Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2231
Revised: September 20, 2011
Accepted: March 10, 2012
Published online: May 14, 2012
AIM: To investigate the relationship between plasma acylated ghrelin levels and the pathophysiology of functional dyspepsia.
METHODS: Twenty-two female patients with functional dyspepsia and twelve healthy volunteers were recruited for the study. The functional dyspepsia patients were each diagnosed based on the Rome III criteria. Eligible patients completed a questionnaire concerning the severity of 10 symptoms. Plasma acylated ghrelin levels before and after a meal were determined in the study participants using a commercial human acylated enzyme immunoassay kit; electrogastrograms were performed for 50 min before and after a standardized 10-min meal containing 265 kcal.
RESULTS: There were no significant differences in plasma acylated ghrelin levels between healthy volunteers and patients with functional dyspepsia. However, in patients with functional dyspepsia, there was a negative correlation between fasting plasma acylated ghrelin levels and the sum score of epigastric pain (r = -0.427, P = 0.047) and a positive correlation between the postprandial/fasting plasma acylated ghrelin ratio and the sum score of early satiety (r = 0.428, P =0.047). Additionally, there was a negative correlation between fasting acylated ghrelin plasma levels and fasting normogastria (%) (r = -0.522, P = 0.013). Interestingly, two functional dyspepsia patients showed paradoxically elevated plasma acylated ghrelin levels after the meal.
CONCLUSION: Abnormal plasma acylated ghrelin levels before or after a meal may be related to several of the dyspeptic symptoms seen in patients with functional dyspepsia.
- Citation: Kim YS, Lee JS, Lee TH, Cho JY, Kim JO, Kim WJ, Kim HG, Jeon SR, Jeong HS. Plasma levels of acylated ghrelin in patients with functional dyspepsia. World J Gastroenterol 2012; 18(18): 2231-2237
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2231
Functional dyspepsia is defined as the presence of dyspeptic symptoms thought to originate in the gastroduodenal region that occur in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms[1,2]. To date, functional dyspepsia has been associated with various physiological abnormalities, including delayed gastric emptying[3], altered antro-duodenal motility[4], impaired gastric accommodation[5], visceral hypersensitivity[6], gastric dysrhythmia[7-10]; functional dyspepsia has also been associated with multiple psychiatric and personal factors, such as somatization[11], depression, anxiety[12], and changes in coping skills[13]. However, a number of studies have failed to find associations between dyspeptic symptoms and the putative pathophysiology of functional dyspepsia in patients. The underlying etiology of functional dyspepsia remains unclear.
Ghrelin is the endogenous ligand for the growth hormone secretagogue receptor, and it has potent growth hormone-releasing activity. Ghrelin is predominately produced by endocrine cells in the oxyntic mucosa of the stomach[14,15]. Ghrelin has two subtypes: a deacylated form and an acylated form[16]. The physiologic functions of ghrelin are pleiotropic as follows. First, ghrelin stimulates food intake. An appetite stimulatory effect is associated with both central regulation and peripheral signals[17,18]. Second, ghrelin regulates gastric acid secretion. Ghrelin acts in the central nervous system to stimulate gastric acid secretion[19]. Third, ghrelin induces the migrating motor complex and promotes gastric emptying. Lastly, ghrelin has also been reported to have a gastroprotective effect in the context of the generation of nitric oxide and prostaglandins[20,21].
Given these diverse functions, ghrelin has been hypothesized to play a role in the pathophysiology of functional dyspepsia. The aim of this study was to investigate the role of acylated ghrelin in the pathophysiology of functional dyspepsia. This study measured plasma acylated ghrelin levels of female subjects, before and after a meal; these data were then compared between females with functional dyspepsia and healthy female volunteers. Also, in patients with functional dyspepsia, we determined the correlation between plasma acylated ghrelin levels, symptom scores, and electrogastrogram (EGG) parameters. In addition, patients with functional dyspepsia were divided into two subgroups according to the Rome III criteria: patients with postprandial distress syndrome (PDS) and patients with epigastric pain syndrome (EPS). Differences in plasma acylated ghrelin levels between these two subgroups were also evaluated.
Female subjects between 18 and 60 years of age were recruited from Sep. 2006 to Jan. 2007 at Soonchunhyang University Hospital, Seoul, South Korea. We recruited healthy volunteers by advertisement. Consecutive patients who were diagnosed with functional dyspepsia were invited to participate in the study. To diagnose functional dyspepsia, patients with dyspeptic symptoms thought to originate in the gastroduodenal region were asked to answer a questionnaire based on the Rome III functional dyspepsia criteria after the exclusion of organic disease using endoscopic examination. In addition, eligible patients were asked to complete a questionnaire regarding the severity of 10 symptoms; severity was determined using a self-devised scale of absent (0), mild (1), relevant (2), moderate (3), and severe (4). The 10 symptoms were epigastric pain, epigastric burning, upper abdominal discomfort, nausea, upper abdominal fullness, gastric retention, upper abdominal distention, early satiation, vomiting, and belching. According to the predominant symptom, patients were classified as either PDS or EPS. Patients were examined for Helicobacter pylori (H. pylori) infection with a rapid urease test (Pronto Dry, Gastrex Corp., Warsaw, Poland) or the 13C-urea breath test (UBiT-IR 300, Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan). The study protocol was approved by the Soonchunhyang University Hospital Institutional Review Board. Written informed consent was obtained from all participants at the time of enrollment.
This prospective case-control study was designed to elucidate the role of plasma acylated ghrelin levels in the pathophysiology of functional dyspepsia. The primary endpoint was to investigate the difference in the plasma levels of acylated ghrelin between the two investigated groups. The second endpoint was to evaluate correlations between fasting and postprandial plasma acylated ghrelin levels, symptom severity, the EGG parameters in patients with functional dyspepsia. The tertiary endpoint was to examine whether there were any differences in plasma acylated ghrelin levels between patients with PDS and EPS.
To be included in the study, patients had to meet the following inclusion criteria: (1) one or more bothersome dyspeptic symptoms, such as postprandial fullness, early satiation, epigastric pain, and epigastric burning, for the last 3 mo, with symptom onset at least 6 mo prior to diagnosis; (2) the ability to cease all medical treatment that could influence the gastrointestinal motility at least 1 wk prior to the test; and (3) informed written consent.
The following exclusion criteria were utilized: (1) subjects who suffered from structural diseases, such as esophagitis, erosive gastroduodenal lesions or ulcers that could explain symptoms; (2) subjects who suffered from systemic diseases, such as diabetes mellitus or thyroid disease; (3) subjects with a history of peptic ulcers or major abdominal surgery; and (4) subjects who were obese, as defined by a body mass index of over 30.
Fasting blood samples were taken and analyzed for plasma levels of acylated ghrelin, glucose, insulin and growth hormone. Postprandial blood samples were obtained fifty minutes after a 10-min meal and analyzed for postprandial plasma levels of acylated ghrelin. For each sample, whole blood was directly drawn into a centrifuge tube that contained 500 U of aprotinin and 1.25 mg of EDTA - 2Na per 1 mL of whole blood. Blood samples in the tubes were immediately centrifuged at 1500 ×g for 15 min at 4 °C. Plasma samples were stored at -80 °C for later use after immediately adding 100 μL of 1 mol/L HCl per 1 mL of collected plasma. The commercially available human acylated ghrelin enzyme immunoassay kit (Cayman Chemical Co., Michigan, United States) was used to measure the acylated ghrelin levels.
Study subjects visited the office in the morning, after an overnight fast. Three EGG electrodes were connected to the subject’s abdomen according to standard method[9,10]. The EGG electrodes were then attached to the Digitrapper EGG recorder (Medtronic Co. WA, United States). A fasting EGG signal was obtained for fifty minutes. After a 10-min break for a standardized meal, the postprandial EGG was also recorded for fifty minutes. Subjects were given a standardized soft diet that contained a total of 265 kcal, composed of 72% carbohydrate, 16% protein, and 12% fat. Nine parameters were measured, namely, the proportions of bradygastria (%), normogastria (%), and tachygastria (%) during the fasting and postprandial periods, the instability coefficient for both periods and the power ratio. The instability coefficient represents the stability of the slow wave and the power ratio represents the fasting electrical power of the slow wave divided by the postprandial electrical power.
SPSS software version 17.0 was used for statistical analyses. The Mann-Whitney U test was utilized to compare plasma acylated ghrelin levels between healthy volunteers and patients with functional dyspepsia. Fisher’s exact test was used to compare the prevalence of H. pylori infection between the two groups. In patients with functional dyspepsia, correlation analysis by the Spearman’s rho correlation coefficient was performed to assess the correlations between plasma acylated ghrelin levels, symptom scores and EGG parameters.
In total, thirty-four female subjects were enrolled. Twelve women were healthy volunteers and twenty-two were patients suffering from functional dyspepsia. Patient baseline characteristics are summarized in Table 1.
| Healthy volunteers (n = 12) | Patients with functional dyspepsia (n = 22) | P value | |
| Age (yr) | 24 (23-34) | 46 (23-59) | 0.0001 |
| Height (cm) | 162 (154-167) | 160 (150-175) | 0.4351 |
| Weight (kg) | 52.8 (44-58) | 55 (45-70) | 0.1701 |
| Body mass index (kg/m2) | 20.2 (18.1-22.9) | 21.2 (17.6-27.3) | 0.1351 |
| Growth hormone (ng/mL) | 0.8 (0.1-3.3) | 0.4 (0.01-14.7) | 0.3391 |
| Insulin (μIU/mL) | 8.3 (2.3-14.6) | 8.3 (4.0-14.4) | 0.6391 |
| Fasting blood sugar (mg/dL) | 82 (72-97) | 88.5 (70-107) | 0.0261 |
| Helicobacter pylori infection rate (%) | 66.7 (8/12) | 36.4 (8/22) | 0.1512 |
The healthy volunteers were significantly younger compared to the patients with functional dyspepsia (P = 0.00). The median fasting blood glucose level was significantly higher in patients with functional dyspepsia compared to healthy volunteers (P = 0.03), because two functional dyspepsia patients exhibited glucose intolerance. However, no significant differences were observed between the two groups for median growth hormone and insulin levels and body mass index values.
For the assessment of H. pylori infection status, thirty participants were tested by upper endoscopy with the rapid urease test, while the remaining four subjects underwent urea breath tests because they had undergone an upper endoscopy within 6 mo preceding study enrollment. H. pylori infection rates were 66.7% (8/12) in healthy volunteers, and 36.4% (8/22) in patients with functional dyspepsia. However, the prevalence of H. pylori between the two groups was not statistically significant.
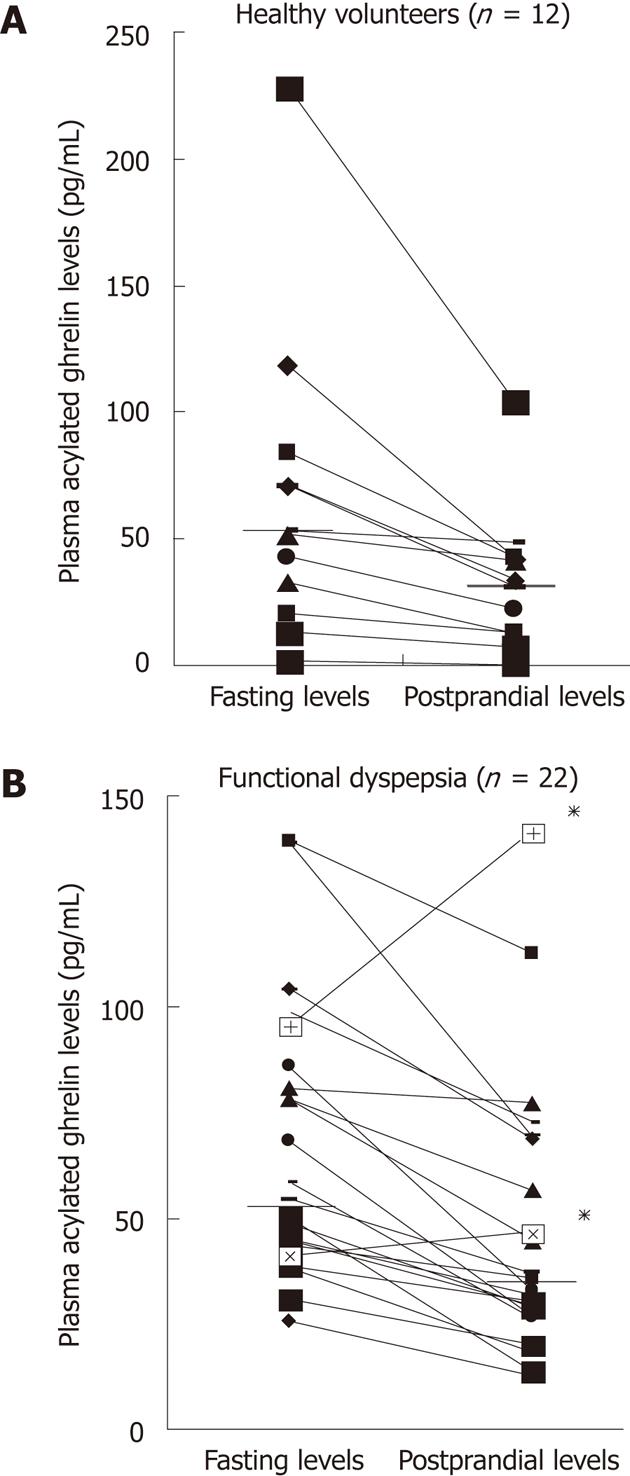
In healthy volunteers, the median level of plasma acylated ghrelin was 52.2 pg/mL (1.6-228) during fasting and 32.1 pg/mL (1.1-104.2) postprandially. In patients with functional dyspepsia, the median levels of plasma acylated ghrelin during fasting and postprandially were 56.4 pg/mL (25.6-139.1) and 34.2 pg/mL (12.4-141.2), respectively. Interestingly, two patients with functional dyspepsia exhibited a paradoxical increase in postprandial plasma acylated ghrelin levels (Figure 1).
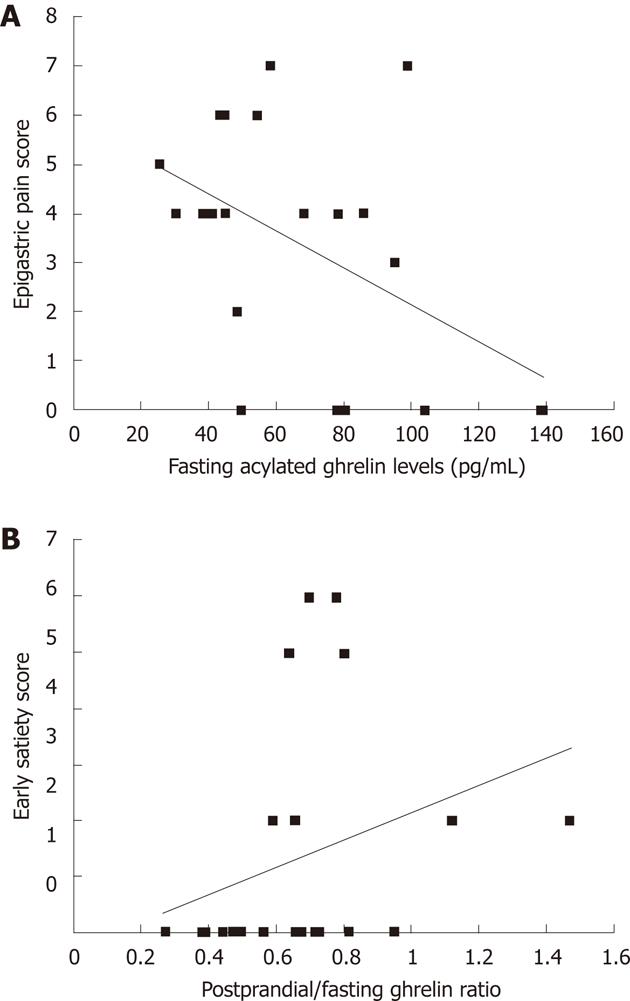
Correlations between plasma acylated ghrelin levels and the symptom scores of the 10-investigated symptoms: There was negative correlation between fasting plasma levels of acylated ghrelin and total epigastric pain scores (r = -0.427, P = 0.047) (Figure 2A). In contrast, we found a positive correlation between the postprandial/fasting acylated ghrelin ratio and the total early satiety scores (r = 0.428, P = 0.047) (Figure 2B).
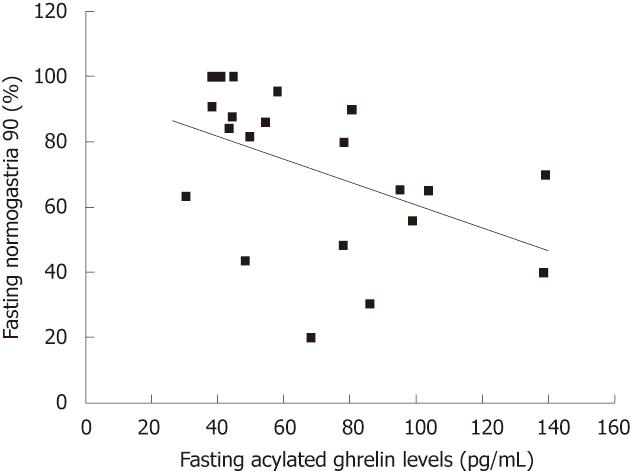
Correlations between plasma acylated ghrelin levels and the nine electrogastrogram parameters: There was a negative correlation between fasting plasma levels of acylated ghrelin and fasting normogastria (%) (r = -0.522, P = 0.013) (Figure 3).
In the thirteen PDS patients, the median plasma level of acylated ghrelin was 58.31 pg/mL (30.53-139.12) during fasting and 35.66 pg/mL (19.61-112.25) postprandially. In the 9 EPS patients, the median plasma level of acylated ghrelin was 49.79 pg/mL (25.56-95.14) during fasting and 32.78 pg/mL (12.35-141.16) postprandially. The differences in plasma acylated ghrelin levels between PDS and EPS patients were not statistically significant (Table 2).
| Postprandial distress syndrome (n = 13) | Epigastric pain syndrome (n = 9) | P value | |
| Age (yr) | 46 (23-59) | 44 (37-58) | 0.7131 |
| Height (cm) | 160 (154-175) | 161 (150-168) | 0.9461 |
| Weight (kg) | 54 (45 -70) | 57 (47-62) | 0.8411 |
| Body mass index (kg/m2) | 20.7 (17.63-27.34) | 22.27 (18.44-26.22) | 0.6401 |
| Growth hormone (ng/mL) | 0.41 (0.03 -9.16) | 0.46 (0.01-14.72) | 0.4041 |
| Insulin (μIU/mL) | 8.99 (4.12-14.38) | 7.88 (4.00-13.80) | 0.6641 |
| Fasting blood sugar (mg/dL) | 92 (81-107) | 86 (70-95) | 0.1141 |
| Helicobacter pylori infection rate (%) | 53.8 (7/13) | 11.1 (1/9) | 0.0742 |
| Fasting plasma levels of acylated ghrelin (pg/mL) | 58.31 (30.53-139.12) | 49.79 (25.56-95.14) | 0.4431 |
| Postprandial plasma levels of acylated ghrelin (pg/mL) | 35.66 (19.61-112.25) | 32.78 (12.35-141.16) | 0.6161 |
Ghrelin has two subtypes: deacylated ghrelin, which lacks an acyl group at third serine residue, and acylated ghrelin, which has the acyl modification necessary for noctanoic acid hormonal activity[16]. Although deacylated ghrelin circulates in far greater amounts compared to the acylated form and is involved in cell proliferation and adipogenesis, only acylated ghrelin exhibits physiologic activity and can stimulate growth hormone release and food intake[22-25]. Therefore, we focused our investigation on acylated ghrelin.
In a study by Takamori et al[26] that focused on two age-matched groups, the authors found that fasting levels of deacylated ghrelin were significantly lower in patients with functional dyspepsia compared to controls; however, they reported that both fasting and postprandial levels of acylated ghrelin and postprandial levels of deacylated ghrelin were similar in the two groups. Consistent with these findings, our study did not show any differences in either fasting or postprandial plasma acylated ghrelin levels between healthy volunteers and patients with functional dyspepsia. These results suggest that plasma acylated ghrelin levels may not be directly associated with the pathophysiology of functional dyspepsia.
Plasma acylated ghrelin levels differ significantly in males and females. A study by Akamizu et al[16] showed that fasting levels of acylated, but not deacylated ghrelin, in female subjects were higher compared to males after adjustment for body mass index. For this reason, we limited our study to female participants.
H. pylori infection was more prevalent in the healthy volunteers compared to the patients with functional dyspepsia in our study; however, the difference in prevalence was not statistically significant. Although the prevalence of H. pylori infection in Korea has been decreasing from 66.9% in 1998 to 59.6% in 2005[27,28], it is still high even in asymptomatic adult subjects. This could be the reason why H. pylori was present more in the healthy volunteers in our study.
In our study, fasting plasma acylated ghrelin levels ranged from 1.61 to 227.98 pg/mL and postprandial plasma acylated ghrelin levels ranged from 1.09 to 141.16 pg/mL. Interestingly, plasma acylated ghrelin levels vary more than 100-fold between the extremes.
Most studies have reported the plasma levels of total ghrelin between 300 and 800 pg/mL[19]. Low total plasma ghrelin levels during fasting are associated with insulin resistance, hypertension, and type 2 diabetes[29]. Plasma ghrelin levels rise before the meal and sharply decline as soon as eating commences[30]. The surge of ghrelin levels before the meal is similar to the increase of gastric acid that occurs in the cephalic phase. Plasma ghrelin levels do not begin to recover until thirty minutes after a meal. This delayed recovery suggests that the mechanism for immediate intragastric inhibition of ghrelin release is not present in the stomach, but is instead associated with feedback inhibition, either via the release of an intestinal hormone or by insulin release in conjunction with food intake[31]. Interestingly, in our study, postprandial plasma acylated ghrelin levels were paradoxically higher compared to fasting levels in two individuals. Test repetition confirmed these results. One individual had a fasting ghrelin level of 95.1 pg/mL with a postprandial ghrelin level of 141.2 pg/mL. In the other individual, the fasting and postprandial plasma ghrelin levels were 41.1 pg/mL and 46.4 pg/mL, respectively. Both individuals listed epigastric pain and burning sensations as their main symptoms. These results suggest that an abnormal acylated ghrelin response after a meal may be one of the mechanisms involved in the pathophysiology of functional dyspepsia. Further study including more subjects will be needed to confirm this hypothesis.
A study by Shinomiya et al[32] revealed that the fasting levels of plasma acylated ghrelin correlated with subjective symptoms of functional dyspepsia in female patients. Similarly, we observed that plasma acylated ghrelin levels were associated with symptom scores and several EGG parameters. First, there was a negative correlation between fasting plasma acylated ghrelin levels and total epigastric pain scores (r = -0.427, P = 0.047). Therefore, patients with higher plasma levels of acylated ghrelin before the meal suffered from less epigastric pain. Taking into consideration a previous report showed that basal gastric acid secretion was normal in patients with functional dyspepsia[33],the relationship between higher fasting plasma levels of acylated ghrelin and decreased epigastric pain appear to result from a gastroprotective effect exerted by ghrelin on the gastric mucosa[19-21]. Second, we found a positive correlation between the ratio of acylated ghrelin level (i.e., the postprandial plasma acylated ghrelin level divided by the fasting plasma acylated ghrelin level) and the total early satiety scores (r = 0.428). Thus, blunted ghrelin decreases after the meal were associated with higher early satiety scores. Our results suggest that abnormal responses following a meal might play a role in the impairment of gastric accommodation. Third, we report a negative correlation between fasting plasma acylated ghrelin levels and fasting normogastria (3 cpm) (%). Accordingly, in patients with functional dyspepsia, higher fasting plasma acylated ghrelin levels were associated with fasting gastric dysrhythmia. Gastric dysrhythmia is reported in 40%-50% of patients with dysmotility-like dyspepsia[7,8]; also,abnormal myoelectrical activity of the stomach is associated with dyspeptic symptoms, especially nausea and vomiting[9,10]. Further study will be required to evaluate the relationship between fasting plasma acylated ghrelin levels and fasting gastric dysrhythmia.
A study by Shindo et al[34] showed that fasting plasma levels of acylated ghrelin in PDS patients were significantly lower compared to healthy volunteers; these levels also tended to be lower compared to EPS patients. However, in our study, no significant differences were observed in either the fasting or postprandial plasma levels of acylated ghrelin between PDS and EPS patients. Interestingly, the two patients who exhibited paradoxical increases in postprandial plasma acylated ghrelin levels had EPS.
The limitations of our study should be noted. These limitations include the small sample, which permitted only the use of non-parametric tests in the statistical analysis. Also, the study group and the control group were not age-matched. Although plasma ghrelin levels are not known to change with age, age may be important factor for this kind of functional study. Additionally, the high level of H. pylori infection in our participants was not ideal. However, H. pylori infection has no direct relationship to the diagnostic criteria for functional dyspepsia[2]. Further, although a study by Isomoto et al[35] showed that the fasting levels of total ghrelin in H. pylori-positive patients were significantly lower compared to H. pylori-negative patients, the relationship of the plasma acylated ghrelin levels to H. pylori infection status has not been evaluated to date. Another factor that was not taken into account was the phase of the participants’ menstrual cycles. This is potentially an important variable to control for because a study from De Souza et al[36] found that fasting ghrelin plasma concentrations were at least 85% greater in subjects with exercise-associated amenorrhea. Finally, as approximately one third of ghrelin is produced in extra-intestinal organs such as the pancreas and the hypothalamus[14,15], further studies measuring exclusively gastric ghrelin levels are needed.
In conclusion, although no significant differences in plasma acylated ghrelin levels between healthy volunteers and patients with functional dyspepsia were observed, we found that abnormal plasma acylated ghrelin levels before and after the meal may be related to several dyspeptic symptoms in patients with functional dyspepsia. We also observed a paradoxical increase in postprandial plasma ghrelin levels in two patients with EPS, suggesting that abnormal plasma acylated ghrelin levels might be involved in the pathophysiology of functional dyspepsia. Further study in a larger sample size is needed to elucidate the complicated pathophysiology of functional dyspepsia.
Dyspepsia occurs in approximately 25 percent of the population each year. The most common cause of dyspepsia is functional dyspepsia. However, the pathophysiology of functional dyspepsia is unclear. As ghrelin, an acylated peptide produced predominantly by the stomach, has a well-established role in increasing appetite and food intake and in stimulating gastric emptying and acid secretion, it may play a role in the pathophysiology of functional dyspepsia.
Ghrelin is the endogenous ligand for the growth hormone secretagogue receptor, which is present on pituitary cells that secrete growth hormone. However, ghrelin exerts many endocrine and extraendocrine biological activities beyond the control of growth hormone secretion. In this study, the authors demonstrate that abnormal plasma acylated ghrelin levels before and after a meal may be related to several dyspeptic symptoms in patients with functional dyspepsia.
The authors found a negative correlation between fasting plasma levels of acylated ghrelin and epigastric pain scores, and a positive correlation between the postprandial/fasting acylated ghrelin ratio and early satiety scores. Further, they also found a negative correlation between fasting plasma acylated ghrelin levels and fasting normogastria. The results regarding the relationship between plasma ghrelin levels and epigastric pain and early satiety scores are interesting.
By revealing which symptoms in patients with functional dyspepsia is associated with plasma ghrelin levels, this study may represent a future strategy for the research on the relationship between plasma ghrelin levels and the pathophysiology of functional dyspespsia.
The authors examined whether fasting and postprandial plasma acylated ghrelin levels exhibited differences in patients with functional dyspepsia compared to healthy volunteers. In addition, they attempted to demonstrate which dyspeptic symptoms and electrogastrogram parameters in patients with functional dyspepsia correlated with fasting and postprandial levels of plasma acylated ghrelin. Overall, this paper is unique despite several weak points.
Peer reviewers: Tomoyuki Shibata, MD, PhD, Associate Professor, Department of Gastroenterology, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukake-cho,Toyoake, Aichi 470-1192, Japan; Ted Dinan, Professor, Department of Psychiatry, Cork University Hospital, C1 Wilton, Cork, C1, Ireland
S- Editor Shi ZF L- Editor A E- Editor Xiong L
| 1. | Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37-II42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 3. | Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 453] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Malagelada JR, Stanghellini V. Manometric evaluation of functional upper gut symptoms. Gastroenterology. 1985;88:1223-1231. [PubMed] |
| 5. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 762] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 6. | Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 410] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 7. | Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Koch KL, Hong SP, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J Clin Gastroenterol. 2000;31:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Chang FY. Electrogastrography: basic knowledge, recording, processing and its clinical applications. J Gastroenterol Hepatol. 2005;20:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Wilhelmsen I, Haug TT, Ursin H, Berstad A. Discriminant analysis of factors distinguishing patients with functional dyspepsia from patients with duodenal ulcer. Significance of somatization. Dig Dis Sci. 1995;40:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Norton GR, Norton PJ, Asmundson GJ, Thompson LA, Larsen DK. Neurotic butterflies in my stomach: the role of anxiety, anxiety sensitivity and depression in functional gastrointestinal disorders. J Psychosom Res. 1999;47:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Cheng C, Hui WM, Lam SK. Coping style of individuals with functional dyspepsia. Psychosom Med. 1999;61:789-795. [PubMed] |
| 14. | Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753-4758. [PubMed] |
| 15. | Krsek M, Rosická M, Haluzík M, Svobodová J, Kotrlíková E, Justová V, Lacinová Z, Jarkovská Z. Plasma ghrelin levels in patients with short bowel syndrome. Endocr Res. 2002;28:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K. Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab. 2005;90:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 472] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 18. | Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 19. | Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 265] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Konturek PC, Brzozowski T, Pajdo R, Nikiforuk A, Kwiecien S, Harsch I, Drozdowicz D, Hahn EG, Konturek SJ. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325-336. [PubMed] |
| 22. | Cassoni P, Papotti M, Ghè C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab. 2001;86:1738-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Bedendi I, Alloatti G, Marcantoni A, Malan D, Catapano F, Ghé C, Deghenghi R, Ghigo E, Muccioli G. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. Eur J Pharmacol. 2003;476:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Takamori K, Mizuta Y, Takeshima F, Akazawa Y, Isomoto H, Ohnita K, Ohba K, Omagari K, Shikuwa S, Kohno S. Relation among plasma ghrelin level, gastric emptying, and psychologic condition in patients with functional dyspepsia. J Clin Gastroenterol. 2007;41:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, Roe IH, Seo JK, Sim JG, Ahn H. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001;16:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Pöykkö SM, Kellokoski E, Hörkkö S, Kauma H, Kesäniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 30. | Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2010] [Cited by in RCA: 1934] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 31. | Schmidt PT, Degerblad M, Lindström E, Sundqvist M, Näslund E, Gillberg PG, Husebye E, Theodorsson E, Hellström PM. Circulating ghrelin levels after food intake during different phases of the migrating motor complex in man. Eur J Clin Invest. 2006;36:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Shinomiya T, Fukunaga M, Akamizu T, Irako T, Yokode M, Kangawa K, Nakai Y, Nakai Y. Plasma acylated ghrelin levels correlate with subjective symptoms of functional dyspepsia in female patients. Scand J Gastroenterol. 2005;40:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Collen MJ, Loebenberg MJ. Basal gastric acid secretion in nonulcer dyspepsia with or without duodenitis. Dig Dis Sci. 1989;34:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Shindo T, Futagami S, Hiratsuka T, Horie A, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis K, Tsukui T. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, Ohnita K, Mizuta Y, Inoue K, Nakazato M. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci. 2005;50:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | De Souza MJ, Leidy HJ, O'Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89:3536-3542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |