Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2253
Revised: February 6, 2012
Accepted: February 16, 2012
Published online: May 14, 2012
AIM: To investigate the relationship and molecular features of CD74/macrophage migration inhibitory factor (MIF)/Toll-like receptor 4 (TLR4) in gastric cancer.
METHODS: CD74, MIF and TLR4 expression in the paraffin-embedded sections of gastric cancer from 120 patients were detected by immunohistochemical staining. Knock down of CD74 expression in gastric cancer cell line MKN-45 was performed by lentivirus transduction and detected by Western blotting. MKN-45 cell proliferation assay under the stimulants was measured by the cell counting kit 8 (CCK8) assay and MIF concentration in the culture medium was detected by enzyme-linked immunosorbent assay. Surface staining of CD74 in the MKN-45 cell line under the stimulation of lipopolysaccharide (LPS) was measured by flow cytometry. MIF, CD74 and TLR4 co-localization in the MKN-45 cell line was performed by the immunoprecipitation.
RESULTS: CD74, MIF and TLR4 were found to be expressed in gastric cancer and increased significantly in the advanced stage, and were also associated with lymph node metastasis. Correlation analysis revealed that CD74 was positively correlated with MIF (r = 0.2367, P < 0.01) and both proteins were also associated with TLR4 (r = 0.4414, r = 0.5001, respectively, P < 0.01). LPS can significantly promote MKN-45 cell proliferation (3.027 ± 0.388 vs 4.201 ± 0.092, P < 0.05), induce MIF production (54.333 ± 2.906 pg/mL vs 29.667 ± 3.180 pg/mL, P < 0.01) and cell surface expression of CD74 (75.6% ± 4.046% vs 9.4% ± 0.964%, P < 0.01) at LPS concentration of 1 μg/mL compared to medium control. Knockdown of CD74 or using anti-CD74 and MIF antagonist ISO-1 significantly reduced LPS-induced MKN-45 cell proliferation (4.201 ± 0.092 vs 3.337 ± 0.087, 4.534 ± 0.222 vs 3.368 ± 0.290, 4.058 ± 0.292 vs 2.934 ± 0.197, respectively, P < 0.01). MIF, CD74 and TLR4 could co-localize in the MKN-45 cell line.
CONCLUSION: Upregulation of MIF, CD74 and TLR4 are associated with increasing clinical stage and provide an opportunity as novel gastric cancer chemoprevention and/or treatment strategy.
- Citation: Zheng YX, Yang M, Rong TT, Yuan XL, Ma YH, Wang ZH, Shen LS, Cui L. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J Gastroenterol 2012; 18(18): 2253-2261
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2253.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2253
CD74 is a transmembrane glycoprotein that associates with MHC II, and is an important chaperone that regulates antigen presentation for the immune response. CD74 is expressed at high levels by antigen-presenting cells (APCs), including B cells, monocytes, macrophages and dendritic cells in normal tissues[1,2]. Although cell surface expression of CD74 is low in many cell types, rapid internalization with concomitant re-expression at the cell surface provides a steady state level of CD74-MHC II complex at the cell surface that is sufficient for biological function[3]. More recently, CD74 expression has been examined in cell types other than APCs, such as epithelial cells, and is particularly important in the complex immunological mechanisms and in the link between chronic inflammation and carcinogenesis in the gastrointestinal tract[4]. Substantial evidence has demonstrated that CD74 protein is upregulated in cancer cells, indicating its role in tumorigenesis and angiogenesis[5]. The contribution of CD74 to carcinogenesis is multifaceted. High levels of CD74 expression associated with class II MHC expression might prevent tumor antigen presentation by blocking the peptide binding cleft and preventing antigenic peptide binding for presentation to T cells, rendering tumors less immunogenic[6]. In addition, CD74 is the receptor for macrophage migration inhibitory factor (MIF), which, when bound to CD74, initiates survival pathways and cell proliferation[7,8] and facilitates adhesion of Helicobacter pylori to gastric epithelial cells (GECs)[9,10].
MIF is an upstream activator of innate immunity that regulates subsequent adaptive responses. In addition to its roles in inflammation and immunity, recent studies have shown that MIF contributes to tumorigenesis. MIF is overexpressed in several tumors including breast cancer, gastric cancer, lung cancer, hepatocellular carcinoma, and cervical cancer[11-15]. MIF binding to CD74 might contribute to carcinogenesis in chronic conditions through the upregulation of proinflammatory cytokines, including interleukin (IL)-8, which upregulates CD74 and has its own mechanisms leading to increased proliferation, tumor growth, and angiogenesis[16]. MIF binding to CD74 affects proliferation and cell cycle events, including antagonism of p53, inhibition of retinoblastoma function, and activation of Akt[17]. This combination of properties suggests that MIF may play a pivotal role in tumor biology.
Pattern-recognition receptors such as Toll-like receptors (TLRs) act as sensors that detect microbial infections and induce a proinflammatory response[18]. TLRs are a family of mammalian homologs of the Drosophila Toll proteins and they recognize pathogen-associated molecular patterns that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity. In mammalian systems, TLR4 confers responsiveness to Gram-negative lipopolysaccharide (LPS), induces cyclo-oxygenase (COX)2, and is important for proliferation and apoptosis in response to gastrointestinal injury[19].
In previous studies, it has been reported that CD74 and MIF are upregulated in gastric cancer[12,20]. However, how CD74 and MIF are elevated in gastric cancer remains unclear. The relationship between MIF/CD74/TLR4 expression by the tumor and clinicopathological factors in gastric carcinoma needs to be further demonstrated. In this study, we examined CD74, MIF and TLR4 expression in gastric cancer and analyzed their correlations with clinicopathological factors. Also, we used the gastric cancer epithelial cell line MKN-45 to confirm that, under LPS stimulation, MIF production and surface CD74 expression increased, thus promoting cell proliferation. These results suggest that the MIF/CD74 pathway may greatly induce gastric tumorigenesis in infection.
One hundred and twenty patients with gastric cancer, who underwent surgery at Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China, were included in this study. Prior to sample collection, appropriate permission was granted from the research ethical committee of Xinhua Hospital. The surgical specimens were fixed in formalin and embedded in paraffin before they were archived. For immunohistochemical staining, paraffin-embedded sections were deparaffinized in xylene and hydrated in 95%, 85%, 75% and 50% ethanol sequentially. Antigens were retrieved by heating for 15 min with 10 mmol citrate buffer (pH 6.0) in a microwave oven. The sections were incubated with 3% hydrogen peroxide to quench endogenous tissue peroxidase activity, and normal goat serum was used as the blocking agent (DakoCytomation, Glostrup, Denmark). The sections were then incubated with CD74 monoclonal antibody (mAb) (1/200 dilution; clone LN2; BD Pharmingen) or MIF Ab (1/100 dilution; clone 2A10-4D3; Sigma-Aldrich) or TLR4 antibody (1/100 dilution; clone 76B357.1; Abcam) at 4 °C overnight. Affinity-purified goat anti-mouse IgG conjugated with peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as secondary antibody. The sections were developed using the liquid diaminobenzidine-substrate chromogen system (DakoCytomation). CD74, MIF and TLR4 expression was separately assessed by two observers who were blinded to the clinical data. CD74 expression was evaluated based on Ishigami’s classification[12], by which, according to the percentage of positive cells, cases were divided into two groups: negative, CD74-positive cells < 10%, and positive, CD74-positive cells ≥ 10%. MIF and TLR4 staining was evaluated as follows: -: undetectable; +: weakly positive; ++: moderately positive; and +++: strongly positive.
The gastric epithelial cell line MKN-45 was obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco) in 5% CO2 at 37 °C.
Four different shRNA sequences for CD74 (NM_001025158.1) were purchased (GeneChem, Shanghai, China). These sequences were inserted into the pGCSIL-green fluorescent protein (GFP) plasmid (Takara Bio Inc, Otsu, Shiga, Japan) and transformed in Escherichia coli for propagation. Purified shRNA plasmids were then used to generate lentiviral particles by expressing them together with a gag-pol-env-encoding plasmid in a HEK293T packaging cell line. Centrifuged cell culture supernatants that contained lentivirus particles were used to infect MKN-45 cells. On day 5, GFP+ cells were sorted by FACSAria (BD Biosciences, NJ, USA) to > 98% purity. Cells that had been infected with the lentiviral shRNA that gave rise to the strongest CD74 knockdown (target sequence: GCATGAAGCTTCCCAAGCCTC) were cultured and used for further experiments.
For surface staining of CD74 in the MKN-45 cell line, cells were harvested and washed with PBS supplemented with 2% FBS. Mouse anti-human FITC-conjugated CD74 and isotype control (BD Biosciences) were used and cultured at 4 °C for 30 min, after two washes and detected by flow cytometry (BD Biosciences).
Two nanograms of recombinant MIF (rMIF) (R&D Systems, Mineapolis, MN, USA) was added to MKN-45 cell lysates, which were rotated for 2 h at 4 °C. Lysate mixtures were precleared with protein A/G beads (GE Healthcare, Pittsburgh, PA, USA) for 2 h at 4 °C. MIF was immunoprecipitated using protein A/G beads that were preincubated with anti-MIF mAb (R&D Systems) for 2 h at room temperature. After washing, beads were incubated with the lysate mixture of MIF and cell lysates. Beads were then washed four times and the bound material was eluted for immunoblotting.
GFP+ MKN-45 cell lysates or eluted antigens were subjected to 10% SDS-PAGE. Immunoblot analysis was performed by transfer of proteins onto nitrocellulose membranes (Schleicher and Schuell Microscience, Dassel, Germany) using a mini Trans-Blot apparatus (Bio-Rad, Hercules, CA, USA). After 2 h blocking, the membranes were incubated overnight at 4 °C with anti-human CD74 (clone EPR4064; Origene, Rockville, MD, USA), anti-human TLR4 (clone 76B357.1; Abcam) specific antibody, and β-actin antibody (Sigma-Aldrich, St Louis, MO, USA). After washing, subsequent incubation with appropriate horseradish-peroxidase-conjugated secondary Antibodies for 1 h at room temperature, and extensive washing, signals were visualized by ECL substrate (Pierce Chemical, Rockford, IL, USA).
Approximately 104 cells/well were grown in 96-well microtiter plates and incubated overnight in 200 μL culture medium. Cells were starved without FCS overnight at 80%-90% confluence and then treated with recombinant human MIF (R&D Systems) and LPS (Sigma-Aldrich) at different concentrations, with or without 2 h pretreatment with ISO-1 [(S,R)-3-(4-hydroxyphenyl)-4,5- dihydro-5-isoxazole acetic acid methyl ester] at 100 nmol (CalBiochem, Darmstadt, Germany), or anti-CD74 5 μg/mL (C-16; Santa Cruz Biotechnology) and isotype control (BD Biosciences). Cells without any treatment were used as controls. After 24 h culture, OD was measured using the microplate computer software (Bio-Rad Laboratories) according to the protocol of the CCK8 assay kit (Dojindo, Kumamoto, Japan).
MKN-45 cells were cultured in 96-well plates and stimulated with the LPS at different concentrations for 24 h. Supernatants from wells were used to quantitate the production of MIF by enzyme-linked immunosorbent assay. The MIF enzyme-linked immunosorbent assay kit was obtained from R&D Systems, and assays were performed according to the manufacturer’s instructions.
Data are expressed as the mean ± SD. Comparison of any two groups was performed by Student’s t test, and one-way ANOVA was performed for multiple comparisons. CD74, MIF and TLR4 protein expression related to clinicopathological parameters was tested using the Mann-Whitney U test and Kruskal-Wallis ANOVA. The relationship between immunohistochemistry scores for CD74, MIF and TLR4 was explored using Spearman’s correlation coefficient. Statistical significance was assumed if the P value was < 0.05. All analyses were performed using SPSS v14.0.
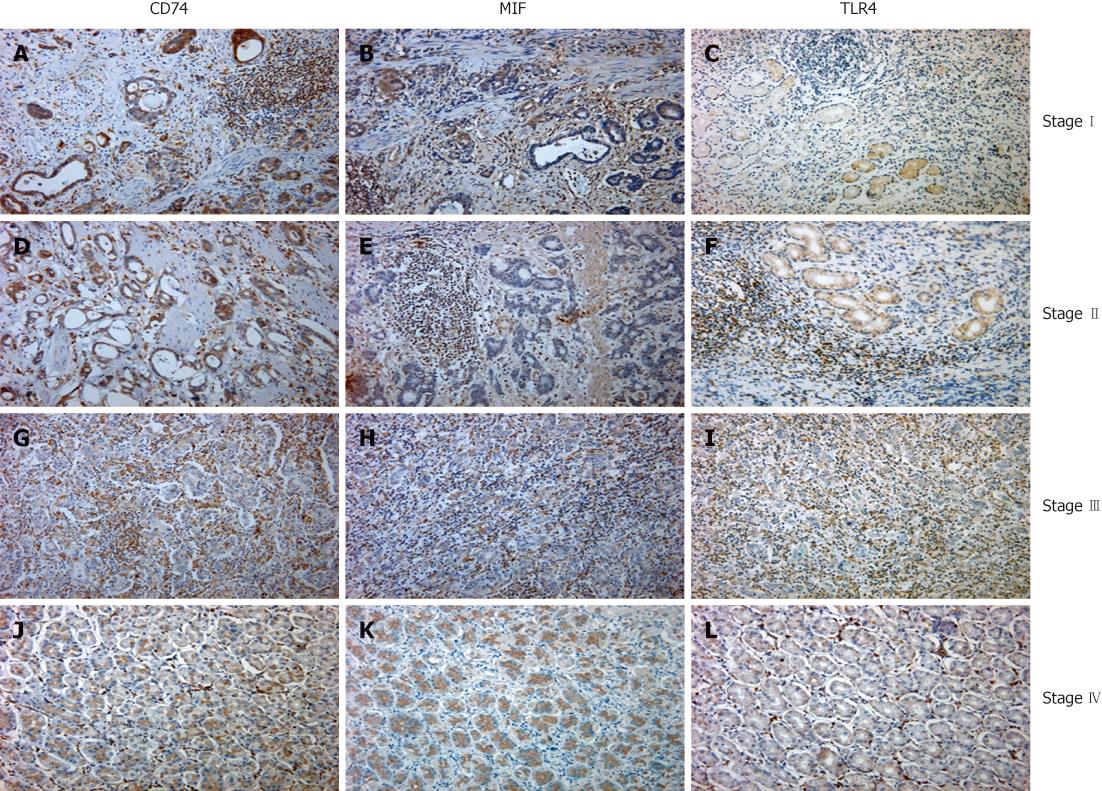
We routinely collected tissue specimens from patients undergoing surgical operation of known gastric cancer and cut 4-μm-thick sections to stain for the presence of CD74, MIF and TLR4 in the adjacent sections. We stained a total of 120 specimens. CD74, MIF and TLR4 immunoreactivity was identified on the surface of the tumor cells. Some populations of tumor-infiltrating lymphocytes were also immunopositive for those markers (Figure 1). Positive antigen expression of CD74 was observed in 100 of 120 specimens (81%), with the overwhelming majority of CD74-positive specimens with localization of the marker to the apical and perinuclear region of the cytoplasm (Figure 1A, D, G and J and Table 1). There was no difference in CD74 scores between adenocarcinoma from patients aged above or below 60 years (Table 1, P = 0.5969). Also, there was no difference between male and female patients (Table 1, P = 0.9910) and cell differentiation (P = 0.3565). However, a significant difference in CD74 scores between adenocarcinoma with different clinical stage was observed (Table 1, P = 0.0141) and lymph node metastasis (P = 0.0158).
| Variables | No. | CD74 expression | P | MIF expression | P | TLR4 expression | P | |||
| Cases | Positive | Negative | Positive | Negative | Positive | Negative | ||||
| Age (yr) | 0.5959 | 0.8612 | 0.6421 | |||||||
| < 60 | 46 | 40 (87) | 6 (13) | 38 (83) | 8 (17) | 38 (83) | 8 (17) | |||
| > 60 | 74 | 60 (81) | 14 (19) | 59 (80) | 15 (20) | 61 (82) | 13 (18) | |||
| Sex | 0.9910 | 0.5817 | 0.6358 | |||||||
| Male | 75 | 61 (81) | 14 (19) | 60 (80) | 15 (20) | 60 (80) | 15 (20) | |||
| Female | 45 | 39 (87) | 6 (13) | 37 (82) | 8 (18) | 39 (87) | 6 (13) | |||
| Histological type | 0.3565 | 0.8440 | 0.2172 | |||||||
| Well | 20 | 16 (80) | 4 (20) | 16 (80) | 4 (20) | 17 (85) | 3 (15) | |||
| Moderate | 40 | 30 (75) | 10 (25) | 33 (83) | 7 (17) | 30 (75) | 10 (25) | |||
| Poor | 60 | 54 (90) | 6 (10) | 48 (80) | 12 (20) | 52 (87) | 18 (13) | |||
| TNM stage | 0.0141 | 0.0281 | 0.0153 | |||||||
| I | 26 | 16 (62) | 10 (38) | 17 (65) | 9 (35) | 17 (50) | 9 (50) | |||
| II | 28 | 20 (71) | 8 (29) | 22 (79) | 6 (21) | 20 (57) | 8 (43) | |||
| III | 33 | 29 (88) | 4 (12) | 28 (85) | 5 (15) | 30 (67) | 3 (33) | |||
| IV | 33 | 31 (94) | 2 (6) | 30 (91) | 3 (19) | 32 (85) | 1 (15) | |||
| Lymph node metastasis | 0.0158 | 0.0251 | 0.0152 | |||||||
| Negative | 50 | 37 (74) | 13 (26) | 36 (72) | 14 (28) | 33 (66) | 17 (34) | |||
| Positive | 70 | 63 (90) | 7 (10) | 61 (87) | 9 (13) | 66 (94) | 4 (6) | |||
Immunohistochemical staining also showed that MIF and TLR4 were primarily localized in the cytoplasm and occasionally on the membrane or nuclei of GECs (Figure 1). The positive staining of MIF and TLR4 was observed in 97 (81%) and 99 (83%) respectively of 120 gastric cancer, and the representative example of positive staining in each stage was shown in Figure 1. Like the CD74 staining, there was no difference in age, sex, and cell differentiation, but there were significant in clinical stage and lymph node metastasis (Table 1).
The function of cell surface CD74 as a receptor for MIF provided the rationale for dual analysis of CD74 and MIF immunoreactivity in gastric cancer. A combined MIF and CD74 epithelial score might have a higher predictive value than either parameter alone. Table 2 shows the distribution of CD74 and MIF epithelial staining. There was a significant correlation between MIF and CD74 epithelial scores in individual adenocarcinomas (r = 0.2367, P < 0.01). TLR4 engagement by ligands such as bacterial LPS leads to proinflammatory cytokine production. Furthermore, from the correlation analysis, we observed that TLR4 had a significant correlation with CD74 (r = 0.4414, P < 0.01) and MIF (r = 0.5501, P < 0.01) (Table 2), which suggests that chronic inflammation might have an important association with gastric carcinogenesis.
| MIF expression | r | P | TLR4 expression | r | P | TLR4 expression | r | P | |||||||||
| CD74 | (+) | (-) | Total | 0.2367 | < 0.01 | CD74 | (+) | (-) | Total | 0.4414 | < 0.01 | MIF | (+) | (-) | Total | 0.5501 | < 0.01 |
| (+) | 85 | 15 | 100 | (+) | 90 | 10 | 100 | (+) | 89 | 8 | 97 | ||||||
| (-) | 12 | 8 | 20 | (-) | 9 | 11 | 20 | (-) | 10 | 13 | 23 | ||||||
| Total | 97 | 23 | 120 | Total | 99 | 21 | 120 | Total | 99 | 21 | 120 | ||||||
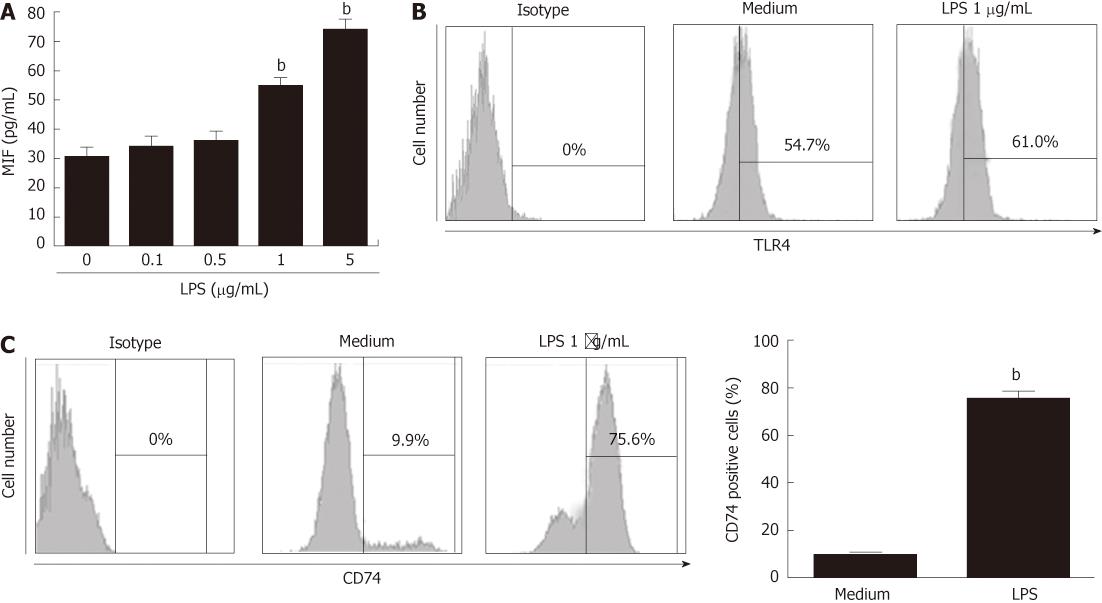
As with immunohistochemical staining, TLR4, CD74 and MIF were highly correlated with the tumor stage and lymph node metastasis, thus, we sought to determine MIF production or CD74 expression by GECs in response to LPS stimulation. Gastric epithelial cell line MKN-45 was cultured in 96-well plates and stimulated with LPS (0.1, 0.5, 1 and 5 μg/mL) for 24 h. LPS significantly induced MIF production (54.333 ± 2.906 pg/mL vs 29.667 ± 3.180 pg/mL, P < 0.01) at a concentration of 1 μg/mL (Figure 2A), suggesting that under conditions of inflammation, such as Gram-negative infection, MIF can be induced. MKN-45 cell line expressed high amounts of TLR4, but LPS stimulation did not significantly induce TLR4 expression (Figure 2B). Although immunohistochemistry confirmed the presence of MIF receptor in gastric tumors, for extracellular MIF signaling to be mediated by CD74 in vivo, it must be present on the cell surface. Therefore, we analyzed the MKN-45 cell line with LPS stimulation, to determine whether surface expression of CD74 was present. We detected CD74 by flow cytometry and revealed a detectable but low level of surface CD74 expression. Stimulation with 1 μg/mL LPS for 24 h increased surface expression of CD74 from the basal level of 9.4% ± 0.964% to 75.6 ± 4.046% (P < 0.01) (Figure 2C), suggesting that surface CD74 expression by GECs is dependent on LPS stimulation. LPS stimulation can greatly induce MIF and surface CD74 expression and enhance the MIF/CD74 pathway.
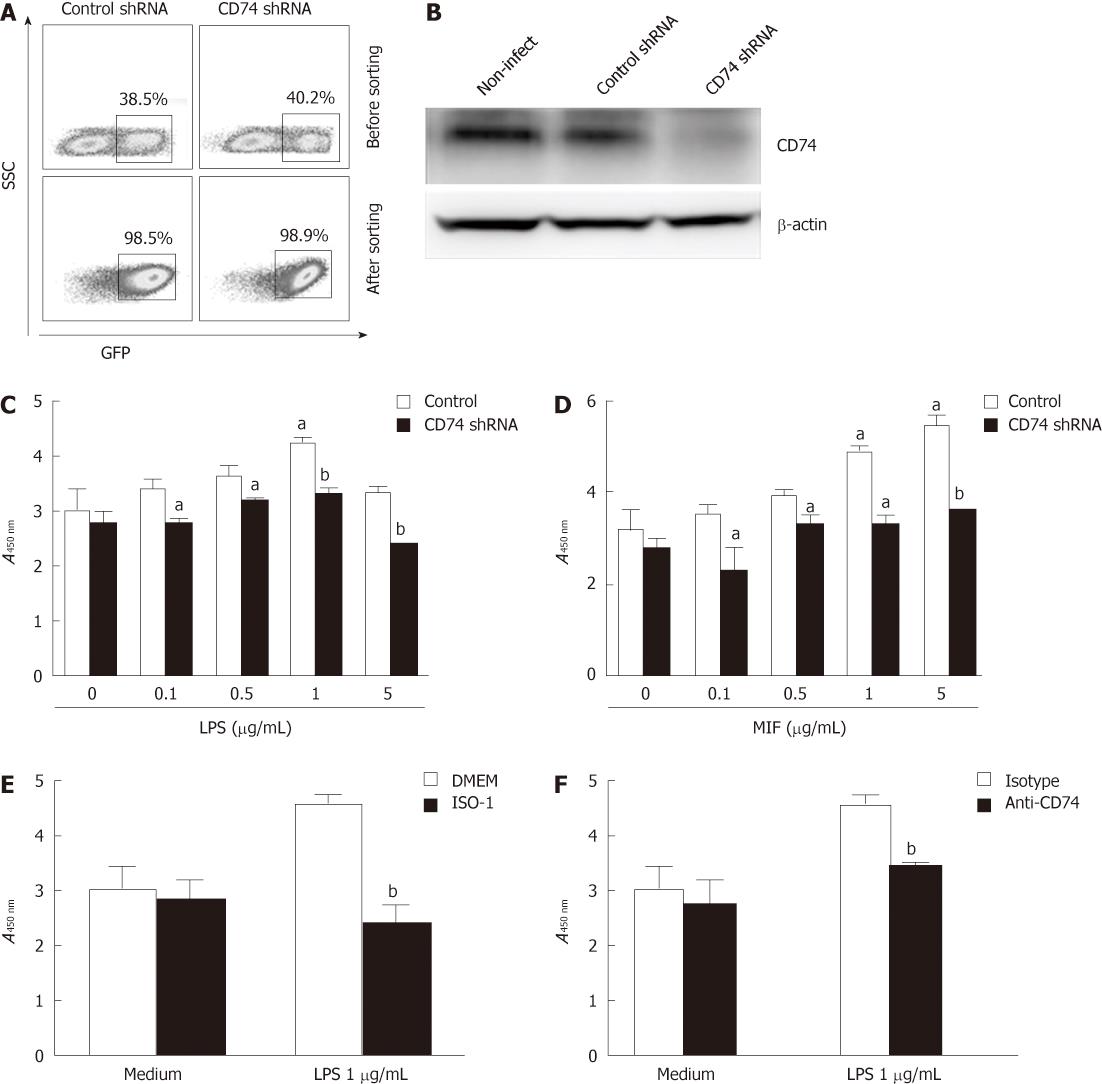
Various reports have shown that MIF or LPS increases proliferation of some cell types[21,22]. We investigated the ability of MIF or LPS to induce proliferation of GECs. rMIF or LPS were incubated with MKN-45 cells for 24 h. Proliferation was measured by nonradioactive cell proliferation colorimetric assay, as used in several recent studies[23]. Standard curves of known numbers of cells were run with each assay to extrapolate cell number from treated samples. As seen in Figure 3B and C, MKN-45 cell proliferation was significantly increased when stimulated with LPS (3.027 ± 0.388 vs 4.201 ± 0.092, P < 0.01) or MIF (3.160 ± 0.054 vs 4.856 ± 0.068, P < 0.05) at 1 μg/mL compared with medium control.
To investigate the role of CD74 in the observed proliferation, we used lentivirus shRNA that targeted CD74. Figure 3A shows that the transduction efficiency of MKN-45 cells between the control and CD74 shRNAs was equal, and after sorting, the GFP+ cells reached 98%. Western blotting showed that CD74 expression was strongly knocked down (Figure 3B). When CD74 expression was knocked down, the proliferation of MKN-45 cells after stimulation by LPS or MIF was greatly inhibited (4.201 ± 0.092 vs 3.337 ± 0.087, 4.856 ± 0.068 vs 3.160 ± 0.054, respectively, P < 0.01) (Figure 3C and D). The same effect was observed when anti-CD74 blocking antibodies were incubated with cells at 2 h before addition of LPS (4.534 ± 0.222 vs 3.368 ± 0.290, P < 0.01), (Figure 3E). Notably, after anti-CD74 treatment, proliferation levels were decreased to levels similar to those of untreated cells. To investigate further the role of MIF in LPS-induced GEC proliferation, using the MIF specific inhibitor ISO-1, MKN-45 cell proliferation was greatly inhibited (4.058 ± 0.292 vs 2.934 ± 0.197, P < 0.01) (Figure 3F). These data suggest that LPS stimulated GEC proliferation through the MIF/CD74 pathway.
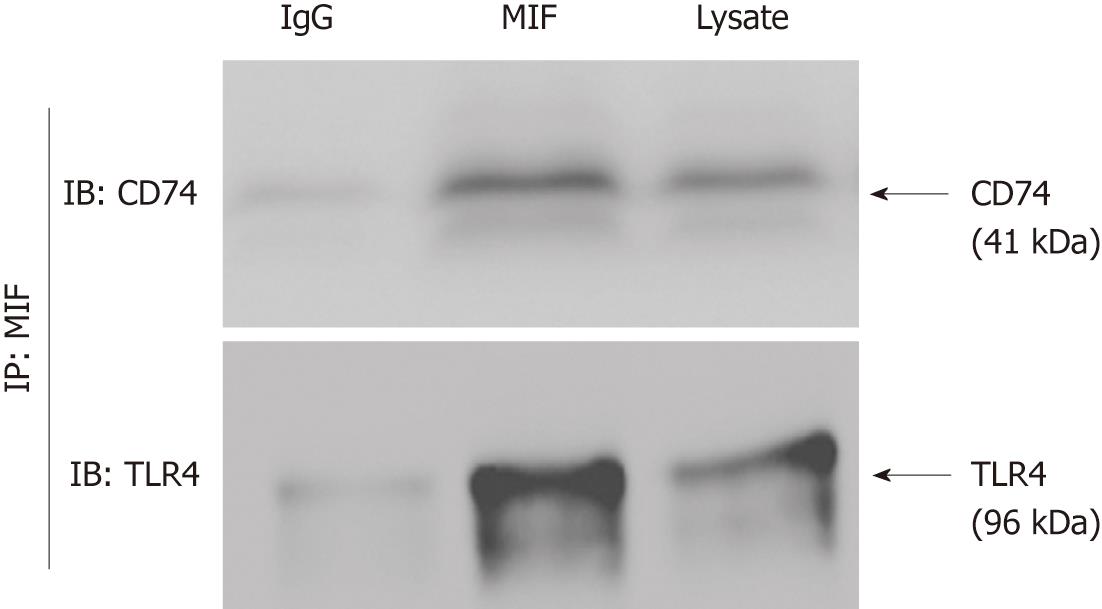
CD74 has been suggested to act as a receptor for MIF in several studies. We have shown that GECs express large amounts of CD74, which is upregulated under inflammatory conditions. Consequently, we examined the role of CD74 as a receptor for MIF on GEC by immunoprecipitation and western blotting. rMIF was incubated with MKN-45 cell lysates. MIF was immunoprecipitated by the MIF antibody along with GEC proteins bound to it. Western blotting using anti-CD74 mAb revealed that CD74 was co-precipitated with MIF, and TLR4 was co-precipitated with MIF (Figure 4). These results suggest that TLR4/CD74/MIF can form a complex to promote cell proliferation.
Recent data have expanded the concept that inflammation is a critical component of tumor progression. Many cancers arise from chronic irritation and inflammation. It is now becoming clear that the tumor microenvironment, which is largely orchestrated by inflammatory cells, is an indispensable participant in the neoplastic process, fostering proliferation, survival and migration[24,25]. TLRs are evolutionarily conserved transmembrane molecules that help the immune system to recognize pathogen-associated molecular patterns, and TLR4 sensitizes immune cells to bacterial LPS. When stimulated by LPS, many intracellular signaling pathways are activated, and lead to the generation of nuclear factor-κB, which in turn promotes proinflammatory cytokine production and release[26]. The unique biological activities of MIF have the potential to contribute to an in vivo microenvironment favoring tumor growth and invasiveness. These functional activities include: tumor suppressor downregulation, COX-2 and prostaglandin E2 upregulation, and potent induction of angiogenesis[27,28]. Recent evidence has suggested another important role for the CD74 molecule in the activation of cell survival pathways. CD74 is a cell receptor for the proinflammatory cytokine, MIF. Although CD74 itself is able to bind MIF, when bound to surface expressed CD44, the CD74-CD44 complex is able to initiate several survival pathways, including the extracellular signal-regulated kinase-1/2-mitogen-activated protein kinase signaling cascade, and to stimulate cell proliferation by enhanced expression of cyclins and other regulatory factors[29].
It has been reported that CD74 surface expression is increased under inflammatory conditions and during H. pylori infection, and the bacterium can also use CD74 as a point of attachment to GECs[30]. The dramatic increase in CD74 expression during infection and the high turnover rate of CD74 suggests that both MIF and H. pylori can use CD74 as a receptor. Our data demonstrate that in gastric cancer, TLR4 expression is increased and has a strong association with disease stage and lymph node metastasis. GEC proliferation was significantly increased by LPS stimulation, suggesting that gastric cancer is strongly correlated with inflammation. Similarly, rMIF induced proliferation of GECs in a dose-dependent manner. Proliferation was decreased when CD74 was blocked by knockdown of CD74 gene or with antibodies or MIF was blocked by the antagonist ISO-1.
Immunohistochemical staining showed that CD74, MIF and TLR4 has a strong association with cancer stage, suggesting that CD74, MIF and TLR4 have a role in tumor progression. Ishigami et al[12] have reported that CD74 expression in gastric cancer is a useful prognostic marker and is correlated with surgical outcome. McClelland et al[13] have observed coexpression of CD74 in close proximity to the ligand MIF in non-small cell lung cancer, and have found that coexpression is associated with higher levels of CXC chemokines. In the current study, we also found positive correlation between MIF and CD74 and TLR4 in gastric cancer through correlation analysis. We further showed that CD74, MIF and TLR4 could form a complex, and under LPS stimulation, greatly induced cell proliferation. These findings suggest that TLR4, MIF and CD74 overexpression may be related to the pathogenesis of gastric cancer, and they could become promising therapeutic targets.
In summary, our study demonstrated the positive correlation of CD74/MIF/TLR4 in gastric cancer, suggesting that inflammation, as induced by LPS stimulation, can enhance the CD74/MIF pathway, promoting GEC proliferation and gastric carcinogenesis. Blocking of CD74 or MIF may provide a novel strategy for gastric cancer chemoprevention and/or treatment.
We thank Guo-Hua Xie, Mei-Xing Li, Lin Deng for providing excellent technical assistance.
CD74 is an important chaperone of MHC II that regulates antigen presentation for the immune response. It is also expressed on epithelial cells, and is particularly important in the complex immunological mechanisms and in the link between chronic inflammation and carcinogenesis in the gastrointestinal tract. Macrophage migration inhibitory factor (MIF) binding to CD74 might contribute to carcinogenesis in chronic conditions, leading to increased proliferation, tumor growth, and angiogenesis. Toll-like receptor 4 (TLR4) confers responsiveness to Gram-negative lipopolysaccharide (LPS), and is important for proliferation and apoptosis in response to gastrointestinal injury.
Recent data have expanded the concept that inflammation is a critical component of tumor progression. In a previous study, it has been reported that CD74 and MIF are upregulated in the gastric cancer. However, how CD74 and MIF are elevated in gastric cancer remains unclear. The relationship between MIF/CD74/TLR4 expression by the tumor and clinicopathological factors in gastric carcinoma needs to be further investigated.
In this study, CD74, MIF and TLR4 were found to be expressed in gastric cancer and increased significantly in the advanced stage; they were also associated with lymph node metastasis. Correlation analysis revealed that CD74 was positively correlated with MIF and both proteins were also associated with TLR4. LPS can significantly promote MKN-45 gastric cancer cell proliferation, and induce MIF production and cell surface expression of CD74. Knockdown of CD74 or using anti-CD74 and MIF antagonist ISO-1 significantly reduces LPS-induced MKN-45 cell proliferation. MIF, CD74 and TLR4 can co-localize in MKN-45 cells.
The study demonstrates the positive correlation of CD74/MIF/TLR4 in gastric cancer, suggesting that inflammation, as caused by LPS stimulation, can enhance the CD74/MIF pathway, promoting gastric epithelial cell proliferation and gastric carcinogenesis. Blocking of CD74 or MIF may provide a novel strategy for gastric cancer chemoprevention and/or treatment.
CD74, also known as the invariant chain, participates in several key processes of the immune system, including antigen presentation, B-cell differentiation and inflammatory signaling. Recently, studies have revealed that CD74 is a receptor for macrophage MIF and is upregulated in inflammation, which has the potential to contribute to an in vivo microenvironment favoring tumor growth and invasiveness. As a participant in several immunological processes and an indicator of disease in some conditions, CD74 has potential as a therapeutic target.
In this study, the authors demonstrated the positive correlation between CD74, MIF and TLR4 in gastric cancer and their association with clinicopathological factors. They revealed that LPS stimulation induced gastric cancer cell proliferation through enhanced MIF production and CD74 expression, and that knockdown of CD74 or using anti-CD74 antibody and MIF antagonist could reduce LPS-induced MKN-45 cell proliferation. This study certainly provides a novel mechanism of gastric carcinogenesis associated with the CD74/MIF pathway. The results suggest that CD74 and MIF could be novel therapeutic and chemopreventive targets in gastric cancer treatment.
Peer reviewer: Satoshi Osawa, Assistant Professor, The First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu 431-3192, Japan
S- Editor Gou SX L- Editor Kerr C E- Editor Zheng XM
| 1. | Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 419] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581-8585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Ong GL, Goldenberg DM, Hansen HJ, Mattes MJ. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology. 1999;98:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets. 2011;15:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, Kitamura M, Takahashi H, Eguchi H, Ohigashi H. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol. 2009;16:2531-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol. 2009;15:2855-2861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 7. | Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Maharshak N, Cohen S, Lantner F, Hart G, Leng L, Bucala R, Shachar I. CD74 is a survival receptor on colon epithelial cells. World J Gastroenterol. 2010;16:3258-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Sekiguchi H, Irie K, Murakami A. Suppression of CD74 expression and Helicobacter pylori adhesion by auraptene targeting serum starvation-activated ERK1/2 in NCI-N87 gastric carcinoma cells. Biosci Biotechnol Biochem. 2010;74:1018-1024. |
| 11. | Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, Hokita S, Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | McClelland M, Zhao L, Carskadon S, Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol. 2009;174:638-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, Lucas DM, Muthusamy N, Goldenberg DM, Lee RJ. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116:2554-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Cheng RJ, Deng WG, Niu CB, Li YY, Fu Y. Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer. 2011;21:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Beswick EJ, Reyes VE. Macrophage migration inhibitory factor and interleukin-8 produced by gastric epithelial cells during Helicobacter pylori exposure induce expression and activation of the epidermal growth factor receptor. Infect Immun. 2008;76:3233-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046-5059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6034] [Cited by in RCA: 6314] [Article Influence: 300.7] [Reference Citation Analysis (0)] |
| 19. | Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Camlica H, Duranyildiz D, Oguz H, Oral EN, Yasasever V. The diagnostic value of macrophage migration inhibitory factor (MIF) in gastric cancer. Pathol Oncol Res. 2008;14:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Bach JP, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 23. | Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335-3340. [PubMed] |
| 24. | Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | DeNardo DG, Johansson M, Coussens LM. Inflaming gastrointestinal oncogenic programming. Cancer Cell. 2008;14:7-9. [PubMed] |
| 26. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Li GQ, Xie J, Lei XY, Zhang L. Macrophage migration inhibitory factor regulates proliferation of gastric cancer cells via the PI3K/Akt pathway. World J Gastroenterol. 2009;15:5541-5548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |