Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2334
Revised: December 7, 2011
Accepted: December 14, 2011
Published online: May 21, 2012
AIM: To investigate the effect of the demethylating reagent 5-aza-2’-deoxycitidine (DAC) on telomerase activity in hepatocellular carcinoma (HCC) cell lines, SMMC-7721 and HepG2.
METHODS: The related gene expression in cell lines was examined by real-time reverse transcription-polymerase chain reaction and Western blotting analysis. The telomerase activity was examined by telomeric repeat amplification protocol-enzyme-linked immunosorbent assay and DNA methylation was determined by methylation-specific polymerase chain reaction.
RESULTS: The telomerase activity was significantly reduced in both cell lines treated with DAC, accompanied by downregulation of telomerase reverse transcriptase (hTERT). We also observed the effect of DAC on the methylation status of hTERT promoter and the expression of regulatory genes, such as c-myc, p15, p16, p21, E2F1, and WT1. The methylation status of hTERT promoter could be reversed in SMMC-7721 by DAC, but not in HepG2 cells. However, p16 expression could be reactivated by demethylation of its promoter, and c-Myc expression was repressed in both cell lines. Moreover, DAC could enhance the sensitivity to the chemotherapeutic agents, such as cisplatin, by induction of apoptosis of HCC cells.
CONCLUSION: The DAC exerts its anti-tumor effects in HCC cells by inhibiting the telomerase activity.
- Citation: Tao SF, Zhang CS, Guo XL, Xu Y, Zhang SS, Song JR, Li R, Wu MC, Wei LX. Anti-tumor effect of 5-aza-2'-deoxycytidine by inhibiting telomerase activity in hepatocellular carcinoma cells. World J Gastroenterol 2012; 18(19): 2334-2343
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2334
The demethylating reagent 5-aza-2’-deoxycitidine (DAC) inhibits DNA methyltransferases and reverses DNA methylation[1]. It has been found that DAC can inhibit cancer cell growth, particularly the leukemia cells, and it has been applied for the treatment of myelodysplastic syndromes. A phase 1 study was finished using DAC as an antineoplastic drug for hematopoietic malignancies[2,3]. However, the mechanisms underlying its anticancer activity and other biological effects are not fully understood. It is believed to reactivate genes, including the tumor suppressor genes p16, E-cadherin, and hMLH1 in cancer cells. Reactivation of these genes is associated with cell cycle arrest and apoptosis, which leads to inhibition of tumor cell growth[4]. However, the mechanisms of telomerase activity and telomerase reverse transcriptase (hTERT) down-regulated by DAC remain unclear. Telomerase is an RNA-dependent DNA polymerase that synthesizes telomeric DNA sequences and almost universally provides the molecular basis for tumor cell proliferative capacity[5]. Telomerase reactivation is a critical step in cellular immortality and carcinogenesis[6,7]. The enzyme consists of three major components: hTERT, telomerase associated protein (TEP1), and telomerase RNA (TERC)[8]. Transcriptional regulation of hTERT is believed to be the major mechanism of telomerase regulation in human cells. Transient transfection experiments with hTERT promoter-luciferase reporters showed that the hTERT promoter is inactive in normal and transformed preimmortal cells but, like telomerase, is activated in immortal cells[9]. Expression of hTERT was observed at high levels in malignant tumors and cancer cell lines, but not in normal tissues or telomerase-negative cell lines, and a strong correlation has been found between hTERT expression and telomerase activity in a variety of tumors[10,11]. These findings suggest that expression of hTERT might be a critical event in carcinogenesis. Thus, the mechanisms of hTERT activation are essential for understanding the molecular basis of telomerase activation and carcinogenesis. The hTERT promoter contains binding sites for many transcription factors that could be involved in its regulation. The abundance of these potential transcription factor binding sites was subjected to various factors in different cellular contexts[12,13]. Several transcription factors are known to participate in hTERT gene expression, including positive regulators: c-Myc, Sp1, human papillomavirus 16 E6, and steroid hormones; and negative regulators: Mad1, p53, p15, p16, p21, E2F, pRB, WT1, interferon-α, tumor growth factor-β and myeloid cell-specific zinc finger protein[14,15]. Our previous studies revealed that methylation status of P21, P15, P16, WTI and E2F-1 was significantly associated with cancer tissues in hepatocellular carcinoma (HCC)[16]. In the present study, we observed the effect of DAC on telomerase activity and hTERT expression in HCC cell lines, and found that DAC down-regulated the telomerase activity and hTERT expression by p16 promoter demethylation.
Human hepatoma cell lines SMMC-7721 and HepG2 were maintained in Dulbecco’s modified Eagle’s medium (high glucose) (Gibco, Invitrogen, United States) and supplemented with 10% fetal bovine serum (GIBCO, Invitrogen, United States), 100 units/mL penicillin, and 100 mg/mL streptomycin in a humidified incubator under 95% air and 5% CO2 at 37 °C. Cells from exponentially growing cultures were used in all the experiments. This study was approved by the Institutional Ethics Committee of the Second Military Medical University, China.
Human hepatoma cell lines (SMMC-7721 and HepG2) were seeded at a density of 5 × 105 cells per well in 6-well tissue culture plates and were allowed to attach over a 24-h period. The demethylating reagent DAC (Merk, Calbiochem, United States) was added to a final concentration of 1 μmol/L, 2 μmol/L and 4 μmol/L and the cells grew for 1 d, 3 d and 5 d. At indicated time intervals, cells were harvested by trypsinization and washed with phosphate-buffered saline.
Telomeric repeat amplification protocol-enzyme-linked immunosorbent assay (TRAP-ELISA) was performed using the telomerase kit Telo TAGGG Telomerase PCR ELISA PLUS (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Extracts from HEK293 cells were used as positive controls for the assay, and cell lysates heat-inactivated for 10 min at 85 °C were used as negative controls. Absorbance values were reported as the A450 nm reading.
RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA, United States). cDNA was synthesized using 2 μg total RNA and a moloney murine leukemia virus-reverse transcriptase kit with random hexamer primers and an RNase inhibitor (Takara Biotechnology Co. Ltd., Dalian, China). Polymerase chain reaction (PCR) amplifications of the respective genes were carried out with 40 ng complementary DNA, 500 nmol/L forward and reverse primer, and iTaqSYBRGreen Supermix (Bio-Rad Laboratories, Hercules, CA) in a final volume of 20 μL. Primer sequences and annealing temperature are summarized in Table 1. Real-time RT-PCR was performed on a Mastercycler cycler (Eppendorf, Hamburg, Germany), and all experiments were performed twice. Amplification of the housekeeping gene β-actin was performed to standardize the amount of sample RNA. Relative quantification of gene expression was performed by the -ΔΔct method.
| Gene | Primer sequence (5’-3’) | Annealing(°C) | Product size (bp) |
| hTERT-F | CGGAAGAGTGTCTGGAGCAA | 58 | 422 |
| hTERT-R | GGATGAAGCGGAGTCTGGA | ||
| c-myc-F | CTTCTCTCCGTCCTCGGATTCT | 65 | 132 |
| c-myc-R | GAAGGTGATCCAGACTCTGACCTT | ||
| p15-F | GAATGCGCGAGGAGAACAAG | 65 | 204 |
| p15-R | CCATCATCATGACCTGGATCG | ||
| p16-F | GCTGCCCACGCACCGAATA | 57 | 179 |
| p16-R | ACCACCAGCGTGTCCAGGAA | ||
| p21-F | GCAGACCAGCATGACAGATTT | 60 | 70 |
| p21-R | GGATTAGGGCTTCCTCTTGGA | ||
| WT1-F | GGCATCTGAGACCAGTGAGAA | 62 | 120 |
| WT1-R | GAGAGTCAGACTTGAAAGCAGT | ||
| E2F1-F | AGCTGGACCACCTGATGAAT | 60 | 95 |
| E2F1-R | GTCCTGACACGTCACGTAGG | ||
| β-actin-F | CCTGTACGCCAACACAGTGC | 60 | 275 |
| β-actin-R | ATACTCCTGCTTGCTGATCC | ||
| hTERT-MF | AGTTTTGGTTTCGGTTATTTTCGC | 58 | 122 |
| hTERT-MR | AACGTAACCAACGACAACACC | ||
| hTERT-UF | AGTTTTGGTTTTGGTTATTTTTGT | 58 | 132 |
| hTERT-UR | AACGTAACCAACGACAACACCT | ||
| p16-MF | TTATTAGAGGGTGGGGCGGATCGC | 56 | 150 |
| p16-MR | GACCCCGAACCGCGACCGTAA | ||
| p16-UF | TTATTAGAGGGTGGGGTGGATTGT | 56 | 151 |
| p16-UR | CAACCCCAAACCACAACCATAA |
The bisulfite modification of DNA was done as described previously[17]. DNA methylation was determined by methylation-specific PCR (MSP). Forty ng of bisulfite-modified DNA was subjected to PCR amplification. The PCR reaction mixture contained 2.5 μL of 10 × PCR buffer, 100 pmol of each primer, 2 mmol/L of each dNTPs, and 1 U of Hotstart Taq DNA polymerase (Takara Biotechnology Co. Ltd., Dalian, China) at a final volume of 25 μL. The PCR was performed in a thermal cycler. Primer sequences and reaction conditions are summarized in Table 1. DNA methylated by SssI methylase (Sss DNA) was used as positive control for methylated alleles.
HepG2 and SMMC-7721 cells were lysed in RIPA lysis buffer (Beyotime, China) with 1 mmol/L phenylmethanesulfonyl fluoride (PMSF). An equal amount of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the nitrocellulose membrane. After blocking with 5% nonfat milk, the membrane was probed with anti-hTERT (Santa Cruz, United States), developed with the BeyoECL Plus substrate system (Beyotime, China). Blots were stripped and re-probed with β-actin antibody (Santa Cruz, United States) to confirm equal protein loading.
The SMMC-7721 and HepG2 cells were seeded in 96-well plates and cultured with chemotherapeutic drugs and DAC for 3 d. The cells were examined by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL, Sigma) assay. The spectrophotometric absorbance was measured using a plate reader at 570 nm. The morphology of apoptosis was observed by 4’,6-diamidino-2-phenylindole (DAPI) staining. The cells were analyzed using a Facial Action Coding System (FACS) Aria flow cytometer (Becton Dickinson, San Jose, CA).
All of the experiments were repeated at least three times. The data were expressed as means ± SD. Statistical analysis was performed using Student’s t test (two tailed). P < 0.05 was considered statistically significant.
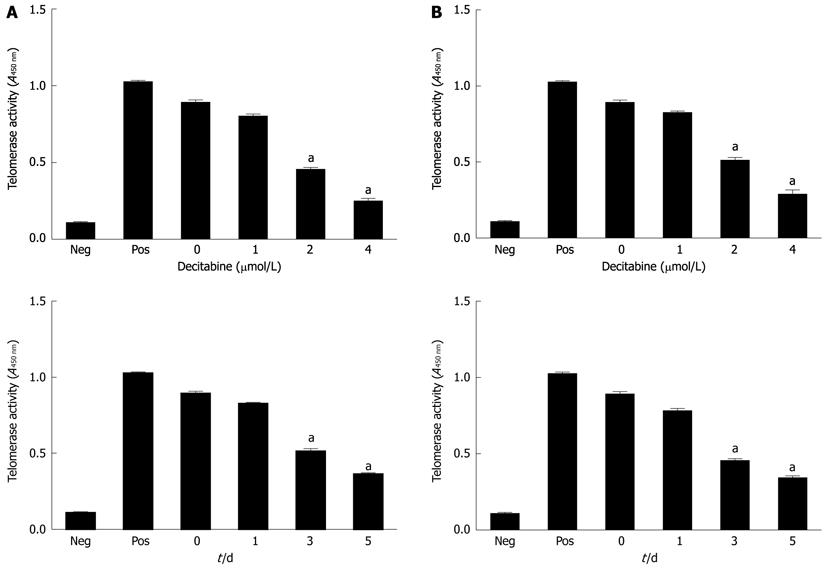
To investigate the effects of DAC on telomerase activity, SMMC-7721 and HepG2 were cultured with 1 μmol/L, 2 μmol/L and 4 μmol/L DAC. Telomerase activity was measured by TRAP-PCR-ELISA assay after 1 d, 3 d and 5 d of exposure to DAC. Inhibition of telomerase activity was observed in both cell lines in a dose-dependent manner, by maximal repression on day 3 at 4 μmol/L or day 5 at 2 μmol/L DAC (Figure 1). There was a 52.7% reduction of telomerase activity in SMMC-7721 cells treated with 4 μmol/L DAC for 3 d, and a 45.6% reduction of telomerase activity in HepG2 cells. The results revealed that the effect of DAC on telomerase activity varied in different cell lines.
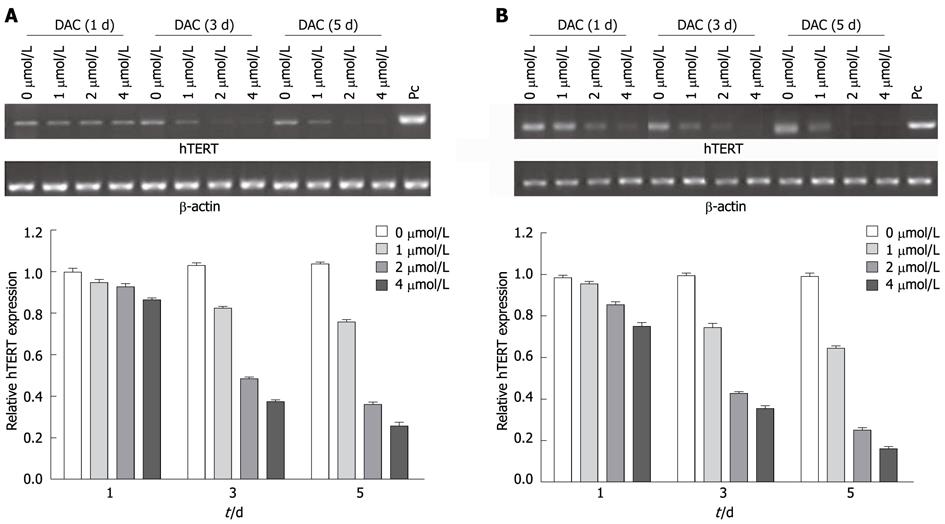
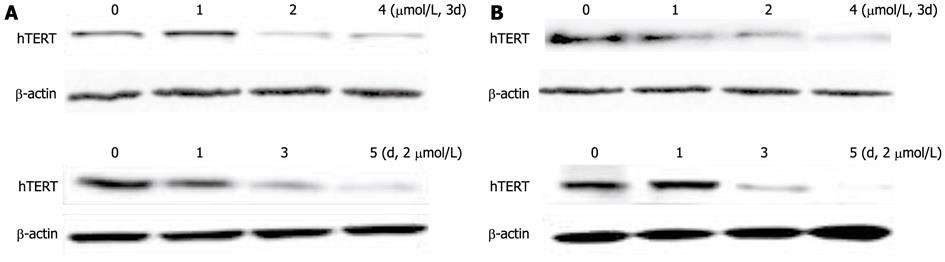
Since the expression of hTERT is closely associated with telomerase activity, we examined whether hTERT expression is suppressed in SMMC-7721 and HepG2 cells by DAC. The expression of hTERT mRNA in SMMC-7721 cells was decreased to 82% on day 1, 34% on day 3, and 26% on day 5 after DAC treatment (2 μmol/L) (Figure 2). A decline in hTERT mRNA was also detected in HepG2 cells treated with DAC. hTERT mRNA expression was maximally down-regulated by 4 μmol/L DAC. The complete down-regulation of hTERT mRNA became apparent on day 3 of treatment and maximal on day 5 in both cell lines. Furthermore, we treated SMMC-7721 and HepG2 cells with 1 μmol/L, 2 μmol/L, 4 μmol/L DAC respectively for 3 d and 2 μmol/L for 1 d, 3 d, 5 d, respectively, then detected the hTERT expression in protein level by Western blotting analysis (Figure 3). The hTERT protein in SMMC-7721 and HepG2 cells was also down-regulated by DAC in a dose- and time-dependent manner, with maximal repression at 4 μmol/L on day 5. The hTERT protein was notably suppressed in both HepG2 and SMMC-7721 cells after treated by 2 μmol/L DAC for 3 d; however, the effect was more significant in SMMC-7721 cells. These results were in accordance with hTERT mRNA expression. The results indicated that inhibition of telomerase activity in HCC cells treated with DAC may contribute to a striking decrease in hTERT mRNA and protein.
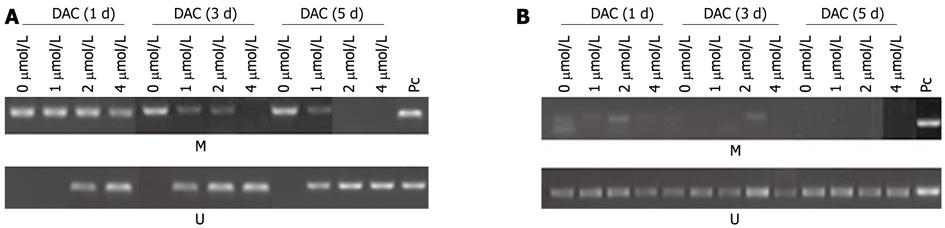
Since promoter methylation may be involved in hTERT repression in HCC cells, we observed the effects of DAC on promoter methylation of hTERT gene using MSP[18]. According to MSP analysis, the hTERT promoter was found to be hypermethylated in SMMC-7721, but not in HepG2 cells (Figure 4). The demethylation of hTERT was found in SMMC-7721 cells treated with DAC in a dose- and time-dependent manner, and there was complete demethylation after treatment with 5 μmol/L DAC for 3 d or 2 μmol/L for 3 d (Figure 4A). However, DAC showed no effects on hTERT methylation in HepG2 cells (Figure 4B). These data suggested that the demethylation of hTERT promoter by DAC could not play an important role in down-regulation of hTERT expression.
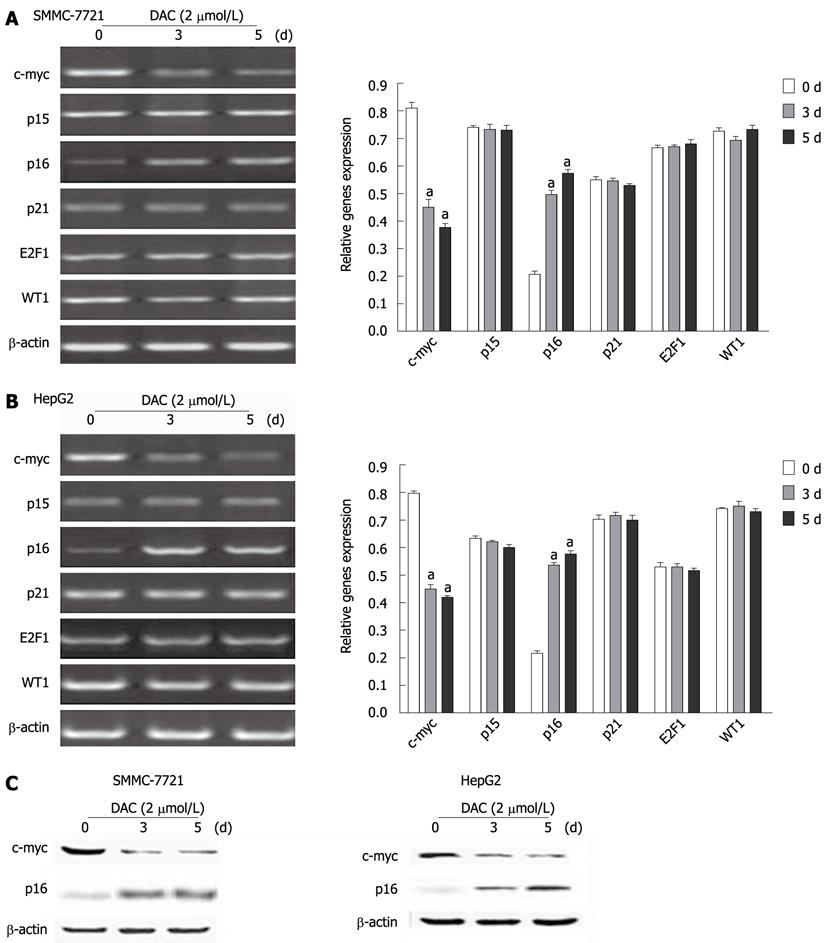
We focused on some regulatory genes of hTERT transcription, such as c-myc, p15, p16, p21, E2F-1, WT1. Using real-time PCR, we examined the mRNA expression of these genes during exposure to DAC at 2 μmol/L for 1 d, 3 d and 5 d. The data showed that c-myc had high expression while p16 had low expression (SMMC-7721) or lost expression (HepG2) in hepatoma cells, and the other genes showed different levels of expression (Figure 5). The down-regulation of c-myc and up-regulation of p16 mRNA expression were found in HCC cell lines treated with DAC. Western blotting analyses further revealed the significant levels of c-myc and p16 in both SMMC-7721 and HepG2 cells. These results suggested that c-myc and p16 could play an important role in down-regulating the hTERT expression by DAC.
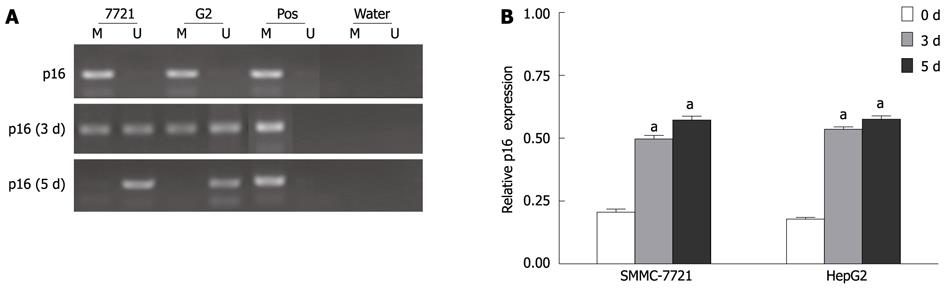
We reproduced the methylation pattern of p16 (Figure 6) using MSP. With DAC treatment, we detected increased demethylation of p16 promoter. The data suggested that promoter hypermethylation could causatively contribute to transcriptional silencing of p16, which up-regulated the hTERT transcription in HCC cells.
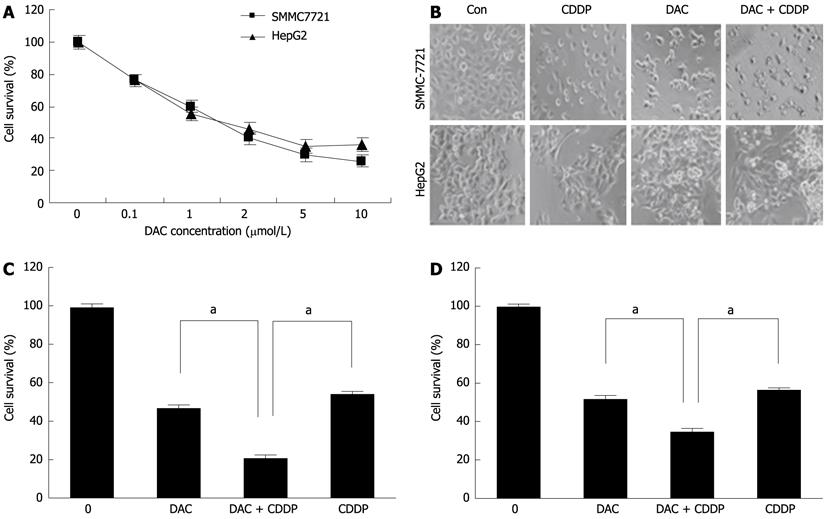
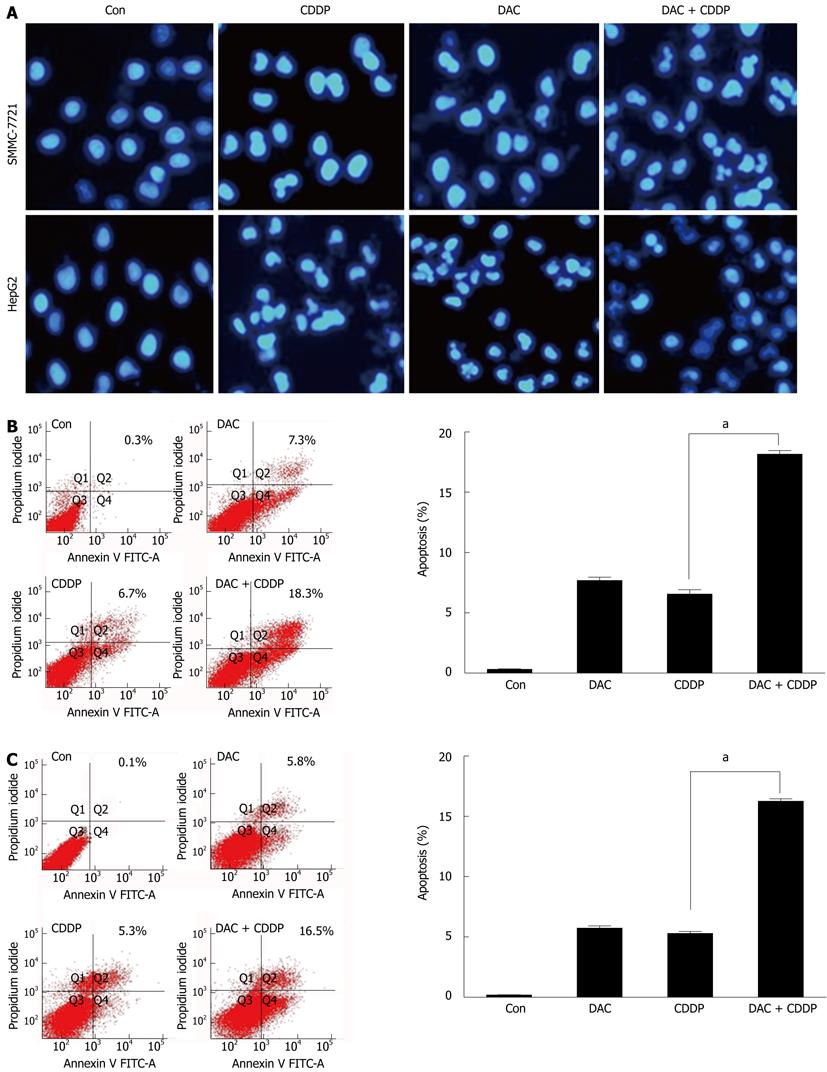
To determine whether DAC could effectively inhibit HCC cell growth, we treated SMMC-7721 and HepG2 cells with various doses of DAC for 72 h. We found that DAC inhibited the growth of cell lines in a dose-dependent manner (Figure 7). To determine whether the growth inhibition by DAC can be enhanced by chemotherapeutic agents, SMMC-7721 and HepG2 cells were treated with DAC in combination with cisplatin. Notably, while dramatic morphological changes were caused by combination of DAC with cisplatin, many cells revealed detachment and shrinkage. The growth inhibition was about 76% in the cells treated with DAC in combination with cisplatin (20 μmol/L) as compared with 54% in the cells treated with cisplatin alone, or 48% treated with DAC alone. These data suggested that DAC could enhance the sensitivity of HCC cells to chemotherapeutic agents such as cisplatin. With the administration of cisplatin and DAC, more cells revealed nuclear condensation and fragmentation of apoptotic cell death. These results were confirmed by Annexin-V and propidium iodide staining and FACS analysis (Figure 8). The cell apoptosis was significantly enhanced by the combined administration of 2 μmol/L DAC and 20 μmol/L cisplatin. These results suggested that the enhanced effects of the combined treatment on cell death were attributed to the augmented induction of apoptosis.
The most widely used demethylating agent, DAC, was first characterized 30 years ago and it functions as a mechanism-dependent suicide inhibitor of DNA methyltransferases, with which genes silenced by hypermethylation can be reactivated[19]. It has been found that telomerase activity in cancer cells was inhibited by differentiation, inducing demethylating agent DAC. We demonstrated that telomerase activity could be inhibited by DAC in HCC cells (HepG2 and SMMC-7721), and the demethylation of p16 promoter could play an important role.
Telomerase reactivation is a critical step in cellular immortality and carcinogenesis and is considered as a target for cancer treatment[8]. Our lab revealed that more than 85% HCC had much stronger telomerase activity than cirrhosis[16]. Drug-induced cell killing of tumor cells is associated with a decline in detectable telomerase activity[20]. In the present study, DAC inhibited telomerase activity and down-regulated hTERT expression in both human hepatoma cell lines SMMC-7721 and HepG2, and DAC suppressed the transcriptional activity of hTERT genes. Targeting telomerase activity is one of the mechanisms responsible for this reagent’s inhibition of cancer cell growth.
On one hand, promoter methylation was associated with transcriptional silencing of the hTERT gene, as treatment of cells with demethylating agent DAC resulted in an increase in hTERT transcription in an immortal fibroblast line SUSM-1[21]. On the other hand, DNA hypermethylation was implicated in the positive regulation of the hTERT promoter because demethylation in several telomerase-positive tumor cell lines reduced hTERT expression and telomerase activity accompanied by telomere shortening[22,23]. Our present study showed that hTERT promoter was methylated in SMMC-7721 cells, but not in HepG2 cells, and almost complete demethylation of hTERT promoter only occurred in the former cell line after DAC treatment. The explanation that CpG methylation likely interfered with the binding of transcriptional repressors, thereby positively regulating the hTERT promoter, may be reasonable for SMMC-7721 but not for HepG2. These findings suggested that hTERT promoter methylation is not the sole regulator of hTERT gene expression in HCC cells treated with DAC.
Several transcription factors are known to be responsible for the regulation of hTERT expression, including c-myc, p21, p16, p15, E2F-1 and Wilms’ Tumor 1 suppressor gene[24]. This evidence prompted us to examine the effect of DAC on the expression of these genes. The c-myc was over-expressed while p16 expression was low or lost in hepatoma cell lines, and DAC repressed c-myc expression while reactivating p16. c-myc plays a critical role in telomerase activation through up-regulating the hTERT transcription, and this could be one mechanism by which 5-aza-CR represses hTERT transcription. Inactivation of p16-dependent pathways possibly in conjunction with telomerase activation might be a critical step for immortalization[25]. Our findings indicated that up-regulation of p16 and subsequent down-regulation of c-myc could be major pathways for hTERT repression by DAC.
Several evidences indicated that p16 expression could be transcriptionally silenced by CpG island hypermethylation in HCC[26]. The absence of expression and promoter methylation of p16 suggested that aberrant methylation is a major mechanism of the inactivation of p16 expression in HCC. Our data showed that DAC reversed p16 promoter methylation status and reactivated its expression, suggesting that p16 plays an important role in the down-regulation of telomerase activity by DAC.
In conclusion, the demethylating reagent 5-aza-CR represses telomerase activity and down-regulates hTERT expression. p16 could play a key role in this regulation. Our findings may provide insights into one of the mechanisms through which 5-aza-CR exerts growth-inhibitory effects on HCC cells.
The inactivation of tumor suppressor genes by aberrant DNA methylation plays an important role in the development of malignancies. An inhibitor of DNA methylation, 5-aza-2’-deoxycitidine (DAC), could inhibit telomerase activity in some cancer cell lines, but the molecular mechanism remains unclear.
DAC can inhibit cancer cell growth, particularly leukemia cells, and it has been applied for the treatment of myelodysplastic syndromes. Targeting telomerase activity is one of the mechanisms responsible for this reagent’s inhibition of cancer cell growth.
The authors demonstrated that the demethylating reagent DAC represses telomerase activity and down-regulates telomerase reverse transcriptase (hTERT) expression. p16 could play a key role in this regulation.
By understanding the mechanism of DAC repressing telomerase activity, this study may represent a future strategy for the treatment of patients with hepatocellular carcinoma (HCC).
Telomerase and hTERT is a critical step in cellular immortality and carcinogenesis and is considered as a target for cancer treatment. And several transcription factors such as c-myc and p16 were responsible for the regulation of hTERT expression. The DNA methylation could play an important role in telomerase activity.
This is a nice paper that describes the role of an inhibitor of DNA methylation DAC on telomerase expression and activity in HCC cell lines. Work has been well designed, effects have been proved in more than one HCC cell lines and in general terms, conclusions are supported by the presented results.
Peer reviewer: Isabel Fabregat, PhD, Associate Professor, Laboratori d’Oncologia Molecular, Institut d’Investigación Biomèdica de Bellvitge, Gran Via, Km 2,7, L’Hospitalet, 08907 Barcelona, Spain
S- Editor Shi ZF L- Editor Ma JY E- Editor Zhang DN
| 1. | Patra SK, Bettuzzi S. Epigenetic DNA-(cytosine-5-carbon) modifications: 5-aza-2'-deoxycytidine and DNA-demethylation. Biochemistry (. Mosc). 2009;74:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Fenaux P, Ades L. Review of azacitidine trials in Intermediate-2-and High-risk myelodysplastic syndromes. Leuk Res. 2009;33 Suppl 2:S7-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Buckstein R, Yee K, Wells RA. 5-Azacytidine in myelodysplastic syndromes: a clinical practice guideline. Cancer Treat Rev. 2011;37:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2210] [Cited by in RCA: 2201] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 5. | Altshuler ML, Severin SE, Glukhov AI. The tumor cell and telomerase. Biochemistry (. Mosc). 2003;68:1275-1283. [PubMed] |
| 6. | Oh BK, Kim H, Park YN, Yoo JE, Choi J, Kim KS, Lee JJ, Park C. High telomerase activity and long telomeres in advanced hepatocellular carcinomas with poor prognosis. Lab Invest. 2008;88:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Hytiroglou P, Theise ND. Telomerase activation in human hepatocarcinogenesis. Am J Gastroenterol. 2006;101:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5233] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 9. | Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551-557. [PubMed] |
| 10. | Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558-1561. [PubMed] |
| 11. | Ito H, Kyo S, Kanaya T, Takakura M, Inoue M, Namiki M. Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res. 1998;4:1603-1608. [PubMed] |
| 12. | Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 365] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Bazarov AV, Hines WC, Mukhopadhyay R, Beliveau A, Melodyev S, Zaslavsky Y, Yaswen P. Telomerase activation by c-Myc in human mammary epithelial cells requires additional genomic changes. Cell Cycle. 2009;8:3373-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407-25, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 15. | Liu YC, Chen CJ, Wu HS, Chan DC, Yu JC, Yang AH, Cheng YL, Lee SC, Harn HJ. Telomerase and c-myc expression in hepatocellular carcinomas. Eur J Surg Oncol. 2004;30:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhang C, Guo X, Jiang G, Zhang L, Yang Y, Shen F, Wu M, Wei L. CpG island methylator phenotype association with upregulated telomerase activity in hepatocellular carcinoma. Int J Cancer. 2008;123:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Zhang C, Xu Y, Zhao J, Fan L, Jiang G, Li R, Ling Y, Wu M, Wei L. Elevated expression of the stem cell marker CD133 associated with Line-1 demethylation in hepatocellular carcinoma. Ann Surg Oncol. 2011;18:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Iliopoulos D, Satra M, Drakaki A, Poultsides GA, Tsezou A. Epigenetic regulation of hTERT promoter in hepatocellular carcinomas. Int J Oncol. 2009;34:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Watanabe Y, Maekawa M. Methylation of DNA in cancer. Adv Clin Chem. 2010;52:145-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Faraoni I, Turriziani M, Masci G, De Vecchis L, Shay JW, Bonmassar E, Graziani G. Decline in telomerase activity as a measure of tumor cell killing by antineoplastic agents in vitro. Clin Cancer Res. 1997;3:579-585. [PubMed] |
| 21. | Devereux TR, Horikawa I, Anna CH, Annab LA, Afshari CA, Barrett JC. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 1999;59:6087-6090. [PubMed] |
| 22. | Guilleret I, Yan P, Grange F, Braunschweig R, Bosman FT, Benhattar J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int J Cancer. 2002;101:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Guilleret I, Benhattar J. Demethylation of the human telomerase catalytic subunit (hTERT) gene promoter reduced hTERT expression and telomerase activity and shortened telomeres. Exp Cell Res. 2003;289:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Shamanin VA, Androphy EJ. Immortalization of human mammary epithelial cells is associated with inactivation of the p14ARF-p53 pathway. Mol Cell Biol. 2004;24:2144-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Bhatia B, Jiang M, Suraneni M, Patrawala L, Badeaux M, Schneider-Broussard R, Multani AS, Jeter CR, Calhoun-Davis T, Hu L. Critical and distinct roles of p16 and telomerase in regulating the proliferative life span of normal human prostate epithelial progenitor cells. J Biol Chem. 2008;283:27957-27972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |