Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2371
Revised: November 28, 2011
Accepted: December 31, 2011
Published online: May 21, 2012
AIM: To compare the effect of percutaneous transhepatic portal vein embolization (PTPE) and unilateral portal vein ligation (PVL) on hepatic hemodynamics and right hepatic lobe (RHL) atrophy.
METHODS: Between March 2005 and March 2009, 13 cases were selected for PTPE (n = 9) and PVL (n = 4) in the RHL. The PTPE group included hilar bile duct carcinoma (n = 2), intrahepatic cholangiocarcinoma (n = 2), hepatocellular carcinoma (n = 2) and liver metastasis (n = 3). The PVL group included hepatocellular carcinoma (n = 2) and liver metastasis (n = 2). In addition, observation of postoperative hepatic hemodynamics obtained from computed tomography and Doppler ultrasonography was compared between the two groups.
RESULTS: Mean ages in the two groups were 58.9 ± 2.9 years (PVL group) vs 69.7 ± 3.2 years (PTPE group), which was a significant difference (P = 0.0002). Among the indicators of liver function, including serum albumin, serum bilirubin, aspartate aminotransferase, alanine aminotransferase, platelets and indocyanine green retention rate at 15 min, no significant differences were observed between the two groups. Preoperative RHL volumes in the PTPE and PVL groups were estimated to be 804.9 ± 181.1 mL and 813.3 ± 129.7 mL, respectively, with volume rates of 68.9% ± 2.8% and 69.2% ± 4.2%, respectively. There were no significant differences in RHL volumes (P = 0.83) and RHL volume rates (P = 0.94), respectively. At 1 mo after PTPE or PVL, postoperative RHL volumes in the PTPE and PVL groups were estimated to be 638.4 ± 153.6 mL and 749.8 ± 121.9 mL, respectively, with no significant difference (P = 0.14). Postoperative RHL volume rates in the PTPE and PVL groups were estimated to be 54.6% ± 4.2% and 63.7% ± 3.9%, respectively, which was a significant difference (P = 0.0056). At 1 mo after the operation, the liver volume atrophy rate was 14.3% ± 2.3% in the PTPE group and 5.4% ± 1.6% in the PVL group, which was a significant difference (P = 0.0061).
CONCLUSION: PTPE is a more effective procedure than PVL because PTPE is able to occlude completely the portal branch throughout the right peripheral vein.
- Citation: Iida H, Aihara T, Ikuta S, Yoshie H, Yamanaka N. Comparison of percutaneous transhepatic portal vein embolization and unilateral portal vein ligation. World J Gastroenterol 2012; 18(19): 2371-2376
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2371
Postoperative liver failure may be induced by major hepatectomy, whereby more than 60%-70% of total liver volume is planned for resection in cases such as hilar bile duct carcinoma, hepatocellular carcinoma and liver metastasis. To reduce the risk of postoperative liver failure due to an insufficient volume of functional liver, the well-established percutaneous transhepatic portal vein embolization (PTPE) procedure can be performed prior to major hepatectomy[1-6]. Unilateral portal vein ligation (PVL) is an alternative method that requires laparotomy[7,8]. Although PTPE has since its introduction become the more popular procedure of the two, PVL remains a viable option during the initial stage of a two-stage hepatectomy procedure (TSHP) for cases such as bilobar multiple liver metastases[9-11]. Both of these techniques occlude the unilateral portal vein, with the aim of inducing atrophy of the ipsilateral liver and thus inducing hypertrophy of the future liver remnant (FLR). In the present study, we retrospectively compared the effectiveness of the two techniques by evaluating the postoperative atrophy rate and hemodynamics of the right hepatic lobe (RHL).
Between March 2005 and March 2009, nine patients were selected for PTPE and four for PVL. The mean patient age was 69.7 ± 3.2 years for the PTPE group and 58.9 ± 2.9 years for the PVL group (P = 0.0002). The clinical characteristics of the patients included hilar bile duct carcinoma (n = 2), intrahepatic cholangiocarcinoma (n = 2), hepatocellular carcinoma (n = 2) and liver metastasis (n = 3) in the PTPE group, and the PVL group had hepatocellular carcinoma (n = 2) and liver metastasis (n = 2). The PTPE group included one patient with hepatitis C, one with hepatitis B, and seven positive for hepatitis B virus (HBV) antigen but negative for hepatitis C virus (HCV) antibody. The PVL group included one patient with hepatitis C, one with hepatitis B, and two positive for HBV antigen and negative for HCV antibody.
Two patients were diagnosed with hilar bile duct carcinoma, based on findings of obstructive jaundice. Endoscopic nasobiliary drainage was first performed. Before conducting an extended right lobectomy, PTPE was performed because the liver volumes to be resected were 70.8% and 70.2% in the two patients.
In the two cases of intrahepatic cholangiocarcinoma, the tumors were 10 cm and 8 cm in diameter, and both were adjacent to the hilar plate. These two patients also underwent a preoperative PTPE because of planned liver resection volumes of 74.2% and 70.5% respectively.
In the two patients with hepatocellular carcinoma, the tumors were 4.0 cm and 3.5 cm in diameter and adjacent to the right Glisson’s capsule. In addition, both the tumors recurred after the transarterial embolization procedure. The liver volume to be resected in these cases was 68.3% and 66.1%, respectively. In addition, indocyanine green retention rate at 15 min (ICG R15) was 17% and 18%, respectively, indicating functional liver impairment. Because of these factors, PTPE was performed prior to resection.
In the three patients with liver metastases, multiple lesions in the right lobes were noted after the initial round of chemotherapy. Preoperative PTPE was scheduled because the liver volumes to be resected in the three patients were 65.2%, 66.6% and 68.8%.
Patients with hepatocellular carcinoma in the PVL group presented with lymph node metastasis. The tumors adjacent to the right Glisson’s capsule were 5 cm and 4 cm in diameter with ICG R15 of 38.8% and 15.2%, respectively. The planned liver resection volumes were 65.1% and 69.8%, respectively. Thus, PVL was performed on the right portal branch only during the implementation of lymphadenectomy.
Regarding the two liver metastasis cases, the patients had undergone chemotherapy and developed multiple synchronous liver metastases to both lobes, disseminated from ascending colon cancer. Because the liver resection volumes were 66.9% and 74.8%, the right portal vein was ligated. In addition, right colectomy and partial resection of the left hepatic lobe were also performed in both patients.
Resectability criteria included an FLR of ≤ 30% of the total liver volume, whereas this criterion was ≤ 35% for the patients who had liver cirrhosis or underwent neoadjuvant chemotherapy. In addition, the following equation established by Yamanaka et al[12,13] and Okamoto et al[14] was used to predict posthepatectomy liver failure: Y = -110 + 0.942 × resection rate (%) + 1.36 × ICG retention rate (%) + 1.17 × patient’s age + 5.94 × ICG maximal removal rate (mg/kg per minute). With this equation, the patients who had a calculated Y value > 50 points were deemed unresectable.
For all patients, PTPE or PVL was performed on the RHL. PVL was indicated for patients who were to undergo laparotomy for lymphadenectomy or right colectomy. In PTPE, the umbilical portion of the portal vein was punctured, and the right branch of the portal vein was embolized using a mixture of fibrin glue (Beriplast P; CSL Behring, Tokyo, Japan) and iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France). In the PVL cases, preoperative multidetector row computed tomography (CT) was routinely performed to check for the presence of anatomical variants of the right portal vein. The right branch of the portal vein was intraoperatively isolated and ligated. After each method, Doppler ultrasonography was used to confirm that portal blood flow had been occluded in the ligated lobe and that it had been sustained in the FLR.
No patients had any postoperative complications. Although a slight postoperative decline in liver function was noted in some patients, all improved with conservative treatments.
Each patient underwent CT volumetry 1 mo before and after each procedure to evaluate volume changes in the RHL, and the values were compared between the two groups. The RHL atrophy rate was estimated by subtracting the RHL volume rate at 1 mo after PTPE or PVL from the preoperative RHL volume rate. The values in each group were compared using the Mann-Whitney test. All analyses were performed using statistical software (JMP 8.0.2 Macintosh; SAS Institute, Japan). Differences were considered statistically significant at P < 0.05.
The mean age of patients was significantly higher in the PTPE group (58.9 ± 2.9 years vs 69.7 ± 3.2 years in the PTPE group), which was a significant difference (P = 0.0002). Among the indicators of liver function, including serum albumin, serum bilirubin, asparate aminotransferase, alanine aminotransferase, platelets and ICG R15, no significant differences were observed between the two groups (Table 1).
| Variables | PTPE group (n = 9) | PVL group (n = 4) | P value |
| Age (yr) | 69.7 ± 3.2 | 58.9 ± 2.9 | 0.0002 |
| Sex (male:female) | 7:2 | 3:1 | |
| Background | |||
| (HCV:HBV:NBNC) | 1:1:7 | 1:1:2 | |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.0 ± 0.4 | 0.61 |
| Bilirubin (mg/dL) | 0.9 ± 0.4 | 1.2 ± 0.7 | 0.35 |
| AST (IU/L) | 30.6 ± 12.2 | 32.3 ± 8.5 | 0.47 |
| ALT (IU/L) | 35.9 ± 29.1 | 29.3 ± 15.6 | 0.92 |
| WBC (/mm3) | 6744 ± 3109 | 5228 ± 1973 | 0.41 |
| PLT (× 104/mL) | 22.1 ± 11.0 | 19.3 ± 9.9 | 0.76 |
| PT (%) | 84.1 ± 10.5 | 84.5 ± 12.2 | 0.86 |
| ICG 15 (%) | 10.7 ± 7.4 | 17.5 ± 13.2 | 0.43 |
| Child pugh (A:B:C) | 7:2:0 | 3:1:0 |
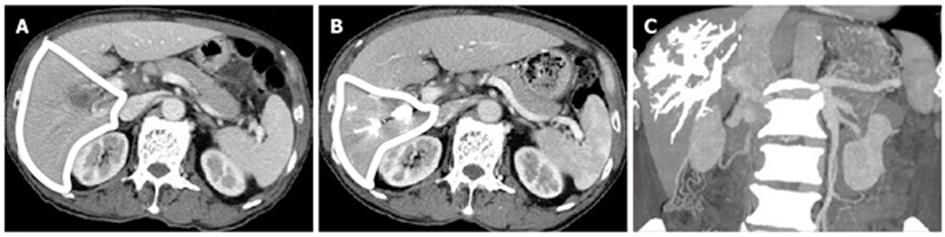
Preoperative RHL volumes in the PTPE and PVL groups were estimated to be 804.9 ± 181.1 mL and 813.3 ± 129.7 mL, respectively, with volume rates of 68.9% ± 2.8% and 69.2% ± 4.2%, respectively. There were no significant statistical differences in RHL volumes (P = 0.83) and RHL volume rates (P = 0.94).
At 1 mo after PTPE or PVL, postoperative RHL volumes in the PTPE and PVL groups were estimated to be 638.4 ± 153.6 mL and 749.8 ± 121.9 mL, respectively, which was not a significant difference (P = 0.14). Postoperative RHL volume rates in the PTPE and PVL groups were estimated to be 54.6% ± 4.2% and 63.7% ± 3.9%, respectively, which was a significant difference (P = 0.0056). At 1 mo postoperatively, the liver volume atrophy rate was 14.3% ± 2.3% in the PTPE group and 5.4% ± 1.6% in the PVL group, which was a significant difference (P = 0.0061) (Table 2).
| Variables | PTPE group (n = 9) | PVL group (n = 4) | P value |
| Preoperative RHL volume (mL) | 804.9 ± 181.1 | 813.3 ± 129.7 | 0.83 |
| Preoperative RHL volume rate (%) | 68.9 ± 2.8 | 69.2 ± 4.2 | 0.94 |
| Postoperative RHL volume (mL) | 638.4 ± 153.6 | 749.8 ± 121.9 | 0.14 |
| Postoperative RHL volume rate (%) | 54.6 ± 4.2 | 63.7 ± 3.9 | 0.0056 |
| RHL atrophy rate 1 mo postoperation (%) | 14.3 ± 2.3 | 5.4 ± 1.6 | 0.0061 |
With respect to the findings from imaging, postoperative CT and Doppler ultrasonography data confirmed residual peripheral portal inflow in the right branch of the ligated portal vein in two cases in the PVL group. In contrast, portal venous flow was confirmed as completely occluded in the PTPE group.
PTPE successfully facilitated liver resection for all nine patients, whereas among the four patients who underwent PVL, two with hepatocellular carcinoma remained unresectable after the procedure. An extended right lobectomy was performed for two hilar bile duct carcinomas and two intrahepatic cholangiocellular carcinomas. A right lobectomy was performed in all the other resectable cases. The 11 patients showed no postoperative complications.
Through experimentation on rabbits in 1920, Rous et al[15] proved that PVL could induce atrophy of the ipsilateral hepatic lobe and hypertrophy of the FLR lobe. Since then, this technique has been clinically applied by Honjo et al[16] and has recently been adopted for TSHP for bilobar multiple liver metastases. In the first stage, partial resection or ablation is performed on the FRL, and PVL is subsequently performed to induce atrophy in the hemiliver to be resected, in preparation for the planned major hepatectomy[17-20]. PTPE has been advocated as a technique for inducing atrophy of the ipsilateral liver without the need for a laparotomy, and is commonly performed for cases such as hilar bile duct carcinoma and hepatocellular carcinoma, in which a substantial liver volume is planned for resection[21-23]. Although PVL and PTPE are both techniques that occlude the portal vein, they differ in approach. PTPE is performed by percutaneously injecting embolic materials, whereas PVL is performed by a laparotomy to ligate the first-order branch of the portal vein. To date, several studies have demonstrated the safety and effectiveness of the two techniques individually; however, there are not enough data available from comparative studies reporting the relative efficacies of the two techniques. In the present study, we compared the atrophic effect and postoperative hepatic hemodynamics associated with PTPE and PVL.
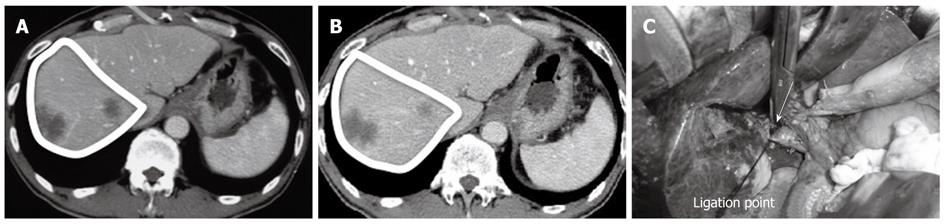
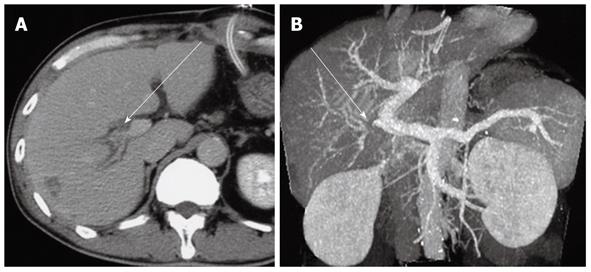
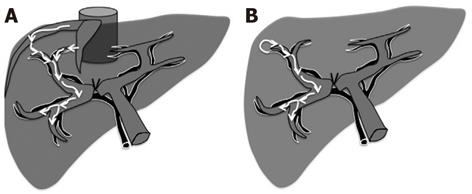
In our study, the PTPE group demonstrated a significantly higher rate of postoperative liver volume atrophy than the PVL group 1 mo after hepatectomy. The mean liver volume atrophy rate was 14.3% ± 2.3% in the PTPE group and 5.4% ± 1.6% in the PVL group (Figures 1 and 2). The CT and Doppler ultrasound findings may explain the reason for this difference. It was confirmed that portal venous flow was completely occluded due to the use of embolic materials acting throughout the peripheral portal vein in the PTPE group. However, portal vein venous flow continued at peripheral sites away from the ligation point in two cases in the PVL group (Figure 3). Residual flow was observed from the anterior to the posterior branch (Figure 4).
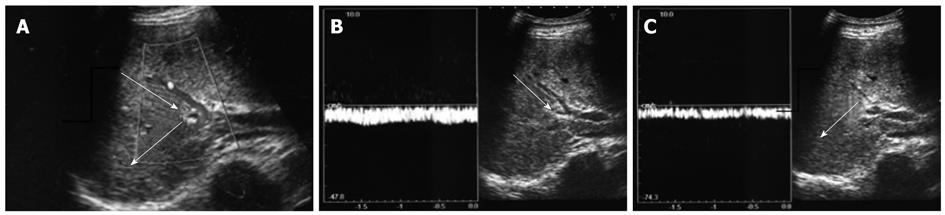
Two explanations can be proposed for the peripheral portal blood flow observed despite the portal vein being ligated at its central site. First, the observed peripheral portal blood flow could be backflow from the ipsilateral hepatic vein (Figure 5A). Normally, portal vein pressure and hepatic venous pressure are approximately equal at 100-150 mmH2O. However, ligating the first-order branch of the portal vein may decrease peripheral portal vein pressure, causing a relative pressure elevation in the hepatic vein and resulting in backflow from the hepatic vein. This backflow was significant for cases where tumors had compressed or obstructed hepatic veins in the presence of high hepatic venous pressure. A second possible explanation for the observed peripheral portal blood flow is related to the arterioportal shunt (AP shunt) (Figure 5B). Blood inflow via the AP shunt diminishes the effectiveness of the technique by allowing some blood to flow in the first-order branch of the portal vein. Consequently, when the AP shunt is preoperatively observed, PVL should not be the preferred treatment option.
In contrast to the PVL group, postoperative CT data confirmed the presence of embolic materials in the peripheral portal vein in all PTPE patients. In addition, postoperative Doppler ultrasound showed no residual blood in the portal vein, leading us to conclude the superiority of PTPE over PVL. However, PVL is currently more often performed in TSHP cases requiring portal vein occlusion. Time between the first stage and PTPE should be minimized because of possible cancer progression[24,25]. Therefore, we recommend PTPE for the first part of TSHP.
In conclusion, the small sample size and the retrospective nature of this study are limitations towards obtaining more conclusive results, compared to a prospective study with a larger population. However, our results show that PTPE can more effectively and rapidly achieve atrophy of ipsilateral liver volume and consequently induce compensatory FLR volume hypertrophy, compared with PVL.
Postoperative liver failure may be induced because of the insufficient remnant liver volume after major hepatectomy where more than 60%-70% of the total liver volume is resected in such cases as hilar bile duct carcinoma or liver metastasis. To achieve safer major hepatectomy, ligation or embolization of the portal vein is preliminarily performed to induce atrophy of the ipsilateral liver and hypertrophy of the future remnant liver.
To occlude unilateral portal vein, either portal vein ligation (PVL) ligating the unilateral portal vein or percutaneous transhepatic portal vein embolization (PTPE) injecting embolic agent to the unilateral portal vein through catheter is performed. PTPE is a procedure advocated after PVL, and PVL cases have decreased in number since the introduction of PTPE. However, as two-stage hepatectomy procedure (TSHP) for liver metastasis has recently become a more popular procedure, PVL has been again increasingly performed in combination with the first resection of TSHP.
The liver atrophy rate of unilateral lobe was compared between the PTPE (n = 9) and PVL groups (n = 4) 1 mo after each procedure. The liver atrophy rate was 14.3% ± 2.3% (PTPE) and 5.4% ± 1.6% (PVL), which was a significant difference (P = 0.0061). To date, the two procedures have been regarded equivalent in effectiveness, however, the results suggest the superiority of PTPE over PVL, and to the best of knowledge, this is the first report to demonstrate such difference.
PVL is a procedure to ligate the unilateral portal vein to induce ipsilateral liver atrophy and consequent hypertrophy of the contralateral liver. PTPE is a procedure to occlude the unilateral portal vein by percutaneously injecting embolic agent to induce ipsilateral liver atrophy and consequent hypertrophy of the contralateral liver. TSHP is a surgical strategy adopted for severe metastatic liver cases where it is impossible to remove all malignant lesions in a single procedure. In the first procedure, resectable tumors are removed and after a period of time for liver regeneration, the second procedure is performed to remove the remaining tumors.
The current study compared PTPE and unilateral PVL. Using small cohorts, the authors concluded that PTPE is a more effective procedure than PVL as the postoperative liver volume atrophy rate was significantly greater in the PTPE group than the PVL group. This study was novel, well written, technically well conducted, and highly clinically important.
Peer reviewers: Philip Rosenthal, MD, Professor of Pediatrics and Surgery, UCSF, 500 Parnassus Avenue, PO Box 0136, MU 4-East, San Francisco, CA 94143-0136, United States; Vance Matthews, PhD, BS, Cellular and Molecular Metabolism Laboratory, Baker University of Texas Medical Branch, IDI, PO Box 6492, St Kilda Road Central, VIC 8008, Melbourne, Australia
S- Editor Gou SX L- Editor Kerr C E- Editor Li JY
| 1. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 247] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000;87:879-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Hirohashi K, Tanaka H, Tsukamoto T, Kubo S, Shuto T, Takemura S, Yamamoto T, Kanazawa A, Ogawa M, Osugi H. Limitation of portal vein embolization for extension of hepatectomy indication in patients with hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1084-1087. [PubMed] |
| 5. | Hiramatsu K, Sano T, Nagino M, Nimura Y. Repeat hepatectomy for colonic liver metastasis presenting intrabiliary growth--application of percutaneous transhepatic portal vein embolization for impaired liver. Hepatogastroenterology. 2007;54:1554-1556. [PubMed] |
| 6. | van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Gouma DJ, Bennink RJ, Laméris JS, van Gulik TM. Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization : recovery after PVE and liver resection. J Gastrointest Surg. 2009;13:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Homayounfar K, Liersch T, Schuetze G, Niessner M, Goralczyk A, Meller J, Langer C, Ghadimi BM, Becker H, Lorf T. Two-stage hepatectomy (R0) with portal vein ligation--towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis. 2009;24:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Lin KJ, Liao CH, Hsiao IT, Yen TC, Chen TC, Jan YY, Chen MF, Yeh TS. Improved hepatocyte function of future liver remnant of cirrhotic rats after portal vein ligation: a bonus other than volume shifting. Surgery. 2009;145:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am. 2004;84:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoridis J, Malliotakis A, Pedonomou M, Solomou EK, Safioleas M. Two-stage liver surgery for advanced liver metastasis synchronous with colorectal tumor. Hepatogastroenterology. 2004;51:413-418. [PubMed] |
| 12. | Yamanaka N, Okamoto E, Kuwata K, Tanaka N. A multiple regression equation for prediction of posthepatectomy liver failure. Ann Surg. 1984;200:658-663. [PubMed] |
| 13. | Yamanaka N, Okamoto E, Oriyama T, Fujimoto J, Furukawa K, Kawamura E, Tanaka T, Tomoda F. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994;219:342-346. [PubMed] |
| 14. | Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586-592. [PubMed] |
| 15. | Rous P, Larimore LD. Relation of the portal blood to liver maintenance: A demonstration of liver atrophy conditional on compensation. J Exp Med. 1920;31:609-632. [PubMed] |
| 16. | Honjo I, Suzuki T, Ozawa K, Takasan H, Kitamura O. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg. 1975;130:296-302. [PubMed] |
| 17. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049; discussion 1037-1049. [PubMed] |
| 18. | Togo S, Nagano Y, Masui H, Tanaka K, Miura Y, Morioka D, Endo I, Sekido H, Ike H, Shimada H. Two-stage hepatectomy for multiple bilobular liver metastases from colorectal cancer. Hepatogastroenterology. 2005;52:913-919. [PubMed] |
| 19. | Mueller L, Hillert C, Möller L, Krupski-Berdien G, Rogiers X, Broering DC. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15:1908-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Capussotti L, Muratore A, Baracchi F, Lelong B, Ferrero A, Regge D, Delpero JR. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008;143:978-982; discussion 982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 22. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 23. | Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, Curley SA, Abdalla EK, Vauthey JN. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery. 2009;145:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |