Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2344
Revised: February 1, 2012
Accepted: February 16, 2012
Published online: May 21, 2012
AIM: To investigate the role of Lactobacillus crispatus (L. crispatus) strain China Center for Type Culture Collection (CCTCC) M206119 in intestinal inflammation.
METHODS: Forty 8-wk-old Balb/c mice (20 ± 2 g) were divided into four groups of 10 mice each. Three groups that had received dextran sulfate sodium (DSS) were administered normal saline, sulfasalazine or CCTCC M206119 strain, and the fourth group received none of these. We assessed the severity of colitis using a disease activity index, measured the colon length and weight, collected stools and mesenteric lymph nodes for bacterial microflora analysis. One centimeter of the proximal colon, middle colon and distal colon were collected and fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. Interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α expression was detected using reverse transcription polymerase chain reaction. Protective factors zonula occludens (ZO)-1 and β-defensin 2 were detected by immunoblotting. The features of CCTCC M206119 strain were identified based on morphology, biochemical profile, and 16S RNA sequencing.
RESULTS: DSS-colitis animals treated with CCTCC M206119 had markedly more severe disease, with greater weight loss, diarrhea, fecal bleeding, and shortened colon length. In addition, the CCTCC-M206119-treated group had comparatively higher histological scores and more neutrophil infiltration than the controls. Expression of protective factors ZO-1 and β-defensin 2 was downregulated due to destruction of the mucosal barrier after CCTCC M206119 strain treatment. An in vitro assay demonstrated that CCTCC M206119 strain increased the nuclear translocation of nuclear factor-κB in epithelial cells. Intestinal proinflammatory or anti-inflammatory cytokine responses were evaluated. Proinflammatory colonic cytokine (IL-1β, IL-6 and TNF-α) levels were clearly increased in CCTCC-M206119-treated animals, whereas anti-inflammatory colonic cytokine (IL-10) level was lowered compared with saline or 5-aminosalicylic-acid-treated DSS-colitis mice. Next, CCTCC M206119 strain was characterized as L. crispatus by microscopic morphology, biochemical tests and 16S rRNA gene level.
CONCLUSION: Not all lactobacilli are beneficial for intestinal inflammation, and L. crispatus CCTCC M206119 strain is involved in exacerbation of intestinal inflammation in DSS-colitis mice.
-
Citation: Zhou FX, Chen L, Liu XW, Ouyang CH, Wu XP, Wang XH, Wang CL, Lu FG.
Lactobacillus crispatus M206119 exacerbates murine DSS-colitis by interfering with inflammatory responses. World J Gastroenterol 2012; 18(19): 2344-2356 - URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2344
Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis (UC), are characterized by an abnormal activation of the gut-associated immune system resulting in a chronic inflammation of the digestive tract[1]. It is widely accepted that a combination of genetic factors, immune disorders and environmental factors could be involved in the etiology of IBD[2]. Recent research has shown that some commensal and pathogenic bacteria are closely related to these diseases[3,4].
In acute dextran sulfate sodium (DSS)-induced colitis, bacteria and/or bacterial products play a major role in the initiation of inflammation but not in chronic DSS-colitis[5]. In the neonatal period, IL-10 gene-deficient mice have decreased levels of colonic Lactobacillus spp. and an increase in colonic mucosal adherent and translocated bacteria. Normalizing Lactobacillus spp. levels reduces colonic mucosal adherent and translocated bacteria and prevents colitis[6]. Also, it is widely accepted that antibiotic treatment can definitely alleviate the clinical manifestation of UC. Various antibiotics have been shown to exhibit varying abilities to prevent and treat colitis in HLA-B27 rats and IL-10-/- mice[7,8]. Some authors recommended that where there are no bacteria, there is no colitis. Various IBD models in rodents reared under germ-free conditions are free of intestinal inflammation[6]. Increasing evidence supports that luminal microflora, or their products, are probably an important initiating factor in the pathogenesis of IBD[9].
Interestingly, facultative anaerobic Gram-positive bacteria and Lactobacillus spp. are decreased in patients with active UC, whereas bifidobacteria are decreased in fecal extracts from patients with Crohn's disease (CD)[10]. Another group has shown that bifidobacteria are reported to be deficient in rectal biopsies from patients with IBD and studies of fecal flora in patients with CD suggest a deficiency in both lactobacilli and bifidobacteria[11]. Furthermore, a marked decrease in intestinal Lactobacillus spp. precede the onset of colitis in IL-10 gene deficient mice, and specific lactobacilli are relevant to disease induction or progression in the IL-10-deficient mouse model of colitis[12]. These studies offer strong evidence to support the view that an imbalance in the intestinal microflora triggers intestinal inflammation.
Microflora considered beneficial to the host includes the genera Bifidobacterium and Lactobacillus, whereas species potentially pathogenic include Enterobacteriacae and clostridia[4]. Therefore, maintaining or recovering the intestinal microflora or mucosa-associated bacteria in specific compartments has been proposed as a potential therapy to treat IBD[13]. For example, the administration of the VSL#3 probiotic cocktail delays relapse of pouchitis after surgical resection[14,15]. Probiotics have undergone investigation for their capacity to reduce the severity of a number of inflammatory conditions including pouchitis and UC. Also, some natural anti-inflammatory effects have recently been shown for Lactobacillus salivarius, Lactobacillus crispatus (L. crispatus), Bifidobacterium and Lactobacillus plantarum and Lactobacillus casei Shirota based on experimental colitis models[16-18].
Despite the wide usage of probiotics in controlling IBD, their efficacy is still controversial[19,20]. Thus, it is very difficult to draw a definitive conclusion in evaluating the role of microflora in pathogenesis of IBD, and subsequently, the efficacy of controlling IBD. Some published data have also questioned the safety of probiotics. The possibilities include possible adverse effects of probiotics in diseased or debilitated patients, rational bacterial selection, bacterial translocation, and microflora shift among individuals. Some published data have shown that lactobacilli isolated from IL-10-deficient mice failed to decrease tumor necrosis factor (TNF)-α production, whereas six lactobacillus isolates from mice without colitis significantly inhibited TNF-α production. This indicates that multiple strains of lactobacteria may be capable of probiotic activity but, conversely, that not all strains of lactobacteria have in vitro immunomodulatory activity[12]. Interestingly, it has been reported that Lactobacillus rhamnosus GG exacerbated acute DSS-induced colitis, demonstrated by increased colitic disease activity and histological damage score, however, in a chronic colitis model, a protective effect was observed[21].
In a previous study, we investigated and compared the effect of different Lactobacillus and Bifidobacterium strains, in a model of DSS-induced colitis[22]. When we screened to find some probiotics that can be potentially therapeutic, we came across a strain L. crispatus China Center for Type Culture Collection (CCTCC) M206119 that is capable of aggravating murine DSS-colitis. What was the possible mechanism? Does it mean that probiotics safety should be reconsidered?
In the present study, the promotive effects of strain CCTCC M206119 on the development of the inflammation were analyzed using a DSS-colitis model in BALB/c mice. We compared the effect of CCTCC M206119 administration, saline, and 5-aminosalicylic acid (5-ASA) treatment on DSS-colitis in mice. Thus, we tested in vivo and in vitro whether and how the CCTCC M206119 strain could aggravate epithelial damage of the colon, and indentified the features of CCTCC M206119 strain based on morphology, biochemical profiles, and 16S RNA sequencing.
L. crispatus strain CCTCC M206119 was isolated from human feces in the Division of Digestive Disease of the Second XiangYa Hospital (Changsha, Hunan, China), and kept at China Center for Type Culture Collection (CCTCC, Wuhan, China). Lactobacillus strains were grown at 37 °C in de Man, Rogosa and Sharpe (MRS) medium (Difco, MD, United States) without shaking.
Eight-week-old BALB/c mice of either sex were purchased from Hunan Agricultural University [SCXK (Xiang 2002-003)], and then bred under specific pathogen-free conditions. Mice were kept in ventilated and filtered cages, fed an irradiated diet, and housed on irradiated bedding. Food and water were supplied ad libitum. All animal experiments were performed in compliance with the guidelines of the Chinese government approved by the College BioResources ethical review board.
Bacterial strains were grown to an optical density at 600 nm of 3 to 4 (early stationary phase) at 37 °C in MRS medium anaerobically, harvested by centrifugation, washed with sterile neutral saline, and resuspended at 109 colony forming units (CFU)/mL in neutral saline. Three hundred microliters of this daily-prepared suspension was fed daily to the mice via intragastric gavage, whereas the controls (both UC-induced and healthy mice) received 300 μL neutral saline via the same procedure.
Eight-week-old Balb/c mice (20 ± 2 g) were divided into a healthy control group, negative control (normal saline) group, positive control (5-ASA, 6 mg/20 g) group, and investigation (CCTCC M206119 strain) group, with 10 mice in each. Normal saline (300 μL), sulfasalazine, CCTCC M206119 strain were administered intragastrically once daily for 2 d before starting DSS and continued for 7 d after DSS induction. Colitis was induced by 5% (w/v) DSS (molecular weight 35 000-50 000; MP Biomedicals, Solon, OH, United States) dissolved in drinking water for 7 d (Table 1). The healthy control group received no DSS. Severity of colitis was assessed daily using a disease activity index (DAI). After the seventh day of induction of colitis, animals were euthanized by CO2 asphyxiation. The colon lengths and weight were measured. Under aseptic technique, 300 mg stools and mesenteric lymph nodes (MLNs) were collected for bacterial microflora analysis. All the samples were placed immediately in sterile tubes containing 5 mL of transport medium. One centimeter of the proximal colon, middle colon and distal colon was collected and fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. The remaining tissues were immediately snap-frozen and stored at -80 °C[23,24].
| Groups | Experimental design |
| Healthy control | No DSS treatments |
| Negative control (saline) | 5% (w/v) DSS dissolved in drinking water for 7 d |
| Saline administered intragastrically once daily for 9 d | |
| Positive control (5-ASA) | 5% (w/v) DSS dissolved in drinking water for 7 d |
| 5-ASA administered intragastrically once daily for 9 d | |
| Investigating group (CCTCC M206119) | 5% (w/v) DSS dissolved in drinking water for 7 d |
| CCTCC M206119 administered intragastrically once daily for 9 d |
The severity of colitis was assessed daily using a DAI based on the scoring system of Hamamoto et al[25], which scores body weight loss, stool consistency and occult/gross fecal bleeding (Table 2). Occult blood in feces was evaluated by means of Hemoccult II test (Beckman Coulter, Palo Alto, CA, United States).
| Score | Weight loss (%) | Stool consistency | Occult/gross fecal bleeding |
| 0 | 0 | Normal | Negative |
| 1 | 1-5 | ||
| 2 | 5-10 | Loose stool | Hemoccult |
| 3 | 10-15 | ||
| 4 | > 15 | Diarrhea | Gross bleeding |
Tissue fixed with 4% (w/v) paraformaldehyde in phosphate buffered solution (PBS) was prepared for light microscopy, and 5-µm-thick sections were stained with hematoxylin and eosin to study histological changes. Grading of intestinal inflammation was determined as follows (Table 3)[26]. All scores were obtained in a blinded fashion by two independent investigators.
| Score | Inflammation | Depth of lesions | Destruction of crypt | Width of lesions (%) |
| 0 | None | None | None | |
| 1 | Mild | Submucosa | 1/3 basal crypt | 1-25 |
| 2 | Severe | Muscularis | 2/3 basal crypt | 26-50 |
| 3 | Sera | Intact epithelium only | 51-75 | |
| 4 | Total crypt and epithelium | 76-100 |
Three-hundred-microgram fecal samples were dispersed in 2 mL PBS. Each pooled sample (0.1 mL) was serially diluted via 10-fold dilutions (from 10-1 to 10-10). Eosin methylene blue agar, KULB agar, TTC azide dextrose agar and mannitol salt agar were used for the enumeration of Enterobacteriaceae, Bacteroides, Enterococcus and Staphylococcus spp., respectively. Standard Nutrient Agar was used for the enumeration of total aerobic bacteria. All the plates were incubated at 37 °C, aerobically, for 24-48 h and the number of colonies were counted. Brain-heart infusion agar and LAMVAB agar were used to enumerate bifidobacteria and lactobacteria, respectively. Anaerobic incubation was carried out in anaerobic jars (Oxoid, Basingstoke, Hants, United Kingdom) at 37 °C for 48-72 h. Anaerobic conditions were obtained using Anaerogen (Oxoid) and were checked using methyl blue strips as oxidation reduction indicator. CFUs were counted and expressed per gram of sample[27,28].
The extent of neutrophil infiltration was determined by myeloperoxidase (MPO) assay[29]. Briefly, 500 mg colon was homogenized in 1 mL iced 0.5% hexadecyltrimethylammonium bromide buffer. The aliquot was centrifuged (4000 g, 15 min) and the supernatant was diluted into 5% homogenates. A mixture of 0.1 mL 0.05% hydrogen peroxide and 0.9 mL 5% homogenates was vortexed to release MPO from the tissue. After incubation in 37 °C for 15 min, 0.2 mL mixture was added to 3 mL O-dianisidine reaction mixture and incubated for 30 min at 37 °C. Sodium azide (50 μL, 2.0%) was added and incubated at 60 °C for 10 min. Absorbance of the reaction mixture was measured at 460 nm by spectrophotometer. Saline was used as a control. MPO activity was expressed as U/g tissue.
HT-29 cells (purchased from Y-Y Chemical Reagent Co. Ltd., Shanghai, China) were cultured in RPMI 1640 medium (Gibco, Rockville, MD, United States) supplemented with 10% fetal calf serum under 5% CO2 at 37 °C and divided into negative control, TNF-α (10 ng/mL) and CCTCC M206119 groups. CCTCC M206119 was diluted to 109 CFU/mL with RPMI 1640 medium. HT-29 (3 × 106 cells) were cultured in 24-well tissue culture plates for 72 h and TNF-α or CCTCC M206119 was added. After 4 h of incubation, HT-29 cells were fixed in cold methanol for 5-15 min. The fixed cells were blocked in 5% bovine serum albumin (BSA)/PBS for 1 h on ice. We remove the blocking buffer and added primary antibody anti-nuclear factor (NF)-κB p65 (10 µg/mL) (MBL, Woods Hole, MA, United States; clone 8F11, rat IgG2a) diluted in PBS/0.3% Triton X100/1%BSA/1% serum overnight at 4 °C in a humidified chamber. After washing three times for 10 min with PBS on ice, the cells were incubated with anti-rabbit Cy3 (10 µg/mL) (Abcam, Cambridge, United Kingdom; polyclonal rabbit IgG) for 1 h at room temperature. The cells were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, United States) with 4',6'-diamidino-2-phenylindole hydrochloride after three washes in PBS at room temperature, and analyzed by a Nikon ECLIPSE E600 fluorescent microscope. The ratio of positive nuclear cells to total cells represented the nuclear translocation of NF-κB. The average number of positive stained cells within at least nine independent high power fields (400 × magnification) were determined microscopically and subjected to statistical analysis as indicated.
Total tissue RNA was isolated using TRIzol reagent following the manufacturer’s instructions For each sample, first-strand cDNA was synthesized using 1.0 µg total RNA with oligo dT23VN primer and M-MuLVuLV reverse transcriptase (Gibco). Five milliliters cDNA samples were amplified in 25 μL of a reaction mixture containing 10 × Taq buffer, 1.5 mmol MgCl2, 5 μmol dNTPs (each), 10 μmol of each 5′ and 3′ primers, and 2 U TaqGold polymerase (Perkin-Elmer Cetus, Waltham, MA, United States). Polymerase chain reaction (PCR) was performed in a thermal cycler (GeneAmp Model 2400; Perkin-Elmer Cetus) for 22 cycles (94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min), followed by a 10-min extension at 72 °C. The PCR products (5 μL) were subjected to electrophoresis on 1.5% agarose gels and stained with 0.5 μg/mL ethidium bromide. Primers used in this paper are illustrated in Table 4.
| Genes | Sequence of primers | |
| GAPDH | Sense | 5’-ATCACCATCTTCCAGGAGCG-3’ |
| Anti-sense | 5’-CCTGCTTCACCACCTTCTTG-3’ | |
| IL-1β | Sense | 5’-TTTTAATCAGCTATCCGGAC-3’ |
| Anti-sense | 5’-TAATGGGAACGTCACACACC-3’ | |
| IL-6 | Sense | 5’-ATGAAGTTCCTCTCTGCAAGAGACT3’ |
| Antisense | 5’-CACTAGGTTTGTTTAATCTC-3’ | |
| TNF-α | Sense | 5’-ACGTGGAACTGGCAGAAGAG-3’ |
| Antisense | 5’-GGTTGTCTTTGAGATCCATGC-3’ |
Colon extracts (40 μg total protein) from each group of mice were resolved by SDS-PAGE under reducing conditions and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, United States). The membrane was blocked with 5% nonfat dry milk overnight and incubated with a rabbit polyclonal antibody to zonula occludens (ZO)-1, β-defensin 2 (Lab Version, Fremont, CA, United States), with a rabbit polyclonal antibody to IL-1β, IL-6 or TNF-α (Santa Cruz Biotechnology, Santa Cruz, CA, United States), with a mouse monoclonal antibody to IL-10 (BD Biosciences, Franklin Lakes, NJ, United States), or with an anti β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Affinity BioRegents, Golden, CO, United States) for loading control. Antigen-antibody binding was detected with horseradish-peroxidase-conjugated anti-rabbit or mouse IgG (Vector Laboratories) using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, United States).
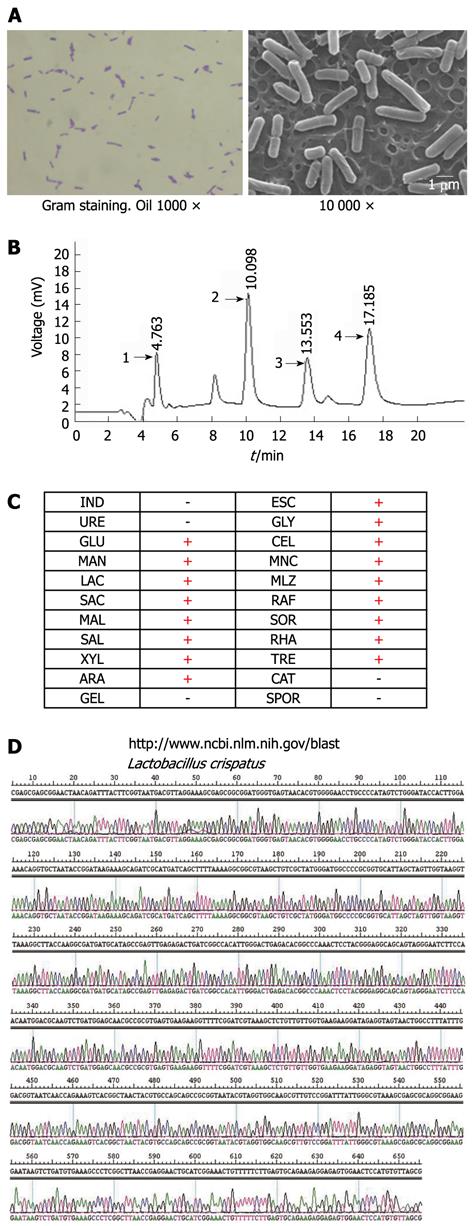
To investigate the characterization of CCTCC M206119 strain, 108 CFU/mL bacteria were grown for 18 h in MRS (Difco), washed with PBS and fixed with 2.5% glutaraldehyde. After two washings with PBS, samples were dehydrated with ethanol to the critical point, coated with 20 nm of gold in the scanning electron microscopy (SEM) coating unit, and examined by a XL30 ESEM scanning electron microscope (Philips, Eindhoven, Netherlands).
CCTCC M206119 strain were grown on MRS agar and incubated under anaerobic conditions at 37 °C for 24-48 h. All isolates were visualized by Gram staining. Biochemical testing was performed with API 20A strips (BioMerieux, Hazelwood, MO, United States) according to the manufacturer’s instructions. Catalase and SPOR tests were performed according to the supplier’s recommendations (Becton Dickinson). G + C percentage was determined using high-performance liquid chromatography (HPLC) method in the Chemistry College of Central South University (China). Ten microliters base standard and 10 μL DNA hydrolase were injected into the HPLC sample injector and analyzed three times. The separating conditions used were as follows: 250 mm Zonbax-C18 column, 0.05 mol/L NH4H2PO4 (pH 4.0) and acetonitrile mixture (20:1) as washing solution, 1 mL/min, moving phase: 2% acetonitrile, 98% 50 mmol/L NaH2PO4 buffered solution (pH 3.94), room temperature, detecting wavelength 254 nm and 270 nm, 0.1 mg/mL dA, dT, dG, dC solution used as external standard.
Approximately 700 bp of the 16S rRNA gene were amplified with primers 16S-F (5’-AGA GTT TGA TCA TGG CTC AG-3’) and 16S-R (5’-CAC CGC TAC ACA TGG AG-3’) under the following PCR conditions: 95 °C for 5 min; 10 cycles of 94 °C for 30 s, 70 °C for 30 s, and 72 °C for 40 s; 26 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min; and 72 °C for 10 min. 16S rDNA amplicons were gel purified by using SDS-PAGE and a gel band purification kit (Amersham Biosciences). The 5’ terminus of the 16S rRNA gene was sequenced with primers 16S-F and 16S-R by using an ABI Prism 3100 (Applied Biosystems) sequencing system and an ABI Prism BigDye Terminator cycle sequencing ready reaction kit (version 2.0, Applied Biosystems). Sequencing traces of amplicons containing ambiguous signals were resubmitted for sequencing. rDNA sequences were analyzed by using Lasergene (version 5.0, DNAStar, Madison, WI, United States). Contigs were generated by using SeqMan. Isolates were identified by using the nucleotide-nucleotide Basic Local Alignment Search Tool (BLASTn) (http://www.ncbi.nlm.nih.gov/blast).
Data were analyzed by Student’s t test or analysis of variance (ANOVA). The Wilcoxon signed-rank test was used to compare data that did not satisfy Student’s t test. P < 0.05 was considered significant.
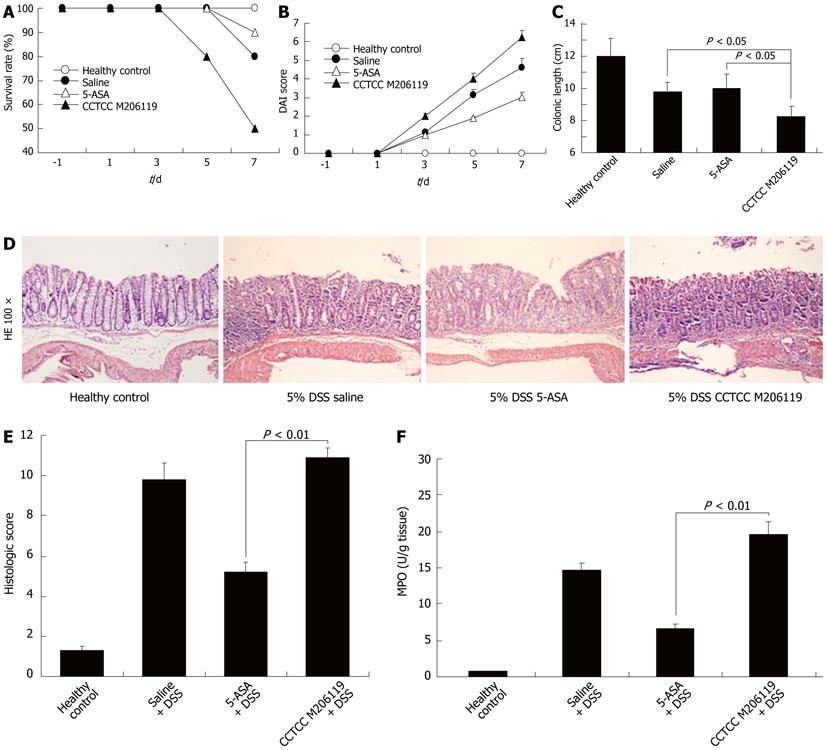
Survival rate of DSS-colitis mice reduced to 80% from day 5 after CCTCC M206119 treatment changes, which differed from other groups significantly (Figure 1A). Body weight loss, stool consistency, and occult/gross fecal bleeding were evaluated and scored individually for each animal according (Table 2). The scores were evaluated statistically by χ2 test and Monte Carlo exact test. CCTCC-M206119-treated DSS-colitis animals showed much higher DAI scores compared with 5-ASA- and saline-treated DSS-colitis mice, starting from day 3 after 5% DSS treatment (Figure 1B).
After 7 d of treatment, all DSS-colitis mice showed a significant reduction in their colon length compared with the healthy controls. CCTCC-M206119-strain-treated DSS-colitis mice had the shortest length (8.2 ± 0.68 cm), followed by saline- (9.8 ± 0.56 cm) or 5-ASA- (10.0 ± 0.89 cm) treated DSS-colitis mice (P < 0.05, Figure 1C). CCTCC-M206119-treated DSS-colitis mice had markedly more severe disease, with greater weight loss, diarrhea, fecal bleeding, and shortened colon length.
The histological lesions were examined blindly and the average scores were evaluated statistically by ANOVA. In the acute study, CCTCC-M206119-treated DSS-colitis mice presented scores of 10.9 ± 0.5 that differed statistically from those in the 5-ASA-treated DSS-colitis mice. It became evident that the CCTCC-M206119-treated DSS-colitis group had comparatively higher DAI scores than the saline controls (P < 0.01, Figure 1D and E).
Furthermore, there was significant difference in MPO enzymatic activity detected in the colons control mice (1.038 ± 0.012 U/g tissue), DSS plus saline group (18.368 ± 1.226 U/g tissue), DSS plus 5-ASA group (8.369 ± 0.652 U/g tissue), and DSS plus CCTCC M206119 group (24.565 ± 2.006 U/g tissue) (Figure 1F). This indicated that more neutrophils infiltrated DSS-colitis mice after CCTCC M206119 treatment.
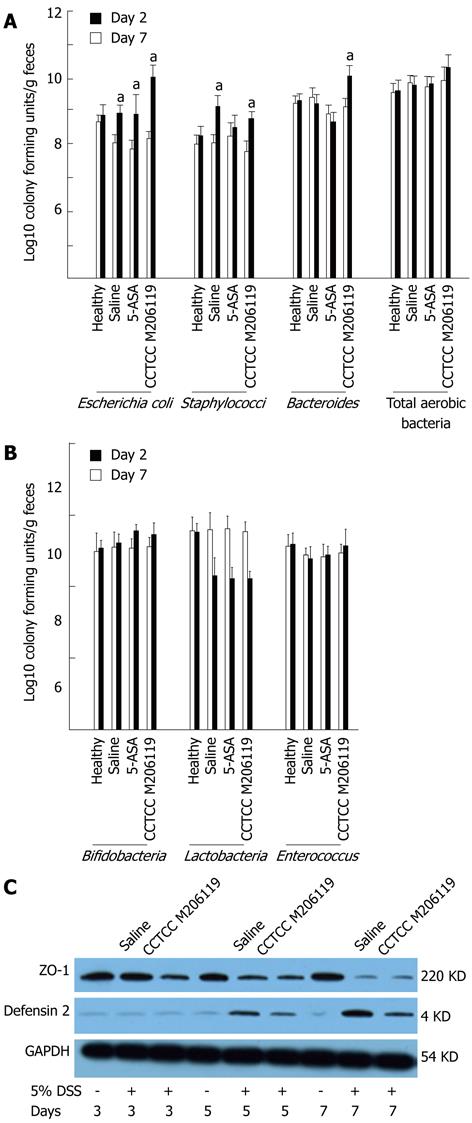
To characterize the role of CCTCC M206119 strain on potential gut flora shifts during DSS-induced barrier damage, we performed a bacterial-culture-based survey of the gut flora in DSS-colitis mice treated with saline, 5-ASA, or CCTCC M206119 strain. No significant differences in total aerobic bacteria or Enterococcus were obtained for any of the groups. Bacterial counts of all fecal aerobic [Escherichia coli (E. coli), Staphylococcus, Bacteroides, or total aerobic bacteria] cultured in DSS-colitis mice increased significantly compared to the healthy group. Also, both E. coli and Staphylococcus increased in the saline group, whereas 5-ASA rebalanced the bacterial counts of Staphylococcus to normal levels (Figure 2A). In contrast, Lactobacillus in the colon lumen was reduced significantly in all DSS-colitis groups, with no significant changes in Bifidobacterium or Enterococcus spp. (Figure 2B).
To determine whether the changes in gut flora after CCTCC M206119 treatment were due to alteration of the epithelial junction or antagonistic agents, we measured the expression levels of ZO-1 and β-defensin 2 from colons of DSS-colitis mice treated with saline or CCTCC M206119 and healthy mice, by western blotting. Expression of ZO-1 in healthy mice remained at an abundant level compared to all DSS-colitis mice. Treatment of mice for 5 d with 5% DSS plus saline reduced ZO-1 expression significantly compared to healthy mice, whereas 5% DSS plus CCTCC M206119 strain reduced ZO-1 expression dramatically at day 3. In contrast, no β-defensin 2 expression was detected in the healthy mice, with relatively high expression of β-defensin 2 detected in the colons in mice that were treated with 5 d of either 5% DSS plus saline or 5% plus CCTCC M206119. After 5 d exposure to 5% DSS plus CCTCC M206119, there was a significant (P < 0.05) decrease in β-defensin 2 level in the colons of mice relative to 5% DSS plus saline treatment (Figure 2C).
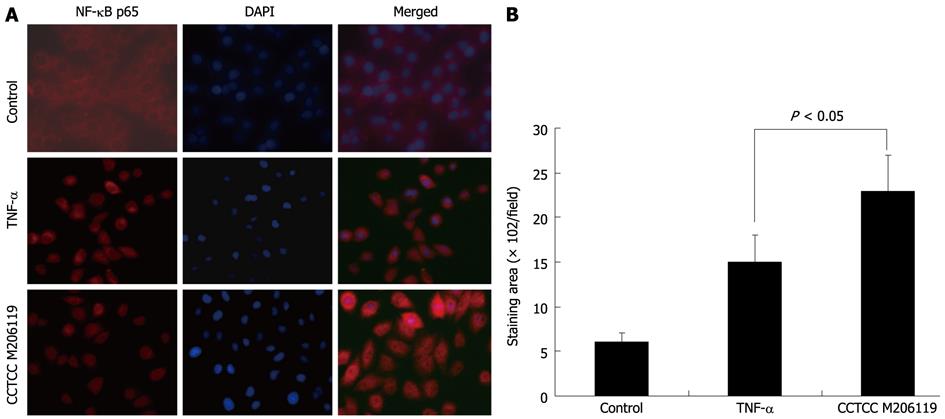
Nuclear translocation of NF-κB p65 represents increased inflammation at the initiation stage. To investigate whether the aggravation of DSS-colitis after CCTCC M206119 strain treatment was due to the initiation of inflammation-related transcription, we detected the translocation of NF-κB p65 in HT-29 cells inoculated with CCTCC M206119 strain. TNF-α was used as a control. In the blank control, no positive staining for NF-κB p65 was detected. Both TNF-α and CCTCC M206119 strain upregulated the translocation of NF-κB p65 in vitro, with more positively stained cells in the latter group (P < 0.05, Figure 3A and B).
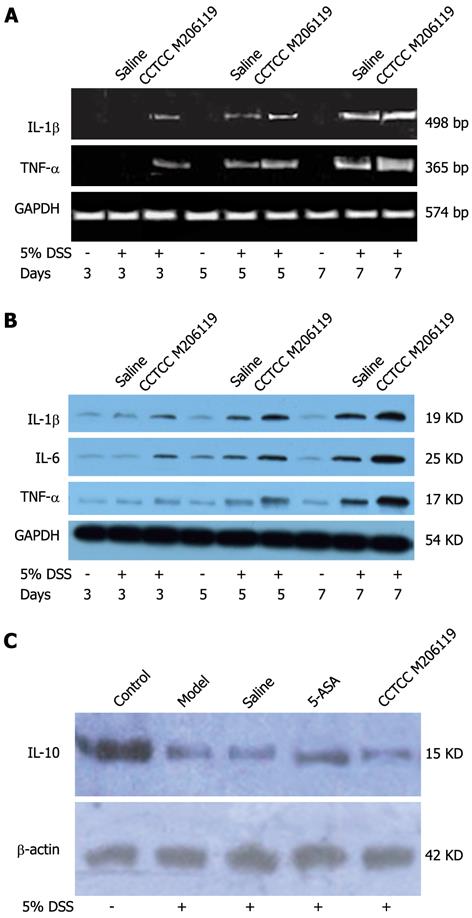
To investigate if CCTCC M206119 administration affected the expression of colonic cytokines, we first screened the mRNA levels of the major colonic proinflammatory cytokines by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). We observed increased mRNA levels for IL-1 β, IL-6 and TNF-α from the colons of DSS-colitis mice treated with CCTCC M206119 strain or saline for 3 d. Also, it was evident that mRNA levels of IL-1 β, IL-6 and TNF-α from colons of CCTCC-M206119-treated DSS-colitis mice were significantly higher than those from colons of saline-treated DSS-colitis mice (Figure 4A). Next, we confirmed the changes in expression of the above cytokines at the protein level, suggesting that DSS treatment resulted in increased expression of proinflammatory cytokines, whereas DSS plus CCTCC M206119 strain treatment resulted in significantly higher expression of the cytokines mentioned above (Figure 4B).
Anti-inflammatory cytokine IL-10 expression from colons of DSS-colitis mice treated with saline, 5-ASA, or CCTCC M206119 was measured by western blotting. IL-10 expression was dramatically downregulated in saline- and CCTCC-M206119-treated DSS-colitis mice, with more significant changes in the latter group. In contrast, 5-ASA upregulated IL-10 expression compared to saline-treated DSS-colitis mice (Figure 4C). It indicated that CCTCC M206119 treatment inhibited the expression of anti-inflammatory cytokine in DSS-colitis mice.
Distinct microscopic morphological features were observed after Gram staining of broth-grown CCTCC M206119 strain. Gram-positive, rod-shaped morphology could be distinguished (Figure 5A). Under SEM, CCTCC M206119 strain showed exclusively smooth rod- or fork-shaped morphology, without spores, flagella or capsules (Figure 5A). Based on analysis of the peak and data of base C, G, T and A, G + C mol% of CCTCC M206119 strain was determined as 58.64% (Figure 5B). All CCTCC M206119 strains tested were unable to utilize indepamide, urea enzymes and gelatin. All strains tested were able to utilize glucose, mannitol, lactose, saccharose, maltose, salicin, L-xylose, arabitol, esculin, glycerol, cellobiose, MNC, melezitose, D-raffinose, sorbitol, rhamnose, and trehalose. All strains were found to be catalase negative [at 3% (v/v) H2O2]. With discriminant and factorial analyses of data from all biochemical tests, a presumptive identification scheme for CCTCC M206119 strain was formulated based on biochemical properties. In conjunction with Gram stain morphology, biochemical profiling can be used to differentiate among major groups of lactobacilli (up to 95% confidence interval). In our abbreviated identification scheme, CCTCC M206119 strain can be presumptively grouped into lactobacilli when biochemical tests are combined with microscopic morphology (Figure 5C). To verify the identities of the reference strains used in this study, 16S rRNA genes were amplified and sequenced. With BLASTn, CCTCC M206119 strain was determined as L. crispatus at the 16S rRNA gene level (Figure 5D).
IBD, including CD and UC, are chronic immune-mediated diseases in which endogenous bacteria are thought to play an important role, as suggested by many clinical observations and experimental studies summarized in recent reviews[1]. Recent studies have also shown that some bacterial strains or mixtures may have the capacity to promote or reduce intestinal inflammation[4].
Although the pathogenesis of IBD remains elusive, the relevance of intestinal luminal bacteria in the initiation and progression of chronic intestinal inflammatory disorders is gaining support[30]. However, no specific microorganism has been associated with the pathogenesis of IBD, suggesting that qualitative/quantitative differences in the intestinal microbiota may play some role in the initiation or perpetuation of intestinal inflammation.
In the current study, we compared the phenotypes of DSS-colitis mice treated by saline, 5-ASA, and CCTCC M206119 strain, trying to determine the role of the probiotic bacterium. We demonstrated that CCTCC M206119 treatment reduced survival rate of DSS-colitis mice. CCTCC-M206119-treated DSS-colitis animals had markedly more severe disease, with greater weight loss, diarrhea, fecal bleeding, and shortened colon length. In addition, the CCTCC-M206119-treated DSS-colitis group had comparatively higher histological scores and more neutrophil infiltration than the controls. To our surprise, CCTCC M206119 strain could be characterized as L. crispatus by microscopic morphology, biochemical tests and 16S rRNA gene level. Hence, our study suggested that not all probiotics are safe for treatment of colitis. Instead, probiotics safety should be carefully evaluated before their application.
Other recent studies also have shown supporting evidence. A strain of Lactobacillus salivarius was isolated from blood and bile pus cultures of a 70-year-old man with bacteremic acute cholecystitis[31]. Administration of different lactobacilli and a Bifidobacterium strain in an acute liver injury rat model has shown different effects on bacterial translocation and hepatocellular damage. Bifidobacterium animalis NM2 increases bacterial translocation to the MLNs but does not affect hepatocellular damage[32]. Together with our data, some probiotics could act as opportunistic pathogens and result in endogenous infection unexpectedly. Also, some researchers have demonstrated that the activities to promote endogenous infections are strain specific.
As we know, most lactobacilli have a remarkable record of safety and have been consumed by humans for decades. However, the possible involvement of certain strains has been described in cases of sepsis, endocarditis, or bacteremia; mostly in association with a severe underlying disease or detrimental condition[33]. In view of these facts, the safety of potential probiotic microorganisms in these conditions should be assessed individually. Bacterial translocation (BT) is most likely the first step in the possible passage of viable (indigenous) bacteria to sterile body sites and is thus important for sepsis, endocarditis, and bacteremia caused by the commensal flora. In animal studies, mono association experiments have shown that the type of colitis is dependent on the bacterial species[12,34].
Based on our research, E. coli and staphylococci were increased in DSS-colitis mice, whereas lactobacilli in the colon luminal contents were reduced significantly in all DSS-colitis groups, with no significant changes in Bifidobacterium or Enterococcus spp. Furthermore, our data indicated that changes in gut flora after L. crispatus CCTCC M206119 treatment were related to reduced ZO-1 expression and increased β-defensin 2 expression. Our in vitro experiment showed that both TNF-α and CCTCC M206119 strain upregulated the nuclear translocation of NF-κB p65, with more positively stained cells in the latter group. Other studies have found that NF-κB can be activated in both intestinal epithelium and intestinal lamina propria mononuclear cells (LPMCs) in acute DSS-colitis mice[35,36]. Intrarectal administration of antisense oligonucleotides to NF-κB P65 could alleviate the inflammation[24,37]. Thus, it is reasonable to conclude that L. crispatus CCTCC M206119 strain exacerbated the imbalance of gut flora; changed the pathogen species or colonies adhered to the colonic mucosa; subsequently altered the expression of tight junction proteins and antibacterial molecules; and activated nuclear translocation of NF-κB p65 in epithelium or inflammatory response cells.
It is thought that IBD might be due to complex mucosal immune responses to antigens of resident enteric bacteria[38-40]. IL-1 and IL-6 have been shown to be the major cytokines secreted by lamina propria cells and play a critical role in the development of Th1-cell-mediated chronic colitis[41]. Recombined IL-1 receptor antagonist can alleviate the mucosal inflammation and necrosis in DSS-coltis mice[42]. DSS-induced colitis was less severe in IL-6 gene knockout mice, whereas transgenic mice with a mutant CIS/SOCS3 gene, encoding a negative regulator for the IL-6/STAT3 (signal transducer and activator of transcription) signaling pathway, had increased susceptibility to DSS-induced colitis[43]. In our study, we found that both mRNA and protein levels of IL-1 β, IL-6 and TNF-α from colons of CCTCC-M206119-treated DSS-colitis mice were significantly higher and earlier than those from colons of saline-treated DSS-colitis mice. In contrast, we found that expression of anti-inflammatory cytokine IL-10 was dramatically downregulated in saline- and CCTCC-M206119-treated DSS-colitis mice, with more significant change in the latter group. This indicated that DSS treatment resulted in increased expression of proinflammatory cytokines and decreased anti-inflammatory cytokines, whereas DSS plus CCTCC M206119 treatment resulted in significantly higher and earlier expression of the proinflammatory cytokines and inhibited the expression of anti-inflammatory cytokine in DSS-colitis mice.
IL-10 can inhibit the expression of IL-1α, IL-1β, IL-6, IL-12, IL-18, granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, M-CSF, TNF, leukemia inhibitory factor and platelet activating factor produced by activated monocytes or macrophages. It exerts strong immune inhibitory function[8] and plays a major role in the immune tolerance of intestinal mucosa[9,10]. Thus, it is possible that administration of L. crispatus CCTCC M206119 strain interrupts the balance between anti-inflammatory and proinflammatory responses in colonic mucosa or lamina propria after DSS treatment, and leads to subsequently aggravated colonic inflammation. These processes are similar to those observed in UC patients[11].
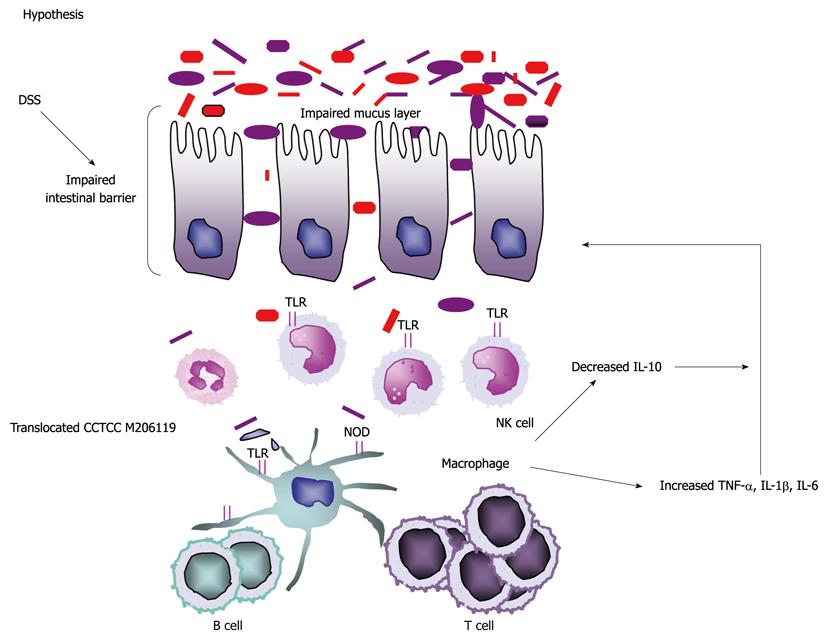
Thus, our hypothesis in this study is as follows: some bacteria or bacterial products, such as L. crispatus CCTCC M206119, may interact directly with colonic epithelial cells or LPMCs after disruption of the mucosal barrier and balance of gut flora by DSS administration. Then neutrophils and mononuclear cells infiltrate the lamina propria and activate NF-κB translocation, which in turn increases proinflammatory cytokines such as IL-1β, IL-6 and TNF-α. Furthermore, TNF-α can augment NF-κB activation in various cell types, and inhibit the production of anti-inflammatory cytokines such as IL-10 (Figure 6).
Overall, human IBD trials to date have suggested that probiotics display no overt side effects, but conflicting reports on probiotic efficacy highlight the importance of selecting well-characterized probiotic strains and in delivering intact pharmaceutical formulations at an appropriate dose level to the inflamed regions of the intestine. These studies have emphasized that certain probiotic strains need to be thoroughly investigated in vitro and in vivo using appropriate disease models. Also, some probiotics such as L. crispatus CCTCC M206119 might act as aggravating factors in the pathogenesis of UC. Future studies to identify associations between microorganisms (and or their genes and gene products) and human physiological or disease processes will lead to the development and testing of hypotheses that address causality as well as a myriad of specific host-microbe interactions.
We thank Jie Zhang (Division of Digestive Disease, Xiangya Second Hospital, Central South University, Changsha, China) for insightful suggestions and Jun Zhang (Department of Medicine, Kingsbrook Jewish Medical Center, NY, United States) for critical reading of the manuscript. We thank Xiao-Xin Liu for technical support. Tissue processing and imaging were performed in the Department of Pathology, 2nd Xiangya Hospital, Central South University, China.
It is widely accepted that a combination of genetic factors, immune disorders and environmental factors could be involved in the etiology of inflammatory bowel disease (IBD). Recent research has illustrated that some commensal and pathogenic bacteria are closely related to IBD. However, to date, the role of bacteria in the pathogenesis of IBD, or the efficacy of probiotics is still controversial.
The efficiency of probiotics in controlling IBD is still controversial. Thus, it is difficult to draw a definitive conclusion in evaluating the role of microflora in the pathogenesis of IBD, and subsequently, the efficacy in controlling IBD. Some published data have also questioned the safety of probiotics.
Microflora considered beneficial to the host include the genera Bifidobacterium and Lactobacillus. In this context, several candidate strains were screened to find some probiotics as therapeutics using dextran sodium sulfate (DSS)-induced colitis in mice, and China Center for Type Culture Collection (CCTCC) M206119 strain led to an exacerbated phenotype of DSS-colitis mice. Expression of protective factors zonula occludens-1 and β-defensin 2 was downregulated after CCTCC M206119 treatment. There was an increase in the nuclear translocation of nuclear factor-κB in epithelial cells. Then, intestinal proinflammatory and anti-inflammatory cytokine responses were evaluated. Proinflammatory colonic cytokines [interleukin (IL)-1β, IL-6 and tumor necrosis factor-α] levels were clearly increased in CCTCC-M206119-treated animals, whereas anti-inflammatory colonic cytokine (IL-10) level was lowered compared with saline- or 5-ASA-treated DSS-colitis mice. Next, CCTCC M206119 strain was identified as Lactobacillus crispatus, so it was concluded that not all lactobacilli strains have beneficial effects on intestinal inflammation and that Lactobacillus crispatus (L. crispatus) CCTCC M206119 is involved in exacerbation of intestinal inflammation in DSS-colitis mice.
The study results suggest that some probiotics such as L. crispatus CCTCC M206119 strain might act as an aggravating factor in the pathogenesis of ulcerative colitis (UC). They also highlight the importance of selecting well-characterized probiotic strains and in delivering intact pharmaceutical formulations at an appropriate dose level to the inflamed regions of the intestine.
IBD is a group of inflammatory conditions of the colon and small intestine, including Crohn’s disease and UC, which are characterized by abnormal activation of the gut-associated immune system, resulting in chronic inflammation of the digestive tract. Lactobacillus is a genus of Gram-positive facultative anaerobic or microaerophilic rod-shaped bacteria. They are a major part of the lactic acid bacteria group, named as such because most of its members convert lactose and other sugars to lactic acid. They are common and usually benign.
In this study, the authors investigated the role of CCTCC M206119 strain on intestinal inflammation using a DSS-induced colitis mice model. They showed that CCTCC M206119 strain was identified as L. crispatus, but this strain was involved in the exacerbation of intestinal inflammation in DSS-colitis mice. From these results, the authors concluded that not all lactobacilli strains have beneficial effects on intestinal inflammation. This paper has been well written and the results are interesting.
Peer reviewer: Jae Hee Cheon, Professor, Yonsei University College of Medicine, 250 Seongsan-ro Seodaemun-gu, Seoul 120-752, South Korea; Masahiro Iizuka, Akita Health Care Canter, Akita Red Cross Hospital, 3-4-23, Nakadori, Akita 010-0001, Japan
S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3353] [Article Influence: 186.3] [Reference Citation Analysis (11)] |
| 2. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1003] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 3. | Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2:e662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1376] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 5. | Hans W, Schölmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 481] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Ohkusa T, Nomura T, Terai T, Miwa H, Kobayashi O, Hojo M, Takei Y, Ogihara T, Hirai S, Okayasu I. Effectiveness of antibiotic combination therapy in patients with active ulcerative colitis: a randomized, controlled pilot trial with long-term follow-up. Scand J Gastroenterol. 2005;40:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kuehbacher T, Rehman A, Lepage P, Hellmig S, Fölsch UR, Schreiber S, Ott SJ. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J Med Microbiol. 2008;57:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Nishikawa J, Kudo T, Sakata S, Benno Y, Sugiyama T. Diversity of mucosa-associated microbiota in active and inactive ulcerative colitis. Scand J Gastroenterol. 2009;44:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take "toll" ? World J Gastroenterol. 2006;12:1829-1841. [PubMed] |
| 11. | Zhang M, Liu B, Zhang Y, Wei H, Lei Y, Zhao L. Structural shifts of mucosa-associated lactobacilli and Clostridium leptum subgroup in patients with ulcerative colitis. J Clin Microbiol. 2007;45:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Peña JA, Li SY, Wilson PH, Thibodeau SA, Szary AJ, Versalovic J. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl Environ Microbiol. 2004;70:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1585-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 16. | Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig Dis Sci. 2004;49:320-327. [PubMed] [DOI] [Full Text] |
| 17. | Geier MS, Butler RN, Giffard PM, Howarth GS. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int J Food Microbiol. 2007;114:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Rochat T, Bermúdez-Humarán L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, Langella P. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact. 2007;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, Cadiot G, Soulé JC, Bourreille A, Metman E. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, DeVos M, Enslen M, Paintin M, Franchimont D. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn's disease after lleo-caecal resection. Inflamm Bowel Dis. 2007;13:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Moon G, Myung SJ, Jeong JY, Yang SK, Cho YK, Lee SM, Chang HS, Byeon JS, Lee YJ, Lee GH. [Prophylactic effect of Lactobacillus GG in animal colitis and its effect on cytokine secretion and mucin gene expressions]. Korean J Gastroenterol. 2004;43:234-245. [PubMed] |
| 22. | Lian GH, Lu FG, Chen HH, You Y, Tan X, Qiu L. Effects of B.adolescentis and L.acidophilus in treating experimental ulcerative colitis in mice and their potential mechanisms. Zhonghua Xiaohua Zazhi. 2008;28:102-104. |
| 23. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 24. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin Exp Immunol. 1999;117:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 926] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 27. | Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzó J. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol. 2002;37:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Brown AC, Shovic A, Ibrahim SA, Holck P, Huang A. A non-dairy probiotic's (poi) influence on changing the gastrointestinal tract's microflora environment. Altern Ther Health Med. 2005;11:58-64. [PubMed] |
| 29. | Fabia R, Ar'Rajab A, Johansson ML, Willén R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155-162. [PubMed] [DOI] [Full Text] |
| 30. | Agius LM. A primary dysregulation in the immunoregulatory role of the intestinal mucosal epithelial cell in inflammatory bowel disease pathogenesis? Biology of inflammatory response as tissue pattern entities in Crohn's versus ulcerative colitis. J Theor Biol. 2004;227:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Woo PC, Fung AM, Lau SK, Yuen KY. Identification by 16S rRNA gene sequencing of Lactobacillus salivarius bacteremic cholecystitis. J Clin Microbiol. 2002;40:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Adawi D, Ahrné S, Molin G. Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol. 2001;70:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Daniel C, Poiret S, Goudercourt D, Dennin V, Leyer G, Pot B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol. 2006;72:5799-5805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, Simonet M, Daniel C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology. 2007;133:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Tessner TG, Cohn SM, Schloemann S, Stenson WF. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology. 1998;115:874-882. [PubMed] |
| 36. | Sakuraba H, Ishiguro Y, Yamagata K, Tagawa Y, Iwakura Y, Sekikawa K, Munakata A, Nakane A. Transforming growth factor-{beta} regulates susceptibility of epithelial apoptosis in murine model of colitis. Ann N Y Acad Sci. 2004;1029:382-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Dijkstra G, Moshage H, Jansen PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand J Gastroenterol Suppl. 2002;37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol. 2005;21:44-50. [PubMed] |
| 39. | Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: why, how, and when. Curr Opin Gastroenterol. 2003;19:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Sartor RB. Induction of mucosal immune responses by bacteria and bacterial components. Curr Opin Gastroenterol. 2001;17:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878-4882. [PubMed] |
| 42. | Peterson RL, Wang L, Albert L, Keith JC, Dorner AJ. Molecular effects of recombinant human interleukin-11 in the HLA-B27 rat model of inflammatory bowel disease. Lab Invest. 1998;78:1503-1512. [PubMed] |
| 43. | Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, Suzuki A, Sata M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |