Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2415
Revised: December 5, 2011
Accepted: March 10, 2012
Published online: May 21, 2012
AIM: To evaluate survival and recurrence after salvage liver transplantation (SLT) for the treatment of hepatocellular carcinoma (HCC) compared with primary liver transplantation (PLT) using a meta-analysis.
METHODS: Literature on SLT versus PLT for the treatment of HCC published between 1966 and July 2011 was retrieved. A meta-analysis was conducted to estimate pooled survival and disease-free rates. A fixed or random-effect model was established to collect the data.
RESULTS: The differences in overall survival and disease-free survival rates at 1-year, 3-year and 5-year survival rates were not statistically significant between SLT group and PLT group (P > 0.05). After stratifying the various studies by donor source and Milan criteria, we found that: (1) Living donor liver transplantation recipients had significantly higher 1-year survival rate, lower 3-year and 5-year survival rates compared with deceased-donor liver transplantation (DDLT) recipients. And in DDLT recipients they had better 1-year and 5-year disease-free survival rate in SLT group; and (2) No difference was seen in 1-year, 3-year and 5-year survival rates between two groups who beyond Milan criteria at the time of liver transplantation.
CONCLUSION: SLT can be effectively performed for patients with recurrence or deterioration of liver function after hepatectomy for HCC. It does not increase the perioperative mortality and has a similar long-term survival rates compared to PLT.
- Citation: Li HY, Wei YG, Yan LN, Li B. Salvage liver transplantation in the treatment of hepatocellular carcinoma: A Meta-analysis. World J Gastroenterol 2012; 18(19): 2415-2422
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2415
In a normal liver, liver resection for hepatocellular carcinoma (HCC) is the primary treatment of choice. But in cirrhotic livers, the presence of HCC and the limited liver capacity are the two intertwined issues rendering the HCC unresectable. Primary liver transplantation (PLT) is the most effective treatment for such HCC patients, especially for those who meet Milan criteria (solitary liver nodule not exceeding 5 cm in maximum diameter, or 2 or 3 tumors not exceeding 3 cm in diameter)[1]. It has been manifested to provide a considerable disease-free survival and to be the first choice for these patients. Due to shortage of available donors, long waiting times may harm the benefit that might be acquired from PLT. Salvage liver transplantation (SLT) has been proposed and performed for those who undergo primary liver resection for HCC or HCC recurrence or deterioration of liver function[2]. SLT proposes liver resection as a bridge to prevent tumor progression in the waiting list. Although SLT might be an alternate choice for HCC patients as the preferred treatment, long-term results are difficult to ascertain. Moreover, few data are available on the overall and disease-free survival of patients. A few researches concern on the comparison between the result of SLT and PLT. In order to reduce research bias and difference, we did a meta-analysis to compare survival and recurrences for SLT strategy versus PLT in the treatment of HCC patients, in order to provide a reference for clinical practice.
Search was applied to the following electronic databases: PubMed (1966 to July 2011), Embase (January 1996 to July 2011), CNKI (January 1996 to July 2011) and Cochrane database. The following key words were used: “liver resection” or “hepatectomy”; “liver transplantation” or “transplantation” or “salvage liver transplantation” or “salvage transplantation”; “hepatocellular carcinoma” or “HCC”. The search was limited to the English language and humans. The relevant reference lists of reviews were also searched at the same time. Abstracts or unpublished studies were not considered. If more than 1 study was published by the same author using the same case series, only the most detailed study was included. And if necessary, authors were contacted to obtain more data on their study.
SLT was defined as a liver transplantation performed for recurrent HCC or deterioration of liver function after primary liver resection.
Inclusion criteria were as follows: (1) having definition of SLT; (2) follow-up 12 mo at least; (3) case-control or cohort design; and (4) sufficient data were obtained to calculate odds ratio (OR) with confidence interval (CI). Reasons for exclusion were: (1) no-control; (2) duplicate; and (3) no useable data reported. We also excluded articles published before 1996 because there was no definition for “SLT”.
The scoring system was adapted from Stahl, the Cochrane Collaboration and others[3-5]. This system suits not only randomized control trial (RCT) but controlled trial or other studies well. Questions were placed on a 3 point scale: unclear/inadequate (0), adequate (1), good (2). Articles were considered for inclusion if their summary score exceeded 30.
All data were extracted independently by 2 reviewers according to the selection criteria. We resolved disagreement through discussion. The following data were extracted: the last name of the first author, study design, publication year, definition of SLT, the type of population described [adults or children (< 18 years)], country of transplant center, number of SLT cases and control (PLT) studies, overall survival, overall recurrence and assessment of risk factors.
Meta-analysis was performed using fixed-effect or random-effect methods, depending on the absence or presence of significant heterogeneity. Statistical heterogeneity between trials was evaluated by the Cochran χ2 test and was considered significant when P < 0.10. In the absence of statistically significant heterogeneity, the Mantel-Haenszel method in the fixed-effect model was used for the meta-analysis. Otherwise, the DerSimonian and Laird method in the random-effect model was selected.
The OR with 95% CI was used to assess treatment efficacy. The combined result was an average OR and 95% CI weighted according to the standard error of the OR of the trial. P < 0.05 was considered statistically significant. We used funnel plots to assess the publication bias, and tested for funnel plot asymmetry using Egger’s test and Begg’s test. All analyses were performed with Review Manager version 5.0.23 (RevMan, Cochrane Collaboration, Oxford, England).
There were 410 papers relevant to the search words. Via steps of screening the title, abstract reviewing and article reviewing, 11 studies which included 141 SLT cases and 872 PLT cases were identified to match our inclusion criteria[6-16]. Studies had been carried out in France, Italy, USA, China, Spain, Korea and Chinese Taiwan. Details of studies and the methodological quality of the studies assessed according to a score system described above are described in Table 1.
| Author | Year | Country | Study design | SLT (cases) | PLT (cases) | Score |
| Adam et al[6] | 2003 | France | Case-control | 17 | 200 | 32 |
| Belghiti et al[7] | 2003 | France | Case-control | 18 | 70 | 33 |
| Concejero et al[8] | 2008 | China | Case-control | 7 | 28 | 31 |
| Del Gaudio et al[9] | 2008 | Italy | Case-control | 16 | 147 | 32 |
| Facciuto et al[10] | 2008 | USA | Case-control | 5 | 32 | 30 |
| Hwang et al[11] | 2007 | Korea | Case-control | 17 | 200 | 31 |
| Kim et al[12] | 2008 | Korea | Case-control | 15 | 31 | 30 |
| Margarit et al[13] | 2005 | Spain | Case-control | 5 | 36 | 31 |
| Sapisochin et al[14] | 2010 | Spain | Case-control | 17 | 34 | 33 |
| Shao et al[15] | 2008 | China | Case-control | 15 | 62 | 30 |
| Vennarecci et al[16] | 2007 | Italy | Case-control | 9 | 37 | 30 |
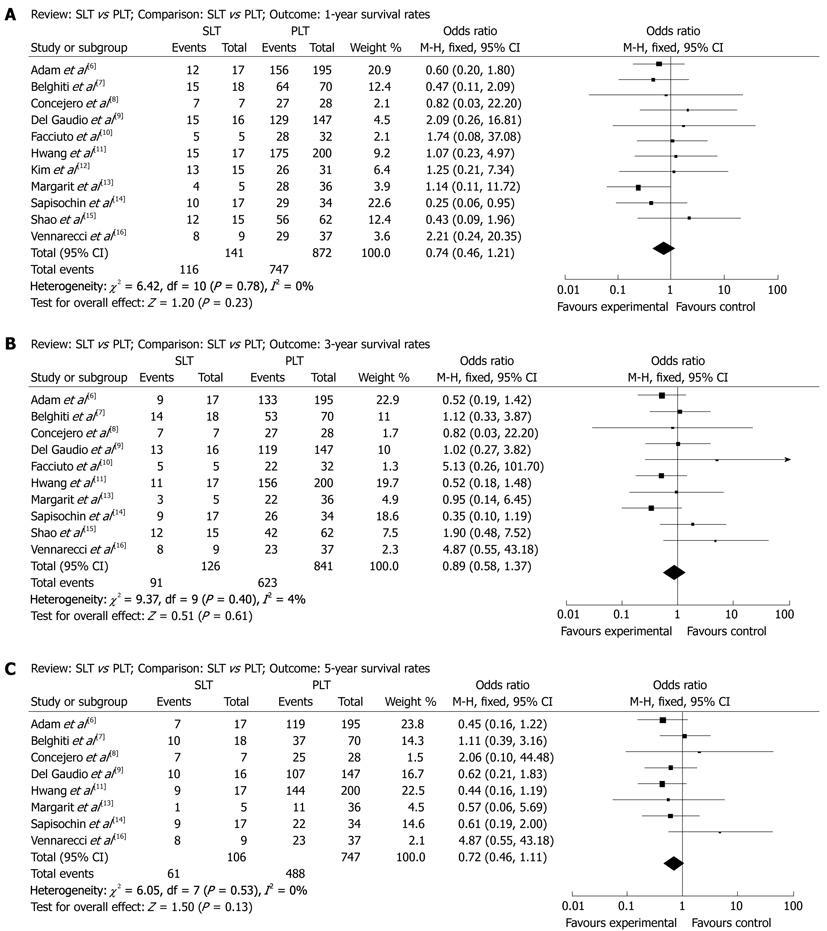
1-year survival after SLT and PLT: A total of 1013 patients were included in 11 articles. According to χ2 test of heterogeneity (P = 0.78), a fixed-effect model was used. No difference between SLT group (82.3%) and PLT group (85.7%) were seen in the 1-year survival rate (OR: 0.74, 95% CI: 0.46-1.21, P = 0.23, Figure 1A).
3-year survival after SLT and PLT: A total of 967 patients were included in 10 articles. According to χ2 test of heterogeneity (P = 0.40), a fixed-effect model was used. No difference between SLT group (72.2%) and PLT group (74.1%) were seen in the 3-year survival rate (OR: 0.89, 95% CI: 0.58-1.37, P = 0.61, Figure 1B).
5-year survival after SLT and PLT: A total of 853 patients were included in 8 articles. According to χ2 test of heterogeneity (P = 0.53), a fixed-effect model was used. No difference between SLT group (57.5%) and PLT group (65.3%) were seen in the 5-year survival rate (OR: 0.72, 95% CI: 0.46-1.11, P = 0.13, Figure 1C).
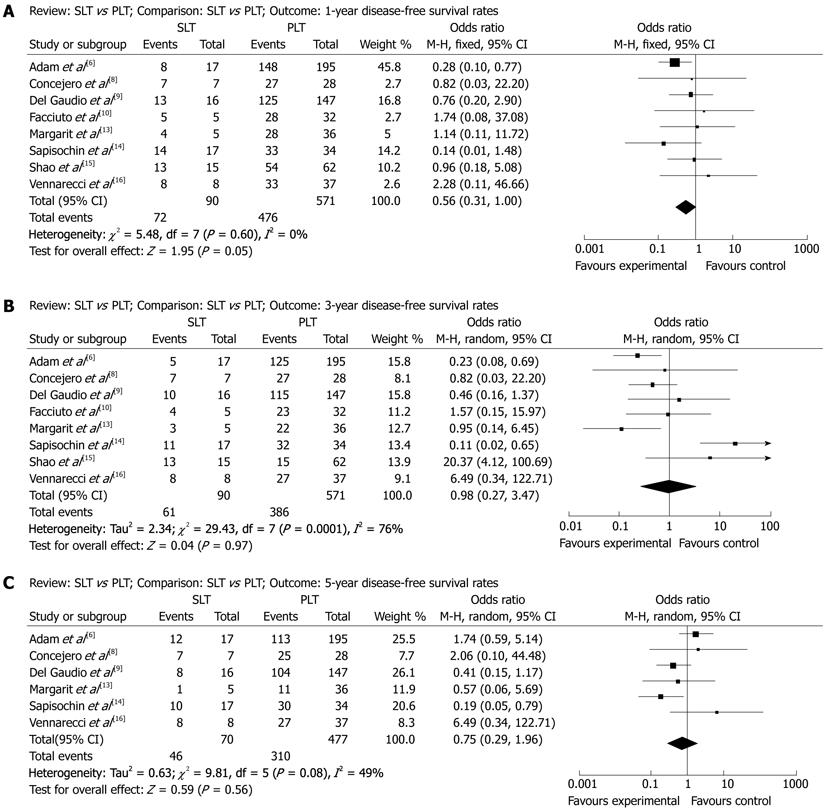
1-year disease-free survival after SLT and PLT: A total of 620 patients were included in 8 articles. According to χ2 test of heterogeneity (P = 0.60), a fixed-effect model was used. No difference between SLT group (80.0%) and PLT group (83.4%) were seen in the 1-year disease-free survival rate (OR: 0.56, 95% CI: 0.31-1.00, P = 0.05, Figure 2A).
3-year disease-free survival after SLT and PLT: A total of 620 patients were included in 8 articles. According to χ2 test of heterogeneity (P = 0.0001), a random-effect model was used. No difference between SLT group (67.8%) and PLT group (67.6%) were seen in the 3-year disease-free survival rate (OR: 0.98, 95% CI: 0.27-3.47, P = 0.97, Figure 2B).
5-year disease-free survival after SLT and PLT: A total of 506 patients were included in 6 articles. According to χ2 test of heterogeneity (P = 0.08), a random-effect model was used. No difference between SLT group (65.7%) and PLT group (65.0%) were seen in the 5-year disease-free survival rate (OR: 0.75, 95% CI: 0.29-1.96, P = 0.56, Figure 2C).
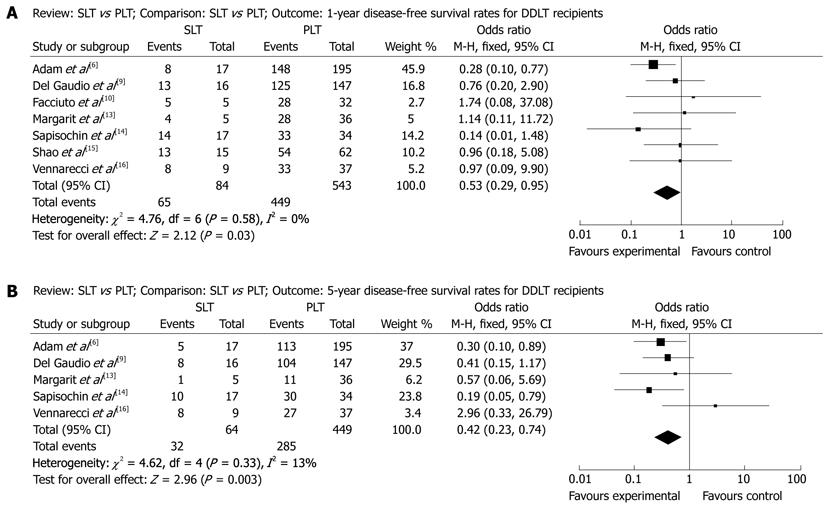
When stratifying for the donor source, compared with deceased-donor liver transplantation (DDLT) recipients, we found that living-donor liver transplantation (LDLT) recipients had significantly higher 1-year survival rate (OR: 1.02, 95% CI: 0.26-4.10, P = 0.97), lower 3-year survival rate (OR: 0.54, 95% CI: 0.20-1.47, P = 0.23) and lower 5-year survival rate (OR: 0.54, 95% CI: 0.21-1.35, P = 0.19). DDLT recipients had significantly lower 1-year survival rate (OR: 0.66, 95% CI: 0.38-1.15, P = 0.14), higher 3-year survival rate (OR: 0.99, 95% CI: 0.62-1.59, P = 0.97) and higher 5-year survival rate (OR: 0.77, 95% CI: 0.47-1.26, P = 0.30). No useable data about disease-free survival rates can be extracted from LDLT researches. And in DDLT recipients they had better 1-year disease-free survival rate (OR: 0.53, 95% CI: 0.29-0.95, P = 0.03, Figure 3A) and better 5-year disease-free survival rate (OR: 0.42, 95% CI: 0.23-0.74, P = 0.003, Figure 3B) in SLT group. No difference between SLT group and PLT group were seen in the 3-year disease-free survival rate (OR: 0.95, 95% CI: 0.26-3.52, P = 0.94).
When stratifying for Milan criteria, we found that no difference was seen in 1-year survival rates (OR: 0.26, 95% CI: 0.01-4.94, P = 0.37), 3-year survival rates (OR: 0.41, 95% CI: 0.01-24.54, P = 0.67) and 5-year survival rates (OR: 0.55, 95% CI: 0.07-4.48, P = 0.57) between SLT group and PLT group who beyond Milan criteria at the time of liver transplantation (LT). No usable data for patients who met Milan criteria at the time of LT.
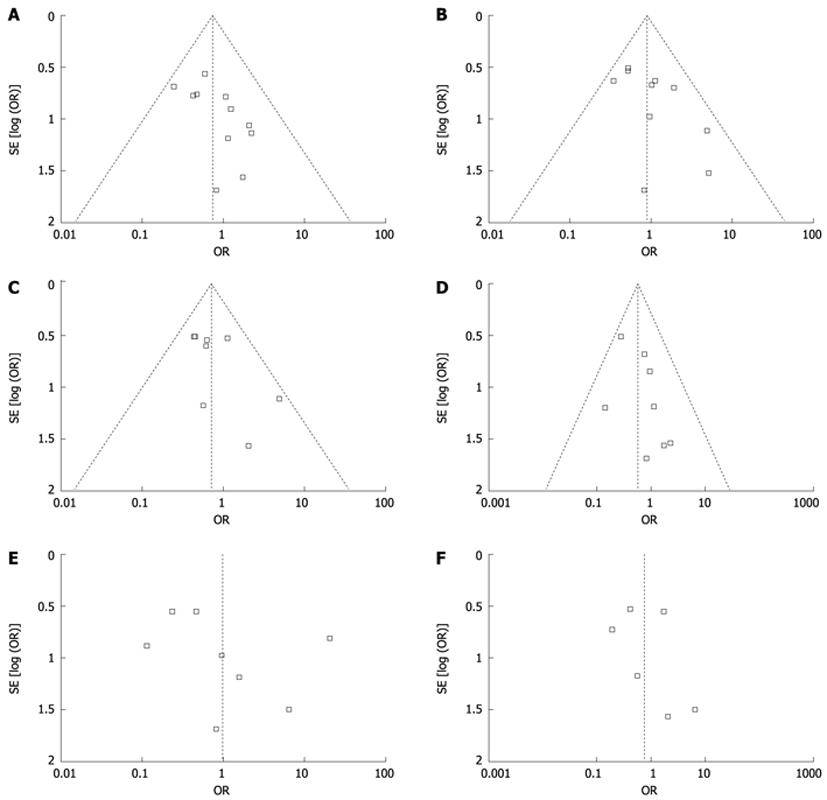
Publication bias may exist when no significant findings remain unpublished, thus artificially inflating the apparent magnitude of an effect. Funnel plots of our study results are shown in Figure 4. The funnel plots on survival and disease-free survival following SLT or PLT for the treatment of HCC showed basic symmetry, which suggested no publication bias.
As one of the radical treatments for HCC, LT is nowadays limited by organ shortage. Due to the prolonged waiting times before transplantation, tumor progression and deterioration of liver function may counteract its benefit[2]. The outcome of liver resection is mainly influenced by a high rate of recurrence that limits long-term survival rates. But previous research noted that most of patients with recurrence after primary liver resection were still eligible for LT[2]. Hence, hepatectomy and LT should be considered as complementary, not competitive, treatments for HCC in cirrhotic patients with well-preserved liver function. Resection of the liver tumor is an optional bridge treatment[17-19]. SLT was proposed in order to reduce the impact of a long waiting times, donor shortage and tumor recurrence after resection in HCC patients.
The increased technical difficulty during SLT and the risk for impaired posttransplant survival worried most of surgeons. Heavy adhesions and portal hypertension are often encountered after prior liver resection. Inattentive dissection of perihepatic adhesions could result in uncontrollable bleeding at the dissection surface. Also due to heavy adhesions, the relationship between hepatic vein and inferior vena cava are hard to identify. Hwang et al[11] found that SLT did not increase the operative risks or postoperative complications. The two major technical concerns-bleeding and reconstruction of the hepatic vein outflow can be solved successfully by steady and meticulous sharp dissection and sufficient dissection of the recipient inferior vena cava. Kim et al[12] showed that end-to-end anastomosis for bile ducts and hepatic artery was feasible, too. Our study showed that SLT had no bad effect on overall survival and disease-free survival in comparison with PLT.
Considering different surgical methods may have an effect on survival rates, the whole patients were stratified to LDLT recipients and DDLT recipients. In each subgroup, no difference between SLT group and PLT group were seen in 1-year survival rates, 3-year survival rates and 5-year survival rates. But LDLT recipients may have a significant higher 1-year survival rates than DDLT recipients, while a significant lower 3-year survival rates and 5-year survival rates. This may be a result of improvement of surgical technique and perioperative management. However, because of a relatively higher incidence (up to 30%) of biliary complication after LDLT[20-23], LDLT recipients may have a lower long-term survival rates than DDLT recipients. Different results in DDLT recipients’ disease-free survival rates seem hard to explain. We consider a better tumor stage at the time of transplantation (meet Milan criteria) may contribute to better 1-year disease-free survival rates and 5-year disease-free survival rates. A lager sample and more randomized controlled studies may resolve this conflict and draw a right conclusion.
The tumors’ stage at the time of resection and LT is another risk factor for postoperative overall survival and disease-free survival. Some studies were theoretical and assessed the salvage transplantability according to the pattern of recurrence after resection for HCC within Milan criteria and found that 76% to 87% of recurrences were considered eligible for SLT on imaging grounds[2,12,24,25]. For HCC patients not meeting Milan criteria, SLT could be applied for those cases with less aggressiveness, namely tumor size less than 6 cm and pathological well differentiation. For those cases meeting Milan criteria, PLT seems to be the first option. SLT could be performed for those patients with recurrence within Milan criteria after primary resection and without delay before recurrence with advanced disease manifestations. But there is no consensus about the survival rates for patients with recurrence beyond Milan criteria. Our result reveals that SLT group has similar survival rates compared with PLT group beyond Milan criteria at the time of LT. Unfortunately, data extracted from our including studies are not enough to do further meta-analysis on patients’ survival rates meeting Milan criteria at the time of LT and the corresponding disease-free survival rates.
Moreover, in countries with a higher incidence of HCC, a higher proportion of HCC patients on the waiting list and/or a longer median time-to-transplant, SLT could offer a gain in life-expectancy to the remaining waiting-list patients[26].
This review has some limitations. Although funnel plots may be suggestive of publication bias with lack of negative small RCTs, a firm conclusion about bias is difficult to make as the asymmetry of the funnel plots is minimal. And funnel plots can show asymmetry for other reasons. Therefore, our pooled OR might be an overestimate of the true effect. Due to data constraints, this meta-analysis could not analyze the quality of life score and was unable to carry out stratified analyses of other possible confounding factors. The method need to be more effective. Larger samples and randomized controlled studies with longer follow-up are required. Our conclusions also need more detailed data to confirm the results. The search language was limited. The integrity of the data was affected to a certain extent.
In conclusion, this new strategy SLT can be effectively performed for patients with recurrence or deterioration of liver function after hepatectomy for HCC. It does not increase the perioperative mortality and has a similar long-term survival rates compared to PLT. When surgical technique is no longer a problem for SLT, more patients will benefit from it.
Due to shortage of available donors, salvage liver transplantation (SLT) has been proposed and performed for the patients who undergo primary liver resection for hepatocellular carcinoma (HCC) or HCC recurrence or deterioration of liver function. This meta-analysis was designed to evaluated survival and recurrence after SLT for the treatment of HCC compared with primary liver transplantation (PLT).
The study evaluated survival and recurrence after SLT for the treatment of HCC compared with PLT using a meta-analysis of all relevant controlled studies.
This is the first systematic review and meta-analysis on the survival and recurrence after SLT for the treatment of HCC compared with PLT. The author made a comprehensive search of studies. Several important conclusions might be used for future selection in SLT or PLT for HCC patients’ treatments.
This meta-analysis shows that SLT has a similar survival rates in comparison with PLT. SLT offers an alternative treatment method for HCC patients in facing a shortage of available donors.
SLT was defined as a liver transplantation performed for recurrent HCC or deterioration of liver function after primary liver resection.
The article should be published as it is a nice overview on the topic after some revisions are performed.
Peer reviewers: Justin H Nguyen, Associate Professor of Surgery, Chair, Division of Transplant Surgery, Department of Transplantation, Vice-chair for Research, Department of Surgery, Mayo Clinic College of Medicine, Mayo Clinic in Florida, 4500 San Pablo Rd, Jackonville, FL 32224, United States; Thilo Hackert, MD, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany
S- Editor Shi ZF L- Editor A E- Editor Zhang DN
| 1. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 2. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [PubMed] |
| 3. | Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31-49. [PubMed] |
| 4. | Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, Lansing K, Bozorgzadeh A. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451-457. [PubMed] |
| 5. | Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. [PubMed] |
| 6. | Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508-518; discussion 518-519. [PubMed] |
| 7. | Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885-892; discussion 892-893. [PubMed] |
| 8. | Concejero A, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation. 2008;85:398-406. [PubMed] |
| 9. | Del Gaudio M, Ercolani G, Ravaioli M, Cescon M, Lauro A, Vivarelli M, Zanello M, Cucchetti A, Vetrone G, Tuci F. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177-1185. [PubMed] |
| 10. | Facciuto ME, Koneru B, Rocca JP, Wolf DC, Kim-Schluger L, Visintainer P, Klein KM, Chun H, Marvin M, Rozenblit G. Surgical treatment of hepatocellular carcinoma beyond Milan criteria. Results of liver resection, salvage transplantation, and primary liver transplantation. Ann Surg Oncol. 2008;15:1383-1391. [PubMed] |
| 11. | Hwang S, Lee SG, Moon DB, Ahn CS, Kim KH, Lee YJ, Ha TY, Song GW. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl. 2007;13:741-746. [PubMed] |
| 12. | Kim BW, Park YK, Kim YB, Wang HJ, Kim MW. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: feasibility of the Milan criteria and operative risk. Transplant Proc. 2008;40:3558-3561. [PubMed] |
| 13. | Margarit C, Escartín A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242-1251. [PubMed] |
| 14. | Sapisochin G, Bilbao I, Balsells J, Dopazo C, Caralt M, Lázaro JL, Castells L, Allende H, Charco R. Optimization of liver transplantation as a treatment of intrahepatic hepatocellular carcinoma recurrence after partial liver resection: experience of a single European series. World J Surg. 2010;34:2146-2154. [PubMed] |
| 15. | Shao Z, Lopez R, Shen B, Yang GS. Orthotopic liver transplantation as a rescue operation for recurrent hepatocellular carcinoma after partial hepatectomy. World J Gastroenterol. 2008;14:4370-4376. [PubMed] |
| 16. | Vennarecci G, Ettorre GM, Antonini M, Santoro R, Maritti M, Tacconi G, Spoletini D, Tessitore L, Perracchio L, Visco G. First-line liver resection and salvage liver transplantation are increasing therapeutic strategies for patients with hepatocellular carcinoma and child a cirrhosis. Transplant Proc. 2007;39:1857-1860. [PubMed] |
| 17. | Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424-432. [PubMed] |
| 18. | Pichlmayr R, Weimann A, Oldhafer KJ, Schlitt HJ, Tusch G, Raab R. Appraisal of transplantation for malignant tumours of the liver with special reference to early stage hepatocellular carcinoma. Eur J Surg Oncol. 1998;24:60-67. [PubMed] |
| 19. | Yamamoto J, Iwatsuki S, Kosuge T, Dvorchik I, Shimada K, Marsh JW, Yamasaki S, Starzl TE. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151-1158. [PubMed] |
| 20. | Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, Yonemura Y, Ikeda T, Shimada M, Maehara Y. Biliary strictures in living donor liver transplantation: incidence, management, and technical evolution. Liver Transpl. 2006;12:979-986. [PubMed] |
| 21. | Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, Yamamoto N, Sasahira N, Yamashiki N, Tada M. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006;101:2230-2236. [PubMed] |
| 22. | Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, Schwartz ME, Miller C. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842-1848. [PubMed] |
| 23. | Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726-732. [PubMed] |
| 24. | Tanaka S, Noguchi N, Ochiai T, Kudo A, Nakamura N, Ito K, Kawamura T, Teramoto K, Arii S. Outcomes and recurrence of initially resectable hepatocellular carcinoma meeting milan criteria: Rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204:1-6. [PubMed] |
| 25. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [PubMed] |
| 26. | Cucchetti A, Vitale A, Gaudio MD, Ravaioli M, Ercolani G, Cescon M, Zanello M, Morelli MC, Cillo U, Grazi GL. Harm and benefits of primary liver resection and salvage transplantation for hepatocellular carcinoma. Am J Transplant. 2010;10:619-627. [PubMed] |