy). Finally, the sections were counterstained with hematoxylin.
Sections were examined using a Leica DM4500B microscope (Leica Microsystems, Wetzlar, Germany) connected to a Leica DFC320 high-resolution digital camera (Leica Microsystems) and a computer equipped with software for image acquisition and analysis (QWin, Leica Microsystems). The densities of CD3- and CD20-positive cells were evaluated at a magnification of 40 ×, and 10 fields per section were examined. The densities were calculated for each section by dividing the number of positive cells by the area of the rectal mucosa analysed.
The rectal mucosa B1a cell concentration (CD5+CD19+) was calculated by multiplying the CD5+/CD19+ ratio, obtained by flow-cytometry, by the B lymphocyte concentration, obtained by immunohistochemistry.
ESR and CRP were measured using the Westergren method and immuno-nephelometry, respectively.
Results are expressed as mean ± SE. Statistical analysis was performed using Mann-Whitney U test for the comparison between the UC patients and controls and by Spearman’s Rank test for correlations. Statistical significance was set at P < 0.05.
Adequate material for flow-cytometry was obtained from 13/15 UC patients (8 males and 5 females, median age 54 years, range 19-71 years) and from 13/17 controls (8 males and 5 females, median age 61 years, range 37-88 years). Of the 13 UC patients included in the study, 5 were taking mesalazine and 8 were not.
Ulcerative colitis was clinically active (Seo score > 150) in 5 patients and endoscopically active (Baron score > 1) in 4. The median histologic activity score was 3 (range 0-5).
The percentage of B lymphocytes (CD19+/CD45+) in the rectal mucosa was higher in UC patients with respect to healthy controls (47.8% ± 3.1% vs 26.1% ± 3.4%, P = 0.001); while the percentage of rectal T lymphocytes (CD3+/CD45+) was significantly lower in UC patients with respect to the controls (53.5% ± 4.2% vs 68.3% ± 3.5%, P = 0.02).
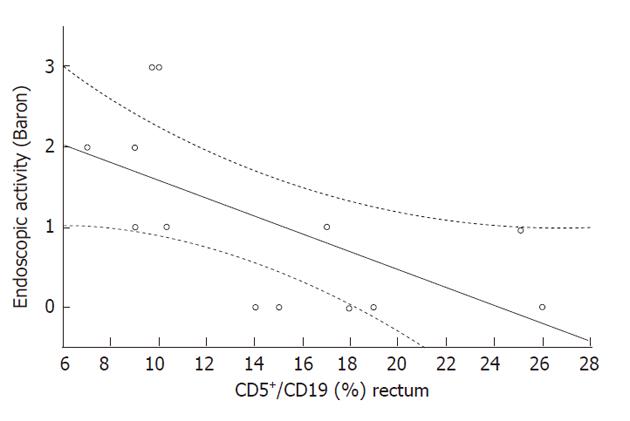
The rectal B1a cell rate (CD5+/CD19+) did not differ significantly in the two groups (Table 1), and was inversely correlated with endoscopic activity (Rs = -0.68, P = 0.01, Figure 3), but not with the clinical SEO disease activity index, ESR and CRP levels, or with age. The mean rectal B1a cell rate was higher, but not significantly different in patients with remission or mild histologic activity (score 0-1), with respect to patients with moderate-severe histologic activity (score 2-5) (22.0% ± 3.0% and 12.7% ± 2.5%, respectively, P = 0.1). The rectal B1a cell rate was not significantly different in the patient group taking mesalazine compared with those not taking mesalazine (11.0 ± 2.1 and 17.2 ± 3.0, respectively, P = 0.13).
Histological analysis confirmed that there was an increased concentration of B lymphocytes CD20+ in the rectal mucosa of ulcerative colitis patients with respect to that in controls (cell density 501 ± 91 cells/mm2vs 117 ± 18 cells/mm2, P < 0.001). T cell density was not significantly different in the UC patients and controls (485 ± 100 vs 445 ± 95, P = 0.6).
The calculated B1a cell density was significantly increased in UC patients with respect to that in controls: 85 ± 15 cells/mm2vs 31 ± 6.7 cells/mm2, P = 0.009.
More than 80% of the body’s activated B cells are located in the gut, where a continuous interaction takes place between the immune system and the trillion bacteria that reside there[9].
IgA generation by B cells is an important mechanism that regulates this homeostasis, contributing to immune protection but without provoking inflammation. A large proportion of the intestinal IgA against cell wall antigens and proteins of commensal bacteria is specifically induced in response to their presence within the microflora, but is independent of T cells or germinal centre formation. This T cell-independent IgA production is derived from B1 lymphocytes which develop in the peritoneal compartment and are distributed diffusely in the intestinal lamina propria[10]. In mice, peritoneal B cells (B1 cells) do not differentiate during migration through the lymphoid organs and finally home to the gut lamina propria where they switch and differentiate to IgA+ plasma cells[11]. The physiological importance of B1 cells in the maintenance of homeostasis at the mucosal surface has been clearly demonstrated[12].
B cells in inflammatory bowel disease have not been as extensively studied as T cells[13], and data on the role of B1 cells in UC are particularly scanty. Except for a smaller sub-group called B1b, B1 cells are distinguishable from B2 cells by expressing CD5 on their surface[14]. Even in the absence of external antigen stimulation, B1 cells produce natural antibodies (Ab) that provide early, broad protection against pathogens[4]. B1 cells are also known to produce auto-reactive Ab, including Ab to cell membrane components, such as phosphorylcholine[15] and phosphatidylcholine[16] to immunoglobulins (rheumatoid factor) and to single-stranded DNA[17].
B1 cells were thus analysed for their role in auto-immunity and high circulating B1a lymphocyte levels have been reported in some autoimmune diseases, such as systemic lupus erythematosus, primary Sjogren’s syndrome[18], rheumatoid arthritis[19], multiple sclerosis[20] and anti-phospholipid syndrome[21]. In the light of recent findings, these cells, and in particular the CD5 molecule on B cells, seem to play a role in preventing autoimmunity[22].
In a previous study we reported that B1a cell (CD5+/CD19+) concentrations are reduced in the blood of patients with UC even after restorative proctocolectomy[5]. Moreover, B1a cell rate was found to be inversely correlated with ESR and CRP levels in UC patients, indicating that these cells play a protective role against inflammation. Low CD5+/CD19+ blood percentages in UC and in Crohn’s disease (CD) have also been reported by other authors[23,24].
The present study focused on the presence of B lym-phocytes, and in particular the B1a sub-group, in the rectal mucosa of UC patients. An increased concentration of B lymphocytes was found in the rectal mucosa of these patients and their percentage within the leukocyte pool was also increased. B1a cell concentrations in the rectal mucosa of UC patients were significantly higher than those in healthy controls, but not their percentage within the whole B lymphocyte pool (CD5+/CD19+). Senju et al[25], who analysed subsets of lamina propria lymphocytes using two-colour flow cytometry, also found no differences in the percentage of CD5+ B lymphocytes in UC, CD patients and controls. They reported that the majority of B cells in the intestinal mucosa did not possess CD5 antigens on their cell surface. In the present study, performed using three colour flow cytometry, we found that the percentage of CD5+ B cells in the rectum was small, but not negligible (between 15% and 23%). The use of anti-CD45 helps to restrict the flow cytometric analysis to leukocytes, excluding from the study the other types of cells homing in the rectal mucosa.
Finding an increased concentration of B1a cells, but not a higher percentage within the whole B lymphocyte population, means that other B lymphocyte subsets were increased in the rectal mucosa of these patients. In addition, finding an inverse correlation between CD5+ B cells and endoscopic disease activity suggests that these cells are recruited to a relatively lower extent when the disease is more severe and possibly, at that point, a more specific immune reaction has already begun. As our findings seem to indicate that there is a loss of tolerance at this disease stage, further studies will clarify this point. The finding of an inverse correlation between rectal mucosa B1a cell rate and endoscopic disease activity is consistent with previous data concerning the inverse correlation of circulating B1a cell rate and blood ESR and CRP levels[5]. The former is a sign of local events, and the latter of a systemic imbalance.
Peterson et al[26] reported that the depletion of CD5+ B1 cells has different effects on the induction phase with respect to the effector phase of experimental autoimmune encephalomyelitis. During the induction phase it increases disease incidence, while in the effector phase it reduces disease severity. The role of CD5+ B cells in UC is not yet clear. It remains to be clarified if they play a protective role by producing polyspecific immunoglobulins, an unleashing role by producing autoreactive Ab, or if there is an even more complex modulating role that differs depending on the disease activity. The findings in this and other studies seem to indicate that their role in UC is not marginal, but more in-depth analyses are warranted to better define their function and to determine if and how their modulation has an impact on disease activity.
The authors are grateful to Linda Inverso Moretti for her assistance in editing the English version of this manuscript.
The aetiology of ulcerative colitis (UC) is still unknown, but several studies have demonstrated that there is an abnormal immunologic response to gut antigens.
B1 lymphocytes are important in maintaining mucosal surfaces in a state of homeostasis, and it has been found that B1 cell concentrations are reduced in the blood of patients with UC even after restorative proctocolectomy. In the present study, B1a cell expression was assessed in the rectal mucosa of UC patients and compared with that in healthy controls using three color flow-cytometry.
Rectal B1a cell density (CD5+CD19+) was increased in ulcerative colitis patients compared with healthy controls, but its rate correlated inversely with endoscopic disease score.
The findings in this and other studies seem to indicate that B1a lymphocytes play a role in the pathogenesis of ulcerative colitis and their modulation could have an impact on the control of disease activity.
B1 lymphocytes are distinguishable from B2 lymphocytes because they express CD5 on their surface. Even in the absence of external antigen stimulation, B1 cells produce natural antibodies that provide early, broad protection against pathogens. B1 cells are also known to produce auto-reactive antibodies. CD5 is a 67 kD trans-membrane glycoprotein that interacts in the B lymphocyte with the B cell receptor, negatively regulating growth signaling.
This study assessed B1a cell concentration in rectal mucosa of 13 UC patients as compared to controls. A possible correlation between these cells and either endoscopic (modified Baron classification) or clinical (Seo clinical score) activity index was also investigated. Data found that B1a cell concentration is increased in UC, but its distribution (i.e., percentage within leucocytes) did not differ between patients and controls. Moreover, a significant, inverse correlation between B1a cell concentration in rectal mucosa and endoscopic activity index was found.
Peer reviewers: Angelo Zullo, MD, Gastroenterologia ed Endoscopia Digestiva, Ospedale Nuovo Regina Margherita, Via E. Morosini, 30, 00153 Roma, Italia; Stephen M Kavic, MD, FACS, Assistant Professor of Surgery, Department of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Room S4B09, Baltimore, MD 21201, United States
S- Editor Tian L L- Editor Webster JR E- Editor Li JY
| 1. | Radford-Smith G. Ulcerative colitis: an immunological disease? Baillieres Clin Gastroenterol. 1997;11:35-52. [PubMed] |
| 2. | Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 303] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut microflora at bay. Science. 2004;303:1624-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 411] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Polese L, De Franchis G, Scarpa M, Sturniolo GC, Ruffolo C, Norberto L, Frego M, D'Amico DF, Angriman I. B1a lymphocytes in ulcerative colitis. Int J Colorectal Dis. 2007;22:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87:971-976. [PubMed] |
| 7. | Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964;1:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 458] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 689] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 9. | Velázquez P, Wei B, Braun J. Surveillance B lymphocytes and mucosal immunoregulation. Springer Semin Immunopathol. 2005;26:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 808] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 328] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2200] [Article Influence: 137.5] [Reference Citation Analysis (6)] |
| 14. | Sen G, Bikah G, Venkataraman C, Bondada S. Negative regulation of antigen receptor-mediated signaling by constitutive association of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur J Immunol. 1999;29:3319-3328. [PubMed] |
| 15. | Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 475] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 381] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Brennan F, Plater-Zyberk C, Maini RN, Feldmann M. Coordinate expansion of 'fetal type' lymphocytes (TCR gamma delta+T and CD5+B) in rheumatoid arthritis and primary Sjögren's syndrome. Clin Exp Immunol. 1989;77:175-178. [PubMed] |
| 19. | Ebo D, DeClerck LS, Bridts CH, Stevens WJ. Expression of CD5 and CD23 on B cells of patients with rheumatoid arthritis, systemic lupus erythematosus and Sjögren's syndrome. Relationship with disease activity and treatment. In Vivo. 1994;8:577-580. [PubMed] |
| 20. | Seidi OA, Semra YK, Sharief MK. Expression of CD5 on B lymphocytes correlates with disease activity in patients with multiple sclerosis. J Neuroimmunol. 2002;133:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Youinou P, Renaudineau Y. The antiphospholipid syndrome as a model for B cell-induced autoimmune diseases. Thromb Res. 2004;114:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Youinou P, Renaudineau Y. The paradox of CD5-expressing B cells in systemic lupus erythematosus. Autoimmun Rev. 2007;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Neil GA, Summers RW, Cheyne BA, Capenter C, Huang WL, Kansas GS, Waldschmidt TJ. CD5+ B cells are decreased in peripheral blood of patients with Crohn's disease. Dig Dis Sci. 1992;37:1390-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Mishima Y, Ishihara S, Amano Y, Oshima N, Kadota C, Otani A, Moriyama I, Li YY, Aziz MM, Kinoshita Y. Alterations of peripheral blood CD5+ B cells in inflammatory bowel disease. Scand J Gastroenterol. 2009;44:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Senju M, Wu KC, Mahida YR, Jewell DP. Two-color immunofluorescence and flow cytometric analysis of lamina propria lymphocyte subsets in ulcerative colitis and Crohn's disease. Dig Dis Sci. 1991;36:1453-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Peterson LK, Tsunoda I, Fujinami RS. Role of CD5+ B-1 cells in EAE pathogenesis. Autoimmunity. 2008;41:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |