Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2408
Revised: March 7, 2012
Accepted: March 29, 2012
Published online: May 21, 2012
AIM: To investigate the diagnostic value of glypican-3 (GPC3) and its relationship with hepatocellular carcinoma (HCC) recurrence after liver transplantation.
METHODS: HCC tissue samples (n = 31) obtained from patients who had undergone liver transplantation were analyzed. GPC3 mRNA and protein expression were analyzed by TaqMan real-time reverse transcription-polymerase chain reaction and immunohistochemistry. Correlation between the GPC3 expression and clinicopathological features was analyzed. The potential prognostic value of GPC3 was investigated by comparing recurrence-free survival between HCC patients with and without GPC3 expression.
RESULTS: Using a cutoff value of 3.5 × 10-2, 20 of 31 cancerous tissues had expression values of > 3.5 × 10-2, whereas 3 of 31 adjacent non-neoplastic parenchyma and 0 of 20 control liver tissues had expression values of > 3.5 × 10-2 (P < 0.001). GPC3 protein was immunoexpressed in 68% of cancerous tissues, but not in adjacent non-neoplastic parenchyma and control liver tissues. Vascular invasion was significantly related to GPC3 expression (P < 0.05). Recurrence-free survival was significantly longer for patients without GPC3 mRNA overexpression (> 3.5 × 10-2) and those without vascular invasion (P < 0.05 for both).
CONCLUSION: GPC3 expression may serve as a valuable diagnostic marker for HCC. GPC3 mRNA overexpression may be an adverse indicator for HCC patients after liver transplantation.
- Citation: Wang YL, Zhu ZJ, Teng DH, Yao Z, Gao W, Shen ZY. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol 2012; 18(19): 2408-2414
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2408
Hepatocellular carcinoma (HCC) is one of the most common tumors in the world. More than 600 000 deaths globally per year have been reported, with 82% of cases (and deaths) occurring in “developing” countries (with 55% in China)[1,2]. HCC is up to four-times more common in men than in women, and 60%-90% of these tumors develop in a cirrhotic liver[3]. About 200 000 patients die from HCC every year in China.
Liver transplantation offers the potential to resect the entire tumor-bearing liver and eliminate the cirrhosis. Liver transplantation therefore holds great theoretical appeal in treating this disease. Furthermore, the liver is entirely removed at liver transplantation, so recurrence must derive from extrahepatic dissemination that occurred before or during transplantation, reflecting highly aggressive tumor biology. Given the current shortage of organs and the risk of aggressive recurrence, selection of candidates for liver transplantation is crucial and controversial[4-6]. Therefore, predicting outcomes for patients is a challenge for clinicians and researchers.
Glypican-3 (GPC3) is a heparan sulfate proteoglycan and locates on the cell surface by a mechanism involving the glycosylphosphatidylinositol anchor[7]. GPC3 has been discovered to be a potential serologic and immunohistochemical diagnostic marker for HCC in general[8-10] and to promote the growth of HCC cells through stimulation of the canonical Wnt signaling pathway[11]. Zhu et al[12] and Sung et al[13] reported that GPC3 mRNA was significantly elevated in most HCC compared with normal liver as well as in livers with focal nodular hyperplasia and liver cirrhosis. This result was later confirmed by Libbrecht et al[14] using reverse transcription-polymerase chain reaction (RT-PCR), who mainly studied small HCC. Despite this clinical interest in GPC3, until now, the relationship between GPC3 expression and post-transplant HCC recurrence has not been clarified.
In our previous study, GPC3 mRNA-expressing cells in peripheral blood had no clinical value in the diagnosis of HCC[15]. The present study is an extension of the study mentioned above. We designed the study to assess the diagnostic value of GPC3 mRNA and protein expression in HCC tissues and to investigate their relationship with HCC recurrence after orthotopic liver transplantation (OLT).
HCC tissue samples (n = 31) were obtained from patients who underwent OLT at the Department of Transplantation Surgery, Tianjin First Central Hospital (Tianjin, China) during 2008. Histological types were assigned according to the classification set by the World Health Organization. The clinicopathological features of OLT patients with HCC are summarized in Table 1. All recipients had undergone successful OLT (livers were from cadaveric donors who had agreed to donate their organs in the event of death). Liver-tissue specimens were obtained intraoperatively from cancerous tissues and adjacent non-neoplastic parenchyma. The controls were normal liver tissues from 20 patients (15 men, 5 women; mean age, 48 years; age range 30-65 years) with hepatic hemangioma. There were no statistically significant differences in age and sex between the study groups (P > 0.05). Liver-tissue specimens were fixed in 10 % formalin and embedded in paraffin for routine histological examination. The remaining tissue was immediately snap-frozen in liquid nitrogen and stored at -80 °C until examination.
| Gender (Male/female) | 29/2 |
| Age (mean/range) (yr) | 49/37-62 |
| Primary liver disease (HBV cirrhosis/HCV cirrhosis) | 30/1 |
| TNM stage (I-II/III-IVa) | 12/19 |
| Milan criteria (within/beyond) | 15/16 |
| Tumor diameter (< 5 cm/≥ 5 cm) | 16/15 |
| Tumor number (< 3/≥ 3) | 26/5 |
| Vascular invasion (present/absent) | 16/15 |
| Serum AFP ( ≤ 20 μg/L/> 20 μg/L) | 11/20 |
| Histological differentiation (good/moderate and poor) | 6/25 |
All patients received standard post-liver transplantation care in the Intensive Care Unit. They received the same immunosuppressive therapy: tacrolimus, daclizumab, mycophenolate mofetil, and methylprednisolone. The diagnosis of HCC recurrences (follow-up of 24 mo) was based on elevation of serum levels of alpha-fetoprotein (AFP) and ultrasonography, hepatic arteriography, or computed tomography. Fine-needle aspiration cytology was used for confirmation of recurrence if necessary.
The present study was approved by our institutional ethics committee. Informed consent was obtained from each patient. The procedure met all applicable guidelines of our institute as well as governmental regulations concerning the ethical use of donated organs.
Total RNA was extracted from frozen liver tissues using Trizol reagents according to manufacturer instructions (Invitrogen, Carlsbad, CA, United States). Reverse transcription to complementary DNA (cDNA) was undertaken using random primer and Superscript RNase H-reverse transcriptase (Invitrogen).
A total of 1 μL of cDNA was mixed with the TaqMan probe[15], RNase-free water, and TaqMan Universal PCR Master Mix. Real-time PCR amplification and data analyses were carried out using an ABI PRISM 7300 Sequence Detector System (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s protocol. β-actin was used as an internal control. Each sample was assayed in duplicate in a MicroAmp optical 96-well plate. The thermo-cycling condition was 2 min at 50 °C and 15 min incubation at 95 °C, followed by 40 two-temperature cycles of 20 s at 95 °C and 1 min at 60 °C. Data were analyzed with Sequence Detection Software. Mean and standard-deviation values were calculated from the data obtained. The level of expression was calculated using the formula: Relative expression = (copy number of target molecule/copy number of β-actin)[16].
Sections of formalin-fixed, paraffin-embedded tissue cell blocks (4 μm) were tested for the presence of monoclonal antibody directed against GPC3 (Santa Cruz Biotechnology, Santa Cruz, CA, United States). The method used for the immunohistochemical analysis has been described previously[17].
For the immunohistochemical analysis of GPC3, we evaluated the area of GPC3-positive staining in one slide for each patient. At first, to analyze GPC3 expression, the results of immunohistochemical staining were classified according to the area of GPC3-positive stained cells as follows: -, Negative (< 10%); ±, Weakly positive (10%-30%); and +, Positive (> 30%). Finally, we classified two groups between GPC3-negative (< 10%) and GPC3-positive (> 10%). The expression of GPC3 was judged to be positive if the percentage of immunoreactive cells was assessed semi-quantitatively as being ≥ 10% in focal lesions.
Data are mean ± SD. For comparisons of continuous variables, one-way analysis of variance with the Bonferroni post-hoc test was used to compare differences among the three groups. We generated receiver operating characteristic curves for GPC3 mRNA levels to determine the cutoff points that yielded the sensitivity and specificity for predicting the diagnosis of HCC. Correlations between GPC3 results and various clinicopathological parameters in the tissue samples were evaluated using the chi-square test. The distribution of time to recurrence was estimated according to the Kaplan-Meier method, and differences assessed using log-rank statistics. P < 0.05 was considered significant. Statistical analyses were done using SPSS ver12 (SPSS, Chicago, IL, United States).
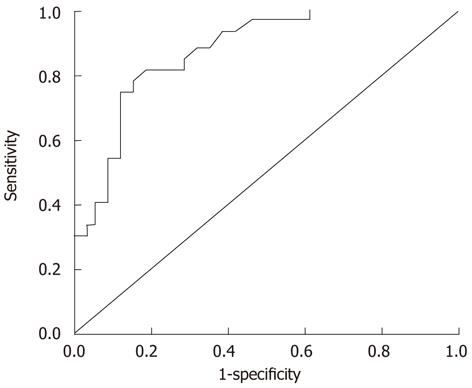
GPC3 mRNA expression in adjacent non-neoplastic parenchyma (n = 31, 2.00 ± 1.91 × 10-2) and in control liver tissues (n = 20, 1.41 ± 1.57 × 10-2) was much lower than in cancerous tissues (n = 31, 5.64 ± 3.06 × 10-2) (P < 0.001). There were no significant differences between adjacent non-neoplastic parenchyma and normal liver tissues with respect to GPC3 mRNA expression (P = 0.270). A cutoff value for GPC3 mRNA expression of 3.5 × 10-2 was diagnostic of HCC with a sensitivity of 68%, specificity of 94%, positive predictive value (PPV) of 87%, and negative predictive value (NPV) of 83% [area under the curve, 0.878; 95% confidence interval (CI): 0.802-0.953, P < 0.001] (Figure 1). Twenty of 31 (64%) cancerous tissues had expression values of > 3.5 × 10-2, whereas 3 of 31 (10%) adjacent non-neoplastic parenchyma and 0 of 20 control liver tissues had expression values of > 3.5 × 10-2 (P < 0.001).
The expression of GPC3 mRNA was evaluated in relation to clinicopathological features. GPC3 mRNA overexpression (> 3.5 × 10-2) was closely associated with vascular invasion (P = 0.006). No significant correlation was found between GPC3 mRNA overexpression and tumor, node, metastasis (TNM) stages, Milan criteria, tumor diameter, tumor number, serum AFP levels, and histological differentiation (Table 2).
| Variable | GPC3 protein expression(n/N) (%) | Pvalue | GPC3 mRNA overexpression (n/N) (%) | Pvalue |
| TNM stage | ||||
| I-II | 7/12 (58) | 6/12 (50) | ||
| III-IVa | 14/19 (74) | 0.373 | 14/19 (74) | 0.179 |
| Milan criteria | ||||
| Within | 11/15 (73) | 8/15 (53) | ||
| Beyond | 10/16 (62) | 0.519 | 12/16 (75) | 0.208 |
| Tumor diameter | ||||
| < 5 cm | 11/16 (69) | 9/16 (56) | ||
| ≥ 5 cm | 10/15 (67) | 0.901 | 11/15 (73) | 0.320 |
| Tumor number | ||||
| < 3 | 18/26 (69) | 17/26 (65) | ||
| ≥ 3 | 3/5 (60) | 0.686 | 3/5 (60) | 0.818 |
| Vascular invasion | ||||
| Present | 14/16 (87) | 14/16 (87) | ||
| Absent | 7/15 (47) | 0.015 | 6/15 (40) | 0.006 |
| Serum AFP | ||||
| ≤ 20 μg/L | 6/11 (54) | 6/11 (54) | ||
| > 20 μg/L | 15/20 (75) | 0.244 | 14/20 (70) | 0.390 |
| Histological differentiation | ||||
| Good | 3/6 (50) | 2/6 (33) | ||
| Moderate and poor | 18/25 (72) | 0.301 | 18/25 (72) | 0.075 |
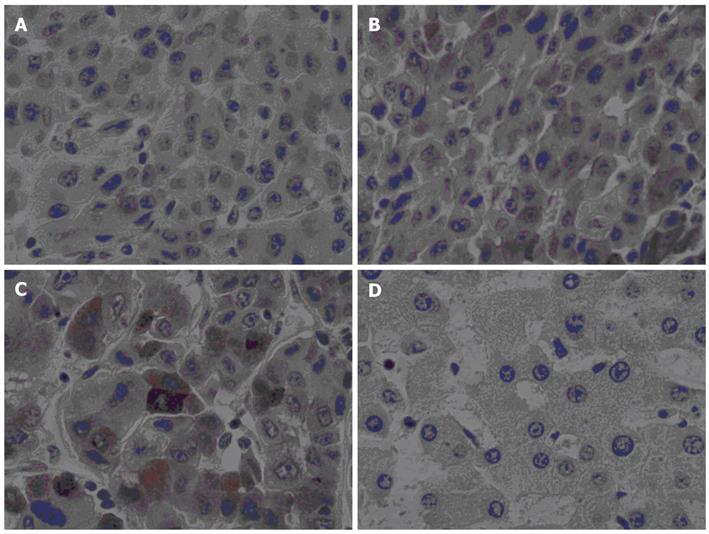
GPC3 protein was immunoexpressed in 21 of 31 (68%) cancerous tissues, but not in any adjacent non-neoplastic parenchyma and control liver tissues (P < 0.001). In GPC3-positive cases, protein expression was localized in the cytoplasm and membrane of cells (Figure 2). The sensitivity, specificity, PPV, and NPV of GPC3 protein expression was 0.68, 1.0, 1.0, and 0.84. Vascular invasion was significantly related to GPC3 protein expression (P = 0.015) (Table 2).
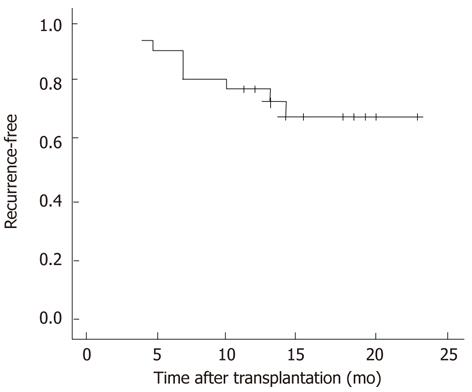
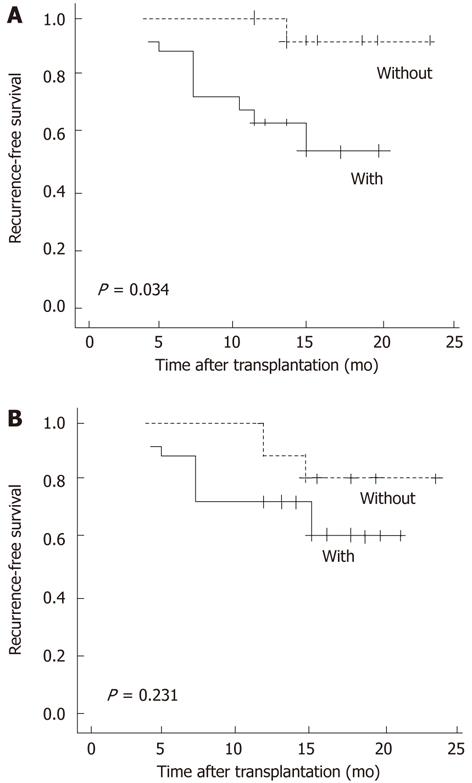
Up-to-date analyses after a follow-up of 24 mo revealed that 10 (32%) patients developed HCC recurrence. The median duration of HCC recurrence was 8.1 (range, 3-18 mo) after OLT (Figure 3). Assessment of Kaplan-Meier curves showed that patients without GPC3 mRNA overexpression had significantly longer recurrence-free survival than those with GPC3 mRNA overexpression (P = 0.034, log-rank test) (Figure 4A). Recurrence-free survival was significantly longer in patients without evidence of vascular invasion compared with those with vascular invasion (P = 0.016, log-rank test). Patients who met the Milan criteria had longer recurrence-free survival than those who did not, though the difference was not statistically significant (P = 0.077, log-rank test). There was no significant difference in recurrence-free survival between patients with positive and negative GPC3 protein expression (P = 0.231, log-rank test) (Figure 4B).
OLT is an excellent therapeutic choice for patients with cirrhosis complicated by HCC because it provides simultaneous treatment for both diseases. However, due to the limited availability of organs, prior selection of candidates most likely to benefit from OLT is very important. Thus, screening for potential early prognostic markers and therapeutic targets is very urgent for better selection of HCC patients for liver transplantation.
More recent studies have demonstrated upregulation of GPC3 in HCC at the protein level. GPC3 expression has been noted in 57% to 90% of HCC cases using immunohistochemistry[17-20]. In the present study, immunohistochemical analyses revealed that HCC expressed GPC3 protein in 68% of HCC tissues tested. RT-PCR analyses showed that, with a cutoff value of 3.5 × 10-2, 20 of 31 HCC tumors showed much stronger expression of GPC3 mRNA than adjacent non-neoplastic parenchyma. It was proposed that GPC3 expression could be a useful tissue marker to distinguish between benign and malignant hepatocellular lesions. Nevertheless, in some studies, HCC and non-malignant liver originated from different patients, so these studies could not exclude the possibility that HCC and adjacent non-neoplastic parenchyma have similar GPC3 expression[12,21]. We can presume that retained carcinoma cells in the adjacent non-neoplastic parenchyma of surgical specimens with large tumors and tumor invasion may be the cause of GPC3 protein expression or GPC3 mRNA overexpression. This suggests that detection of GPC3 expression in adjacent non-neoplastic parenchyma could indicate whether or not the tumor is completely resected. It may also serve as a reference value in deciding if further treatment after resection is required.
Although GPC3 mRNA and protein show expression in HCC tissues, in our previous study, we examined GPC3 mRNA-expressing cells in peripheral blood in liver-transplant recipients during the transplant period, and found no clinical diagnostic value for HCC. Studies showed that GPC3 was a potential serologic diagnostic marker for HCC. However, the diagnostic value of GPC3 protein in sera remains questionable. Yasuda et al[22] found that GPC3 is not a serologic marker for detection of HCC. We speculate that GPC3 serves as a potential tissue-specific diagnostic marker for HCC. Some liver-transplant candidates undergo locoregional tumor treatments, so obtaining tissue samples for preoperative molecular marker analyses would not be problematic.
In the clinicopathological analysis, TNM stages, Milan criteria, tumor diameter, tumor number, or serum AFP levels were not clearly associated with increase in GPC3 expression. GPC3 may therefore play an important part in the malignant transformation of cells in HCC. Up until now, AFP has been regarded as the most useful marker of HCC, although its sensitivity is limited[23]. Our data show that GPC3 mRNA and protein expression in HCC without serum AFP elevation (≤ 20 μg/L) were 54% and 54%, respectively, and in HCC of diameter < 5 cm were 56% and 69%, respectively. These findings suggest that GPC3 is a sensitive marker in small HCC and negative-serum AFP patients not only in resection[24,25] but also in OLT. Vascular invasion has been consistently reported to be a significant factor for a poor prognosis after liver transplantation for HCC[15,26,27]. Interestingly, in the present study, vascular invasion was significantly related to GPC3 expression. Zhu et al[12] using in-situ hybridization signals, showed that pushing HCC expressed significantly more GPC3 mRNA than the invading HCC. Their observations suggested that GPC3 may promote the growth of local cancer cells, and may also inhibit tissue invasion and metastasis. Their finding is different to ours. In addition, expression of GPC3 was lower in well-differentiated HCC than in moderately and poorly differentiated HCC, though the difference was not statistically significant, a finding consistent with previous reports[17,19]. The present study suggests that GPC3 expression may facilitate the growth of cancer cells, the degree of malignancy, and contribution to HCC progression.
Recurrence of HCC after transplantation remains a formidable problem despite refined selection criteria and exhaustive preoperative staging. In the present study, recurrence-free survival was longer in patients who had tumors with no evidence of vascular invasion. Microscopic vascular invasion in the explant is known to be a risk factor for HCC recurrence after transplantation. In addition, recurrence-free survival was longer in patients who met the Milan criteria, though the difference was not statistically significant. This could be because (1) the sample size in the present study was relatively small to allow appreciation of significant differences in the frequency of HCC recurrence in the groups; and (2) the patients in the Milan group had worse pathological stages. In our previous study, GPC3 mRNA-expressing cells in peripheral blood carried no predictive value for HCC recurrence after liver transplantation[15]. Hence, we used liver tissue instead of peripheral blood to determine if GPC3 expression in tumor tissues was associated with HCC recurrence after liver transplantation. In the present study, HCC patients with GPC3 mRNA overexpression had a significantly shorter recurrence-free survival than those with lower GPC3 mRNA expression after liver transplantation. This finding indicated that GPC3 mRNA overexpression could be an independent factor of a poor prognosis in HCC. Thus, we hypothesized that GPC3 may have a role in promoting carcinogenesis and in the development of HCC.
In general, GPC3 has been reported to interfere with different pathways and growth factors, and has a tissue- and stage-specific role in the development and tumor growth[28]. Ishiguro et al[29] showed that anti-GPC3 antibody can be used as a potential antitumor agent for human liver cancer, and can provide a novel treatment option for liver-cancer patients with GPC3-positive tumors. Evaluation of GPC3 as a diagnostic and immunotherapeutic target may be worthwhile for the prevention and treatment of HCC. Further studies must be completed to clarify these issues.
In conclusion, GPC3 is a valuable diagnostic marker sensitive and specific for HCC in liver-transplant patients. The most valuable finding in the present study was that GPC3 mRNA overexpression may act as a prognostic factor for recurrence-free survival in HCC after liver transplantation. The number of samples used in the present study was small. Confirmation of the findings of our study using more samples is warranted.
Given the current shortage of organs and the risk of aggressive recurrence, selection of candidates for liver transplantation is crucial and controversial. Establishment of direct and accurate methods to detect hepatocellular carcinoma (HCC) and predict HCC recurrence after liver transplantation is needed.
Glypican-3 (GPC3) has been discovered to be a potential serologic and immunohistochemical diagnostic marker for HCC. Despite clinical interest in GPC3, until now, the relationship between GPC3 expression and post-transplant HCC recurrence has not been clarified.
In the previous study, the level of GPC3 mRNA in peripheral blood had no clinical value in assessing tumor recurrence after liver transplantation. Therefore, the present study further examined the diagnostic value of GPC3 expression as retrospectively analyzed by real-time reverse transcription- polymerase chain reaction and immunohistochemistry in explant tumor samples. The principal finding was that GPC3 mRNA overexpression may act as an adverse indicator for HCC patients after liver transplantation because it was associated with significantly shorter recurrence-free survival.
The results of the present study suggest that GPC3 is a valuable diagnostic marker sensitive and specific for HCC in liver-transplant patients. Also, GPC3 mRNA overexpression may act as a prognostic factor for recurrence-free survival in HCC after liver transplantation.
GPC3 is a heparan sulfate proteoglycan which is bound to the cell surface by a mechanism involving the glycosylphosphatidylinositol anchor. GPC3 is highly expressed in fetal but not in normal adult liver.
Authors focused on the role of GPC3 mRNA and protein expression in the diagnosis of recurring HCC after liver transplantation. For this purpose, patient samples were analyzed according to clinicopathological features, mRNA expression levels, protein expression, and recurrence-free survival. They concluded that GPC3 expression could serve as a diagnostic marker for HCC and as an adverse indicator for HCC patients after liver transplantation.
Peer reviewers: Mark De Ridder, MD, PhD, Dienst Radiotherapie, UZ Brussel, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium; Dr. Wolfgang Mikulits, Professor, Medical University of Vienna, Borschkegasse 8a, 1090 Vienna, Austria
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [PubMed] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4259] [Article Influence: 236.6] [Reference Citation Analysis (2)] |
| 3. | Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418-424. [PubMed] |
| 4. | Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, Airoldi A, Giacomoni A, Rondinara G, Tinelli C. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Silva MF, Wigg AJ. Current controversies surrounding liver transplantation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Schwartz ME, D'Amico F, Vitale A, Emre S, Cillo U. Liver transplantation for hepatocellular carcinoma: Are the Milan criteria still valid? Eur J Surg Oncol. 2008;34:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573:241-246. [PubMed] |
| 8. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [PubMed] |
| 9. | Kandil DH, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-6254. [PubMed] |
| 12. | Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Büchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558-564. [PubMed] |
| 13. | Sung YK, Hwang SY, Park MK, Farooq M, Han IS, Bae HI, Kim JC, Kim M. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259-262. [PubMed] |
| 14. | Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J, Roskams T. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Shen Z, Zhu Z, Han R, Huai M. Clinical values of AFP, GPC3 mRNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon. 2011;11:195-199. [PubMed] |
| 16. | Wang YL, Li G, Wu D, Liu YW, Yao Z. Analysis of alpha-fetoprotein mRNA level on the tumor cell hematogenous spread of patients with hepatocellular carcinoma undergoing orthotopic liver transplantation. Transplant Proc. 2007;39:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and survivin in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in liver fine-needle aspiration biopsies. Diagn Cytopathol. 2009;37:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol. 2009;34:649-656. [PubMed] |
| 20. | Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, Li N, Ding HG. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410-4415. [PubMed] |
| 21. | Midorikawa Y, Ishikawa S, Iwanari H, Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M, Aburatani H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Yasuda E, Kumada T, Toyoda H, Kaneoka Y, Maeda A, Okuda S, Yoshimi N, Kozawa O. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol Res. 2010;40:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC, Chen MF, Tsai CN. Recurrence and Poor Prognosis Following Resection of Small Hepatitis B-Related Hepatocellular Carcinoma Lesions Are Associated with Aberrant Tumor Expression Profiles of Glypican 3 and Osteopontin. Ann Surg Oncol. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Sauer P, Kraus TW, Schemmer P, Mehrabi A, Stremmel W, Buechler MW, Encke J. Liver transplantation for hepatocellular carcinoma: is there evidence for expanding the selection criteria? Transplantation. 2005;80:S105-S108. [PubMed] |
| 27. | Cheung ST, Fan ST, Lee YT, Chow JP, Ng IO, Fong DY, Lo CM. Albumin mRNA in plasma predicts post-transplant recurrence of patients with hepatocellular carcinoma. Transplantation. 2008;85:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008;7:2787-2790. [PubMed] |
| 29. | Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832-9838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |