Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2203
Revised: September 9, 2011
Accepted: March 28, 2012
Published online: May 14, 2012
AIM: To evaluate the effects of soy supplementation on insulin resistance, fatty liver and alanine aminotransferase (ALT) levels in non-diabetic patients with chronic hepatitis C (CHC).
METHODS: In a prospective, randomized and single-blinded clinical trial, we compared patients with CHC who had casein as a supplement (n = 80) (control group), with patients who consumed a soy supplement diet (n = 80) [intervention group (IG)]. Both groups received 32 g/d of protein for 12 wk.
RESULTS: Patients’ baseline features showed that 48.1% were overweight, 43.7% had abdominal fat accumulation, 34.7% had hepatic steatosis and 36.3% had an homeostasis model assessment index of insulin resistance (HOMA-IR) ≥ 3.0. Descriptive analysis showed that protein supplementation diet reduced hepatic steatosis in both groups; however, significant reductions in ALT levels occurred in the soy group. Multiple regression modeling indicated that in the presence of severe fibrosis (F3/F4), γ glutamyl transferase elevation and high density lipoprotein (HDL) reduction, the intervention group had 75% less chance of developing hepatic steatosis (OR= 0.25; 95% CI: 0.06-0.82) and 55% less chance of presenting with an ALT level ≥ 1.5 × the upper limit of normal (ULN) (OR = 0.45, 95% CI: 0.22-0.89). Soy treatment did not have any effect on insulin resistance (OR = 1.92; 95% CI: 0.80-4.83), which might be attributed to the fact that the HOMA-IR values at baseline in most of our patients were in the normal range. Advanced hepatic fibrosis, an ALT level > 1.5 × ULN and visceral fat were predictors of an HOMA-IR ≥ 3. The IG group had a reduced risk of an ALT level > 1.5 × ULN. An HOMA-IR ≥ 3.0 and HDL < 35 mg/dL were also risk factors for increased ALT.
CONCLUSION: Soy supplementation decreased ALT levels and thus may improve liver inflammation in hepatitis C virus (HCV) patients; it also reduced hepatic steatosis in a subgroup of patients but did not change insulin resistance. It should be considered in the nutritional care of HCV patients.
- Citation: Oliveira LP, Jesus RP, Boulhosa RS, Mendes CMC, Gnoatto MC, Lemaire DC, Toralles MBP, Cavalcante LN, Lyra AC, Lyra LG. Effect of soy protein supplementation in patients with chronic hepatitis C: A randomized clinical trial. World J Gastroenterol 2012; 18(18): 2203-2211
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2203
Hepatitis C virus (HCV) infection is considered an important public health problem[1] and is the leading cause of liver transplantation in the Western world. Chronic HCV infection increases the risk for hepatic steatosis, insulin resistance, glucose intolerance and type 2 diabetes[2-4].
The pathophysiology of nonalcoholic fatty liver disease (NAFLD) involves histology ranging from fat alone (hepatic steatosis) to fat plus inflammation (nonalcoholic steatohepatitis, NASH) to fat plus hepatocyte injury (ballooning degeneration) with or without fibrosis or Mallory’s bodies which can lead to liver failure[5]. NAFLD and NASH have been associated with insulin resistance resulting in glucose intolerance and hyperglycemia[6]. Insulin resistance also contributes to increased lipolysis, which reduces fat uptake and oxidation by peripheral tissues. Both mechanisms lead to fat influx and accumulation in hepatic tissue[7].
Insulin resistance induced by HCV may involve several mechanisms, such as an immune response mediated by Th1 lymphocytes, the action of pro-inflammatory cytokines [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1 and IL-6), the degradation of intracellular components that participate in the insulin signaling system, including insulin receptor substrates (IRS-1 and IRS-2), and the reduced activation of phosphatidyl inositol 3-kinase (PI3-K) and protein kinase B (AKT)[8-12].
Considering these factors, appropriate nutrition becomes an essential tool to minimize HCV’s comorbidities such as fatty liver, inflammation and insulin resistance. Recently, functional foods have been considered essential for promoting and maintaining health. According to some reports, soy protein and its derivatives might be able to lower insulin resistance in patients with chronic liver diseases due its constituents, such as fiber, isoflavones and high biological value protein[13], and modulation of hepatic lipid metabolism[14,15]. The aim of this study was to evaluate the influence of soy protein supplementation on insulin resistance, liver fat content and alanine transaminase (ALT) levels in non-diabetic patients with chronic hepatitis C.
Non-diabetic patients with chronic hepatitis C were recruited from a reference ambulatory unit of the Federal University of Bahia’s Hospital between June 2008 and December 2009. The diagnosis of HCV infection was made by the presence of serum anti-HCV, which was confirmed by qualitative determination of HCV RNA. Inclusion criteria were the following: patients aged over 18 years, with or without liver cirrhosis; patients with ethanol consumption below 20 g/d; patients with normal liver function (Child-Pugh A); and patients who were not under antiviral therapy or who had discontinued antiviral therapy for at least three months.
Patients co-infected with HIV and/or HBV with renal failure as well as those with heart disease, decompensated cirrhosis, pregnancy, any malignancy, diabetes mellitus or obesity (BMI > 30 kg/m2) were excluded. The subjects gave written informed consent before participating in the study. The Ethics Committee of the Federal University of Bahia approved the study.
The study was a prospective, randomized and single-blinded clinical trial. Patients who were regularly followed in the Hepatology outpatient clinic were informed about the protocol and referred to the Nutrition clinic. Subjects who met the inclusion criteria of the study were randomly allocated into one of two study groups. The study was single-blinded (only blinded for patients). The estimated sample size was 160 patients.
The 160 patients were equally divided into two groups (n = 80), and each group received isonitrogenous protein supplementation with 32 g of protein per day for twelve weeks. The control group (CG) was supplemented with animal protein (casein), and the intervention group (IG) was supplemented with vegetable protein (soy). The nutritional composition of the supplements used in this study is reported in Table 1.
| Whole soy powder1 | Calcium caseinate | |
| Energy (Kcal) | 392 | 371 |
| Carbohydrate (g) | 14 | 0.2 |
| Protein (g) | 40 | 97.4 |
| Total fat (g) | 18.8 | 2.4 |
| Alpha linolenic acid (mg) | 1252 | 0 |
| Linoleic acid (mg) | 9332 | 0 |
| Dietary fiber (g) | 18 | 0 |
| Isoflavones (mg) | 53.82 | 0 |
Patients were instructed to dissolve the protein supplement in water, juice, soup, porridge or to consume it with fruits. Additionally, considering their nutritional status and dietary habits, patients received dietary guidelines to promote healthy eating and weight control. Diet counseling aimed to promote the ingestion of a normocaloric, normoglycidic and high protein (1.5 g/kg per day) diet by both groups. Patients returned monthly to receive their supplements.
Clinical survey data such as clinical diagnosis, viral genotype, necroinflammatory activity index and fibrosis (METAVIR classification) were either collected from medical records or from patient examinations.
Patients underwent ultrasonography of the upper abdomen with a team of three examiners using a single piece of equipment at the University Hospital’s radiology service. Hepatic steatosis was graded as mild, moderate or severe according to the classification of Saverumuttu et al[16].
Measurement of waist circumference was performed according to the World Health Organization recommendations using an inelastic tape measure (TBW Import Ltd.) that was 0.5 cm wide and 200 cm in length. Waist circumference was measured at a level midway between the superior aspect of the iliac crests and the lower lateral margins of the ribs. The cutoff points adopted for classifying central obesity and increased risk of metabolic complications were above 80 cm for women and 94 cm for men[17]. Socio-demographic and lifestyle information was also collected using a structured questionnaire during the first appointment of follow-up (baseline).
Patients underwent follow-up visits once a month with registered dietitians to elucidate the adherence to the diet prescription and protein supplementation. Schedule monitoring also included weekly telephone calls in the first month and biweekly thereafter. After 12 wk of supplementation, physical, biochemical and anthropometric tests as well as a questionnaire were applied to evaluate possible changes during the intervention program. Ultrasound was also performed in patients with a diagnosis of hepatic steatosis at baseline. All procedures were performed within a maximum interval of ten days after the nutrition counseling.
After a 12-h fast, a blood sample was collected for the determination of aspartate aminotransferase (AST), ALT, gamma glutamyl transferase (γGT), alkaline phosphatase, plasma glucose, insulin, total cholesterol and cholesterol fractions. Analyses were performed on a Beckman Coulter LX-20 PRO and CX-9 equipment. Serum insulin was measured using an electrochemiluminescence method with an Elecsys 2010 device.
The insulin resistance index was predicted according to the homeostasis model assessment index of insulin resistance (HOMA-IR). The formula was as follows: insulin resistance (HOMA-IR) = fasting insulinemia (microU/mL) × fasting glycemia (mmol/L)/22.5[18]. We considered ≥ 3.0 as the cutoff point to define insulin resistance.
An enzyme linked immunosorbent assay was performed on all serum samples to detect the presence of anti-HCV using third generation commercial kits (anti-HCV Hepatitis C® Wiener Lab.) following the manufacturer’s instructions. Reverse transcription-polymerase chain reaction (RT-PCR) was performed on all samples for qualitative determination of HCV RNA. The method used was the nested PCR (HCV-RNA detectable using the COBAS® AMPLICOR HCV Test, v2.0, Roche). HCV genotyping was performed using the technique of restriction fragment polymorphism (RFLP-PCR).
The stages of fibrosis and inflammation were determined according to the METAVIR scoring system: F0 = no fibrosis, F1 = expansion of fibrosis in portal areas without septa, F2 = fibrous portal expansion with septa, F3 = numerous septa or fibers with nodular transformation, and F4 = cirrhosis[19].
Descriptive analysis was performed to characterize the population. Mann-Whitney U and Wilcoxon’s rank sum tests were used to compare the biochemical values in the intervention and control groups and to evaluate the differences obtained at baseline and after intervention. Logistic regression analysis was used to evaluate risk predictor factors for hepatic steatosis, insulin resistance (HOMA-IR ≥ 3.0) and changes in ALT levels (1.5 above the upper limit of normal). Confounding and interaction analyses were performed to select the final models. Multiple regression analysis was performed after the intervention considering the groups had similar demographic, clinical and laboratory features before protein supplementation.
The sample size was calculated using an estimated 20% loss to follow-up, a confidence level of 95% and 80% power. A statistical significance was inferred at P < 0.05. In some biochemical analyses, the Bonferroni method was applied to adjust the multiple comparisons P-value between the groups at the significance level of 0.007. Statistical analysis was performed with the statistical package R version 2.12[20].
The characterization of the study population is presented in Table 2. Males (63.8%) were predominant. The prevalence of HCV genotype 1 infected patients was 83.5%. Advanced fibrosis (Metavir F3/F4) was detected in 40.5% of patients. The average age was 52.2 (± 10.5) years, 48.1% were overweight and 51.9% had a BMI < 25 kg/m2; 43.7% individuals had abdominal fat accumulation. It was also observed that 34.7% of the patients had hepatic steatosis, and 36.3% had an HOMA-IR ≥ 3.0 at baseline.
| CG-casein | IG-soy | Total | P value | |
| Demographic characteristics | ||||
| Gender | ||||
| Male | 49 (61.2) | 53 (66.2) | 102 (63.8) | 0.621 |
| Female | 31 (38.8) | 27 (33.8) | 58 (36.2) | |
| Marital Status | ||||
| Married | 56 (70.0) | 55 (68.7) | 111 (69.4) | 1.001 |
| Single, widowed or divorced | 24 (30.0) | 25 (31.3) | 49 (30.6) | |
| Anthropometric data | ||||
| Body mass index | ||||
| < 25 kg/m2 | 40 (50.0) | 43(53.8) | 83 (51.9) | 0.7511 |
| ≥ 25 kg/m2 | 40 (50.0) | 37(46.2) | 77 (48.1) | |
| Waist circumference | ||||
| Adequate | 43 (53.7) | 46 (59.0) | 89 (56.3) | 0.5251 |
| Inadequate | 37 (46.3) | 32 (41.0) | 69 (43.7) | |
| Clinical data | ||||
| Genotype | ||||
| 1 | 66 (88.0) | 61 (79.2) | 127 (83.5) | 0.1891 |
| 2/3 | 9 (12.0) | 16 (20.8) | 25 (16.5) | |
| Necroinflammatory activity | ||||
| A0 | 26 (41.3) | 29 (46.8) | 55 (44.0) | 0.7921 |
| A1 | 26 (41.3) | 22 (35.5) | 48 (38.4) | |
| A2 e A3 | 11 (17.4) | 11 (17.7) | 22 (17.6) | |
| Stage of fibrosis | ||||
| F0/F1/F2 | 35 (55.6) | 40 (63.5) | 75 (59.5) | 0.4681 |
| F3/F4 | 28 (44.4) | 23 (36.5) | 51 (40.5) | |
| Hepatic steatosis | ||||
| Yes | 24 (40.0) | 19 (29.7) | 43 (34.7) | 0.2601 |
| No | 36 (60.0) | 45 (70.3) | 81 (65.3) | |
| HOMA-IR | ||||
| < 3.0 | 55 (53.9) | 47 (46.1) | 102 (63.7) | 0.251 |
| ≥ 3.0 | 25 (43.1) | 33 (56.9) | 58 (36.3) | |
| Biochemical data | Median (iq r) | Median (iq r) | ||
| Glucose (mg/dL) | 91.0 (14.2) | 91.5 (14.2) | 0.702 | |
| HOMA-IR | 2.35 (2.24) | 2.26 (2.59) | 0.992 | |
| Triglycerides (mg/dL) | 96.5 (60.2) | 96.0 (67.2) | 0.572 | |
| Total cholesterol (mg/dL) | 149.0 (49.7) | 160.0 (44.0) | 0.422 | |
| HDL (mg/dL) | 44.0 (14.2) | 44.0 (15.5) | 0.302 | |
| LDL (mg/dL) | 90.5 (40.7) | 90.0 (43.0) | 0.942 | |
| AST (U/L) | 59.5 (40.7) | 52.0 (44.5) | 0.142 | |
| ALT (U/L) | 76.5 (54.5) | 73.5 (53.0) | 0.382 | |
| γGT (U/L) | 102.0 (118.0) | 87.0 (114.0) | 0.712 | |
| Alkaline phosphatase (U/L) | 79.0 (33.5) | 80.0 (53.2) | 0.492 | |
Biochemical data presented in Table 2 show that glucose and lipid profiles were not altered; however, high transaminase levels were observed in both groups. It is also observed that the median value for γGT is greater in the CG than in the IG (102.0 U/L vs 87.0 U/L); however, no significant difference was detected between the groups. Median alkaline phosphatase was in the normal range for both groups. Baseline demographic, anthropometric, clinical and laboratory data show that the study population had a homogeneous distribution between the groups.
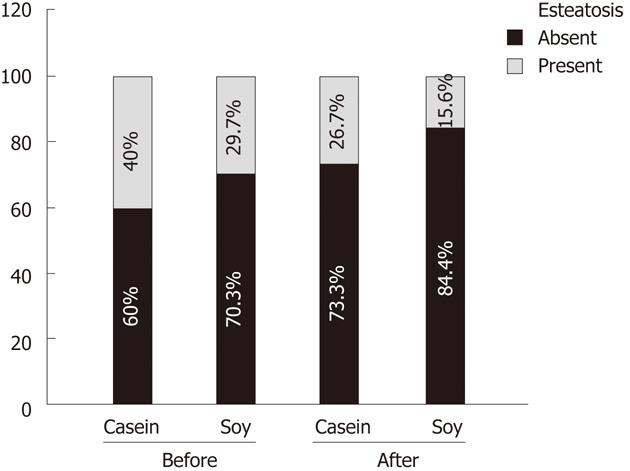
Table 3 shows the comparison of the prevalence of hepatic steatosis before and after the intervention in the IG and CG. The prevalence of hepatic steatosis was different between the groups at baseline (29.7% vs 40.0%) and remained different after the interventions (15.6% vs 26.7%), but without statistical significance in both instances (Figure 1).
| Hepatic Steatosis | n | Yes | No | P value |
| Before | 0.26041 | |||
| IG-soy | 64 | 19 (29.7) | 45 (70.3) | |
| CG-casein | 60 | 24 (40.0) | 36 (60.0) | |
| After | 0.18501 | |||
| IG-soy | 64 | 10 (15.6) | 54 (84.4) | |
| CG-casein | 60 | 16 (26.7) | 44 (73.3) | |
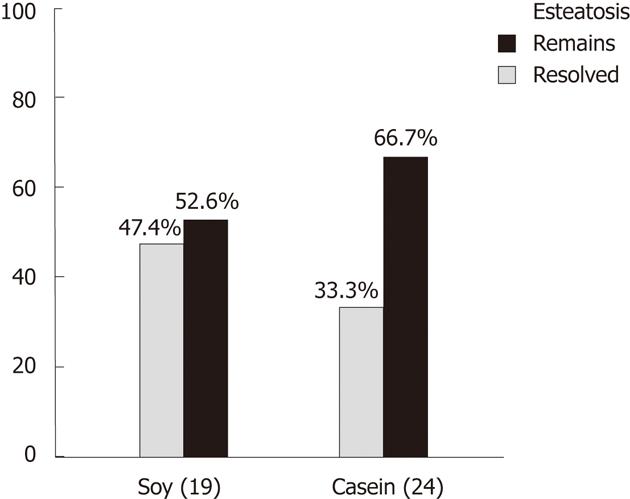
| IG-soy: before vs after | 0.0076 2 | |||
| With hepatic steatosis | 19 | 10 (52.6) | 9 (47.4) | |
| Without hepatic steatosis | 45 | 0 (0.0) | 45 (100.0) | |
| GC-casein: before vs after | 0.01332 | |||
| With hepatic steatosis | 24 | 16 (66.7) | 8 (33.3) | |
| Without hepatic steatosis | 36 | 0 (0.0) | 36 (100.0) |
Paired analysis, within groups (before and after protein supplementation), showed the reduction in hepatic steatosis was significant for both groups. The IG showed a reduction from 19 to 10 cases (47.4% reduction, P = 0.0076) and the CG decreased from 24 to 16 cases (33.3% reduction, P = 0.0133) (Figure 2 and Table 3).
After protein supplementation, a significant reduction was observed in the transaminase levels of the IG vs the CG (AST: 49.5 U/L vs 61.5 U/L, P = 0.02; ALT: 64.0 U/L vs 73.0 U/L, P = 0.007). A reduction in the levels of γGT was observed in the IG compared to the CG (84.0 U/L vs 108.0 U/L, P = 0.09) but without statistical significance (Table 4).
| CG-casein | IG-soy | Treatment difference | |||
| Exams | n | median (iq r) | n | median (iq r) | P value1 |
| Total cholesterol (mg/dL) | 80 | 152.0 (61.5) | 80 | 153.0 (44.7) | 0.54 |
| HDL (mg/dL) | 80 | 46.0 (17.0) | 80 | 43.5 (15.2) | 0.21 |
| LDL (mg/dL) | 80 | 89.5 (45.0) | 80 | 84.5 (41.5) | 0.39 |
| AST (U/L) | 78 | 61.5 (40.5) | 80 | 49.5 (44.2) | 0.02 |
| ALT (U/L) | 78 | 73.0 (65.7) | 79 | 64.0 (50.0) | 0.007 |
| γGT (U/L) | 75 | 108.0 (120.5) | 73 | 84.0 (107.0) | 0.09 |
| HOMA-IR | 79 | 2.4 (2.7) | 80 | 2.6 (2.2) | 0.91 |
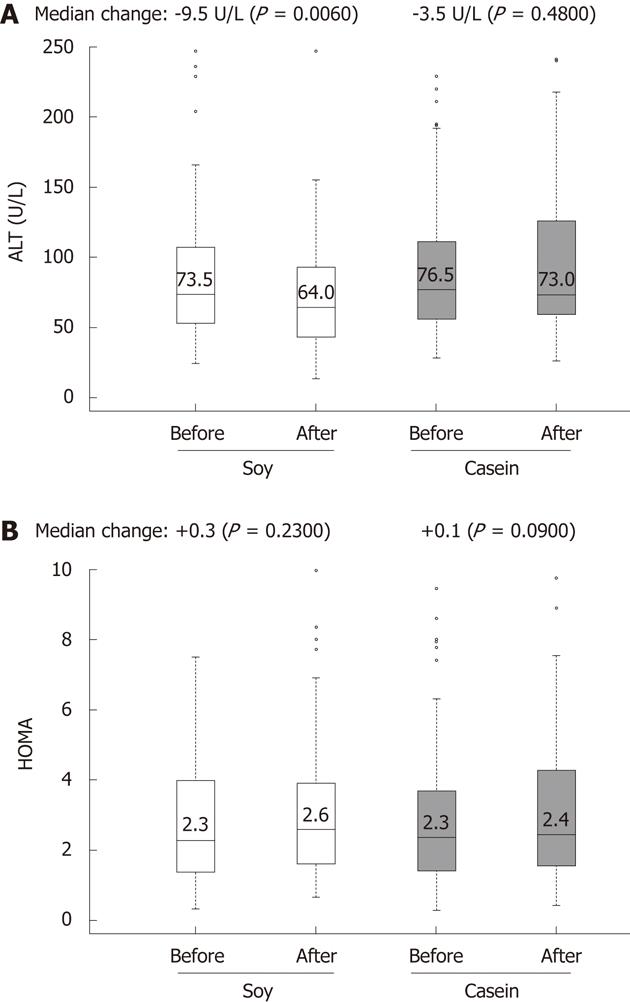
The IG had a significant reduction in ALT levels (73.5 U/L vs 64.0 U/L; 12.92% change, P = 0.006) (Figure 3) and γGT levels (87.0 U/L vs 84.0 U/L; 3.45% change, P = 0.007) after 12 wk of intervention. Reductions were also observed in total cholesterol (160.0 mg/dL vs 153.0 mg/dL; 4.37% change), but without statistical significance (Table 5). It is noteworthy that the CG did not present improvements in these biochemical tests.
| IG-soy | CG-casein | |||||||||||
| Baseline | 12 wk | Baseline | 12 wk | |||||||||
| Exams | n | Median (iq r) | Median (iq r) | change | % change | P value1 | n | Median (iq r) | Median (iq r) | change | % change | P value1 |
| Total cholesterol (mg/dL) | 80 | 160.0 (44.0) | 153.0 (44.7) | -7.0 | -7.37 | 0.05 | 80 | 149.0 (49.7) | 152.0 (61.5) | +3.0 | +2.01 | 0.63 |
| HDL (mg/dL) | 79 | 44.0 (15.5) | 43.5 (15.2) | -0.5 | -1.14 | 0.69 | 80 | 44.0 (14.2) | 46.0 (17.0) | +2.0 | +5.54 | 0.13 |
| LDL (mg/dL) | 78 | 90.0 (43.0) | 84.5 (41.5) | -5.5 | -6.11 | 0.03 | 80 | 90.5 (40.7) | 89.5 (45.0) | -1.0 | -1.10 | 0.66 |
| AST (U/L) | 80 | 52.0 (44.5) | 49.5 (44.2) | -2.5 | 4.81 | 0.32 | 78 | 59.5 (40.7) | 61.5 (40.5) | +2.0 | +3.36 | 0.04 |
| ALT (U/L) | 76 | 73.5 (53.0) | 64.0 (50.0) | -9.5 | -12.92 | 0.006 | 78 | 76.5 (54.5) | 73.0 (65.7) | -3.5 | -4.57 | 0.48 |
| γGT (U/L) | 73 | 87.0 (114.0) | 84.0 (107.0) | -3.0 | -3.45 | 0.007 | 75 | 102.0 (118.0) | 108.0 (120.5) | +6.0 | +5.88 | 0.19 |
| HOMA-IR | 80 | 2.3 (2.6) | 2.6 (2.2) | +0.3 | +13.04 | 0.23 | 79 | 2.3 (2.2) | 2.4 (2.7) | +0.1 | +4.35 | 0.09 |
Table 6 presents the logistic regression analysis for predictive factors of hepatic steatosis. Multiple regression modeling indicates that, in the presence of severe fibrosis (F3/F4), γGT elevation and HDL reduction, the IG had a 75% less chance of developing hepatic steatosis (OR = 0.25; 95% CI: 0.06-0.82). Nevertheless, in the IG, those with an HOMA-IR ≥ 3 were three times more likely to develop hepatic steatosis (OR = 3.49; 95% CI: 1.10-11.90) (Table 6). The bivariate analysis also revealed that an age ≥ 60 years (crude OR = 3.81; 95% CI: 1.50-9.70), abdominal fat accumulation (crude OR = 3.87; 95% CI: 1.57-10.29) and BMI ≥ 25.0 kg/m2 (crude OR = 1.32; 95% CI: 1.12-1.61) were independent risk factors for hepatic steatosis (data not shown).
| Bivariate analysis | Multivariate analysis | |||
| Crude OR (95% IC) | P value | Adjusted OR1 (95% IC) | P value | |
| Groups | ||||
| Control-casein | 1 | 1 | ||
| IG-soy | 0.51 (0.20-1.22) | 0.135 | 0.25 (0.06-0.82) | 0.032 |
| HOMA-IR | ||||
| < 3.0 | 1 | 1 | ||
| ≥ 3.0 | 1.97 (0.82-4.77) | 0.129 | 3.49 (1.10-11.90) | 0.037 |
| Stage of fibrosis | ||||
| F0/F1/F2 | 1 | 1 | ||
| F3/F4 | 1.78 (0.68-4.69) | 0.239 | 1.59 (0.50-5.40) | 0.436 |
| γGT | ||||
| < 85 U/L | 1 | 1 | ||
| ≥ 85 U/L | 2.07 (0.81-5.64) | 0.135 | 1.52 (0.47-5.18) | 0.487 |
| HDL-C | ||||
| ≥ 35 mg/dL | 1 | 1 | ||
| < 35 mg/dL | 1.25 (0.41-3.40) | 0.677 | 2.18 (0.56-8.21) | 0.247 |
Predictors of insulin resistance (HOMA-IR≥3.0) after intervention
Soy treatment did not have any effect on insulin resistance (OR = 1.92; 95% CI: 0.80-4.83). In patients with advanced fibrosis, an ALT level ≥ 1.5 times upper limit of normal (ULN) and increased abdominal fat accumulation were independent risk factors for insulin resistance. Logistic regression analysis showed that the presence of severe fibrosis promoted a five-fold increase in the chance (OR = 5.25; 95% CI: 2.17-13.67) for an HOMA-IR ≥ 3.0 as well as accumulation of abdominal fat (OR = 5.57; 95% CI: 2.23-15.27). Those with an ALT level ≥ 1.5 × ULN had a three times greater chance of developing insulin resistance. Being single, widowed or divorced was a protective factor (OR: 0.24; 95% CI: 0.08-0.65) for an HOMA-IR ≥ 3.0 (Table 7).
| Bivariate analysis | Multivariate analysis | |||
| Crude OR (95% IC) | P value | Adjusted OR1 (95% IC) | P value | |
| Groups | ||||
| Control-casein | 1 | 1 | ||
| Intervention-soy | 0.90 (0.47-1.70) | 0.745 | 1.92 (0.80-4.83) | 0.15 |
| Stage of fibrosis | ||||
| F0/F1/F2 | 1 | 1 | ||
| F3/F4 | 4.21 (1.99-9.19) | 0.0002 | 5.25 (2.17-13.67) | 0.0004 |
| Alanine aminotransferase | ||||
| 1.5 times below ULN | 1 | 1 | ||
| 1.5 times above ULN | 2.22 (1.16-4.30) | 0.017 | 3.26 (1.30-8.71) | 0.014 |
| Marital status | ||||
| Married | 1 | 1 | ||
| Single, widowed or divorced | 0.52 (0.24-1.07) | 0.081 | 0.24 (0.08-0.65) | 0.007 |
| Waist circumference2 | ||||
| Adequate | 1 | 1 | ||
| Inadequate | 2.62 (1.37-5.16) | 0.004 | 5.57 (2.23-15.27) | 0.0004 |
The independent predictive factors for changes in ALT levels (≥ 1.5 × ULN) were an HOMA-IR ≥ 3.0, HDL < 35 mg/dL and being a male subject (Table 8). Severe fibrosis and alterations in AST and γGT levels were also independent predictors of an increased ALT level (≥ 1.5 × ULN). Multivariate analysis showed that supplementation with soy protein per se represents a protective factor; the IG had a 55% less chance of presenting with an ALT level ≥ 1.5 × ULN (OR = 0.45, 95% CI: 0.22-0.89), and subjects with an HOMA-IR ≥ 3.0 were three times more likely to have an increased ALT level (OR = 3.16, 95% CI: 1.51-6.93). However, females had 72% less chance to have an increased ALT level (OR = 0.28, 95% CI: 0.12-0.60) (Table 8).
| Bivariate analysis | Multivariate analysis | |||
| Crude OR (95% IC) | P value | Adjusted OR1 (95% IC) | P value | |
| Groups | ||||
| Control-casein | 1 | 1 | ||
| IG-soy | 0.55 (0.29-1.04) | 0.068 | 0.45 (0.22-0.89) | 0.024 |
| HOMA-IR | ||||
| < 3.0 | 1 | 1 | ||
| ≥ 3.0 | 2.22 (1.16-4.30) | 0.017 | 3.16 (1.51-6.93) | 0.001 |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 0.39 (0.20-0.77) | 0.007 | 0.28 (0.12-0.60) | 0.003 |
| HDL-C | ||||
| ≥ 35 mg/dL | 1 | 1 | ||
| < 35 mg/dL | 3.15 (1.37-7.76) | 0.008 | 2.85 (1.18-7.40) | 0.024 |
Our population was predominantly male, infected with HCV genotype 1, overweight and presented abdominal fat accumulation. Both studied groups had similar characteristics. These clinical conditions increase the chances of developing insulin resistance and hepatic steatosis, which have a negative impact in patients with chronic hepatitis C[21]. In patients infected with HCV genotype 1, steatosis is frequently associated with metabolic syndrome and insulin resistance and is also called “metabolic steatosis”[4,8,21].
A large proportion of our patients had increased liver enzymes (i.e., ALT, AST, γGT) and had not yet been subjected to antiviral treatment. In our population, at baseline, there was a 34.7% prevalence of hepatic steatosis and a 36.3% prevalence of an HOMA-IR ≥ 3.0. The prevalence of hepatic steatosis associated with HCV varies widely in the literature[22,23] and may differ depending on the population profiles[24].
In this study, protein supplementation caused a significant reduction of hepatic steatosis in both groups; however, this reduction was not significant between the groups. The probable mechanism is not associated with the quality of protein supplementation (animal or vegetable) but likely the nutritional care offered to both groups, which promoted changes in eating habits and consequently improved the overall quality of the diet. Of note, there was no change in body mass index (BMI) or in the pattern of physical activity of these patients.
It is controversial whether insulin resistance is a cause or consequence of steatosis, however the literature suggests that it seems to work more like a cause than a consequence of steatosis in patients infected with HCV genotype 1[2]. In the present study, the regression model showed that an HOMA-IR ≥ 3 increased the chances of hepatic steatosis more than three-fold. The regression model also revealed that advanced age (≥ 60 years) and a higher waist circumference and BMI were independent predictor factors for hepatic steatosis. These results are in agreement with other studies which have observed a direct correlation between BMI, visceral obesity and liver steatosis[25-27].
Clinical and experimental studies suggest that soy protein and isoflavones can synergistically act to promote a greater benefit in controlling hypercholesterolemia, hypertriglyceridemia, insulin resistance and steatosis[13,28,29]. We observed that consumption of soy protein had a protective effect and was associated with 75% less chance of having hepatic steatosis.
In an experimental study with obese Zucker rats, a diet with isolated soy protein favored reduced triglycerides in the liver. The proportions of AST/ALT, alkaline phosphatase, bile acids in plasma and pro-inflammatory cytokines (TNF-α and IL1) were also reduced. The authors suggested that soy protein enriched with isoflavones has a favorable effect on the inflammatory status of obese mice, which may promote a favorable outcome in NAFLD patients[28]. It is known that oxidative stress is a decisive factor in the progression of steatosis[30,31]; thus, if isoflavones can act as an antioxidant, then they may minimize the negative progression of steatosis[32,33]. The morbidity of hepatic steatosis is increasing and has been recognized as a liver component of the metabolic syndrome, which also has a negative effect on HCV treatment[8,26,34-36].
Our data revealed a significant decrease in ALT values after supplementation with soy protein compared to the control patients who consumed casein. These findings agree with experimental studies, which have found that soy protein enriched with isoflavones reduces plasma aminotransferase levels[32] and the proportion of AST/ALT[28]. However, a reduction in HOMA-IR levels after supplementation with soy was not observed in our study, which can be attributed to the fact that the HOMA-IR values at baseline in most of our patients were in the normal range. In contrast, Jayagopal et al[13] in a study conducted with diabetic women in which the mean value of the HOMA-IR was 5.54 in the intervention group and 5.14 in the control group, supplementation with soy protein enriched with isoflavones significantly reduced serum insulin and the HOMA-IR.
We found that insulin resistance (HOMA-IR ≥ 3.0) and lower HDL values were predictors for increased ALT. Soy protein intervention in female subjects presented per se as a protective factor for increased ALT levels. Our data are in agreement with a recent study that showed higher levels of ALT were significantly associated with gender, a low HDL level and a high HOMA-IR[25]. When evaluating patients with HCV with and without changes of ALT levels and healthy controls, Addel-Azziz et al[37] found a higher value of HOMA-IR in patients with abnormal ALT levels compared with those with no change in ALT levels and healthy controls (3.98 vs 2.69 vs 1.92, respectively), with a significant difference between those with abnormal ALT levels and controls (3.98 vs 1.92).
In our study, abdominal fat concentration, an ALT level ≥ 1.5 the upper limit of normal and the presence of advanced fibrosis were independent predictors of insulin resistance, and even in a multivariate model, they remained significant. Addel-Azziz et al[37] also detected a positive correlation between the HOMA-IR and fibrosis. We detected that marital status was a predictor for insulin resistance as well; single, widowed or divorced patients were less susceptible to inadequacy of the HOMA-IR. This could be associated with the fact that a higher prevalence of overweight and abdominal fat accumulation has been described among married subjects.
In patients with hepatitis C, the presence of a high BMI, insulin resistance and high cholesterol are important predictors for mortality. Multivariate analysis has shown increased mortality associated with metabolic disorders such as diabetes, hypertension and a higher BMI[36,38]. Mehta et al[39] found that individuals with HCV and an age above 40 years had a three-fold higher chance of presenting with type 2 diabetes. Therefore, it is recommended that all patients with chronic hepatitis C avoid excess weight and maintain blood glucose, cholesterol levels and blood pressure within normal ranges[40].
Soy supplementation decreased ALT levels and thus may improve liver inflammation in HCV patients. It also reduced hepatic steatosis in a subgroup of individuals with advanced fibrosis, insulin resistance, increased γGT levels and low HDL. On the other hand, soy supplementation did not change insulin resistance, which might be attributed to the fact that the HOMA-IR values at baseline in most of our patients were in the normal range. To our knowledge, this is the first study to show that soy protein supplementation reduces hepatic steatosis and decreases ALT levels in chronic hepatitis C patients. Control of insulin resistance, hepatic steatosis, abdominal obesity and body weight seems to play an essential role in nonpharmacological therapies for chronic hepatitis C treatment. These practices should therefore be encouraged by a multidisciplinary team. Supplementation with soy protein should be considered as an important choice of nutritional management of patients with chronic hepatitis C.
Hepatitis C virus (HCV) infection is an important public health problem and is the leading cause of liver transplantation in the Western world. Chronic HCV infection increases the risk for hepatic steatosis, insulin resistance, glucose intolerance and type 2 diabetes. The improvement of these comorbidities may benefit the clinical course of the patients.
Several studies have shown that soy protein may stimulate peroxisome proliferator-activated receptors-α and thus might increase liver fatty oxidation and decrease hepatic steatosis. It also may inhibit sterol regulatory element-binding transcription factor 1 and decrease hepatic lipogenesis. Clinical studies have previously demonstrated that soy consumption may reduce plasma lipid levels, promote insulin resistance reduction and maintain normal glucose levels. HCV infection may be associated with hepatic steatosis and increased insulin resistance. The morbidity of hepatic steatosis is increasing and it has been recognized as a liver component of the metabolic syndrome, which also has a negative effect on HCV treatment. The role of soy supplementation in the improvement of liver diseases is still a matter of debate and there are no studies that have evaluated its effect on insulin resistance, liver fat content and alanine transaminase (ALT) levels in non-diabetic patients with chronic hepatitis C.
Our work is characterized by its originality since it evaluated the impact of soy nutritional intervention in a population of patients infected with hepatitis C. To our knowledge, this is the first study to show that soy protein supplementation decreases ALT levels and reduces hepatic steatosis in a subgroup of individuals with advanced fibrosis, insulin resistance, increased γGT levels and low HDL in chronic hepatitis C. In an experimental study with obese Zucker rats, a diet with isolated soy protein favored reduced triglycerides in the liver. The AST/ALT ratio, alkaline phosphatase, bile acids in plasma and pro-inflammatory cytokines (tumor necrosis factor-α and interleukin-1) were also reduced. However, there are no clinical studies that have evaluated the role of soy supplementation on liver enzymes levels, insulin resistance and hepatic steatosis of patients with chronic HCV infection. The authors emphasize the need for further clinical trials to confirm the soy effects on ALT levels and hepatic steatosis of patients with chronic HCV infection.
This study showed that soy supplementation may improve liver inflammation (decrease ALT level), and may improve steatosis in a sub-group of patients with HCV. Therefore, supplementation with soy protein should be considered in the nutritional management of patients with chronic hepatitis C. However, further clinical trials are necessary to confirm our results.
HOMA-IR: Homeostasis model assessment index of insulin resistance; PPARs: Peroxisome proliferator-activated receptors. These are nuclear receptors that function as transcription factors regulating the expression of genes. PPARs play essential roles in the regulation of cellular differentiation and metabolism (carbohydrate, lipid, protein); SREBP-1: Sterol regulatory element-binding transcription factor 1 is a transcription factor involved in sterol biosynthesis.
The topic of the study is interesting and based on rational logic. The design of the study is appropriate, but to be sure of that we need to know some more information about the recruitment, randomization and blinding method. In a clinical trial design the conclusions are based only in the analysis performed for the main objectives of the study, otherwise the conclusion from sub-analysis must be biased.
Peer reviewer: Juan-Ramón Larrubia, PhD, Gastroenterology Unit and Liver Research Unit, Guadalajara University Hospital, Donante de Sangre s/n, 19002 Guadalajara, Spain
S- Editor Cheng JX L- Editor Logan S E- Editor Xiong L
| 1. | Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22:991-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 3. | Delgado-Borrego A, Liu YS, Jordan SH, Agrawal S, Zhang H, Christofi M, Casson D, Cosimi AB, Chung RT. Prospective study of liver transplant recipients with HCV infection: evidence for a causal relationship between HCV and insulin resistance. Liver Transpl. 2008;14:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Zekry A, McHutchison JG, Diehl AM. Insulin resistance and steatosis in hepatitis C virus infection. Gut. 2005;54:903-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S34-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285-300. [PubMed] |
| 7. | McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 8. | Romero-Gmez M. Hepatitis C and insulin resistance: steatosis, fibrosis and non-response. Revista Espanola de Enfermedades Digestivas. 2006;98:605-615. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care. 2006;29:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014-5019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | He Q, Graham CS, Durante Mangoni E, Koziel MJ. Differential expression of toll-like receptor mRNA in treatment non-responders and sustained virologic responders at baseline in patients with chronic hepatitis C. Liver Int. 2006;26:1100-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Barnes S. Evolution of the health benefits of soy isoflavones. Proc Soc Exp Biol Med. 1998;217:386-392. [PubMed] |
| 15. | Park D, Huang T, Frishman WH. Phytoestrogens as cardioprotective agents. Cardiol Rev. 2005;13:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13-15. [PubMed] |
| 17. | Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401-1405. [PubMed] |
| 18. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24499] [Article Influence: 612.5] [Reference Citation Analysis (0)] |
| 19. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1415] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 20. | R Foundation For Statistical Computing R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: http: //www.R-project.org (accessed October 2010). |
| 21. | Parise ER, Oliveira AC. [Insulin resistance in chronic hepatitits C]. Arq Gastroenterol. 2007;44:178-184. [PubMed] |
| 22. | Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, Adinolfi LE, Ruggiero G, Carulli N, Loria P. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Giannini C, Giannelli F, Monti M, Careccia G, Marrocchi ME, Laffi G, Gentilini P, Zignego AL. Prevalence of mixed infection by different hepatitis C virus genotypes in patients with hepatitis C virus-related chronic liver disease. J Lab Clin Med. 1999;134:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kobayashi Y, Kawaguchi Y, Mizuta T, Kuwashiro T, Oeda S, Oza N, Takahashi H, Iwane S, Eguchi Y, Anzai K. Metabolic factors are associated with serum alanine aminotransferase levels in patients with chronic hepatitis C. J Gastroenterol. 2011;46:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Lo Iacono O, Venezia G, Petta S, Mineo C, De Lisi S, Di Marco V, Rodolico V, Amato M, Ferraro D, Giordano C. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, Brun JM, Hillon P. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Gudbrandsen OA, Wergedahl H, Berge RK. A casein diet added isoflavone-enriched soy protein favorably affects biomarkers of steatohepatitis in obese Zucker rats. Nutrition. 2009;25:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Peluso MR, Winters TA, Shanahan MF, Banz WJ. A cooperative interaction between soy protein and its isoflavone-enriched fraction lowers hepatic lipids in male obese Zucker rats and reduces blood platelet sensitivity in male Sprague-Dawley rats. J Nutr. 2000;130:2333-2342. [PubMed] |
| 30. | Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135-G1139. [PubMed] |
| 31. | Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VW, Chan HL, Farrell GC, Sung JJ. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology. 2010;138:694-704, 704.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Gudbrandsen OA, Wergedahl H, Mørk S, Liaset B, Espe M, Berge RK. Dietary soya protein concentrate enriched with isoflavones reduced fatty liver, increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Br J Nutr. 2006;96:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Wiseman H, O'Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, Sanders TA. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72:395-400. [PubMed] |
| 34. | Negro F. Peroxisome proliferator-activated receptors and hepatitis C virus-induced insulin resistance. PPAR Res. 2009;2009:483485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Younossi ZM, McCullough AJ. Metabolic syndrome, non-alcoholic fatty liver disease and hepatitis C virus: impact on disease progression and treatment response. Liver Int. 2009;29 Suppl 2:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Abdel-Azziz MY, Zalata KR, El-Bendary MM. Insulin resistance and liver fibrosis progression in patients with chronic hepatitis C virus infection. Arab J Gastroenterol. 2010;11:30-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Oh MK, Winn J, Poordad F. Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] |
| 40. | Kim CH, Kallman JB, Bai C, Pawloski L, Gewa C, Arsalla A, Sabatella ME, Younossi ZM. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes Surg. 2010;20:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |