Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2127
Revised: February 3, 2012
Accepted: February 16, 2012
Published online: May 7, 2012
AIM: To investigate the possible reasons and suggest therapeutic plan of stress-induced intestinal necrosis resulting from the severe trauma.
METHODS: Three patients in our study were trapped inside collapsed structures for 22, 21 and 37 h, respectively. The patients underwent 3-4 operations after sustaining their injuries. Mechanical ventilation, intermittent hemodialysis and other treatments were also provided. The patients showed signs of peritoneal irritation on postoperative days 10-38. Small intestinal necrosis was confirmed by emergency laparotomy, and for each patient, part of the small bowel was removed.
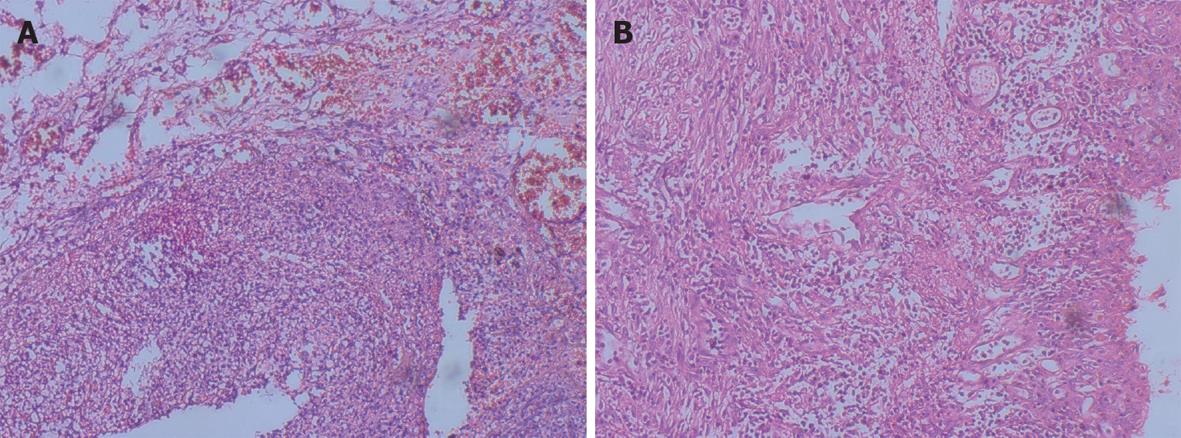
RESULTS: Two patients who all performed 3 operations died of respiratory complications on the first and second postoperative days respectively. The third patient who performed 4 operations was discharged and made a full recovery. Three patients had the following common characteristics: (1) Multiple severe trauma events with no direct penetrating gastrointestinal injury; (2) Multiple surgeries with impaired renal function and intermittent hemodialysis treatment; (3) Progressive abdominal pain and tenderness, and peritoneal irritation was present on post-traumatic days 10-38; (4) Abdominal operations confirmed segment ulcer, necrosis of the small intestine, hyperplasia and stiffness of the intestinal wall; and (5) Pathological examinations suggested submucosal hemorrhage, necrosis, fibrosis and hyalinization of the vascular wall. Pathological examinations of all 3 patients suggested intestinal necrosis with fistulas.
CONCLUSION: Intestinal necrosis is strongly asso-ciated with stress from trauma and post-traumatic complications; timely exploratory laparotomy maybe an effective method for preventing and treating stress-induced intestinal necrosis.
- Citation: Gong JQ, Zhang GH, Tian FZ, Wang YH, Zhang L, Cao YK, Wang PH. Stress-induced intestinal necrosis resulting from severe trauma of an earthquake. World J Gastroenterol 2012; 18(17): 2127-2131
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2127
Intestinal necrosis is a common condition that is frequently seen in clinical practice[1-6]. It is generally associated with mesenteric thrombosis or bacterial infection after eating contaminated foods[5,6]. However, few cases of stress-induced intestinal necrosis have been reported. Although animal studies have confirmed that stress can cause intestinal mucosal barrier disturbances[7,8], stress-induced intestinal necrosis has not been confirmed. We analyzed the clinical data of 3210 patients who were admitted to our hospital after the Wenchuan earthquake on May 12, 2008. In the laparotomy examinations of 3 patients with no penetrating abdominal trauma, intestinal necrosis was confirmed on postoperative days 10-38. Two of these patients died, while 1 survived and made a full recovery. For these 3 cases, we made the diagnosis of “stress-induced intestinal necrosis” after repeated careful consideration.
The 3 male patients were 27, 36 and 42 years old, respectively (average age of 35 years). They were trapped inside collapsed structures for 22, 21 and 37 h, respectively (average time of 26.3 h). They were admitted into our hospital at 37, 44 and 39 h, respectively, after trapped in the buildings. Their primary earthquake-related traumatic injuries mainly occurred on the head and extremities, including 1 case with a left thorax crush injury. The physician specialists in various departments of our hospital consulted and discussed the cases with experts from The Liberation Army General Hospital of Beijing immediately after the patients’ admission. Treatment plans were designed individually by a multi-disciplinary team. The patients underwent 4, 3 and 3 surgeries, respectively, according to their clinical features. The final operation for each patient, which was a small bowel resection, was performed on postoperative days 38, 21 and 10, respectively. Antibiotic therapy, intravenous fluid hydration, mechanical ventilation and intermittent hemodialysis were administered to all 3 patients after admission.
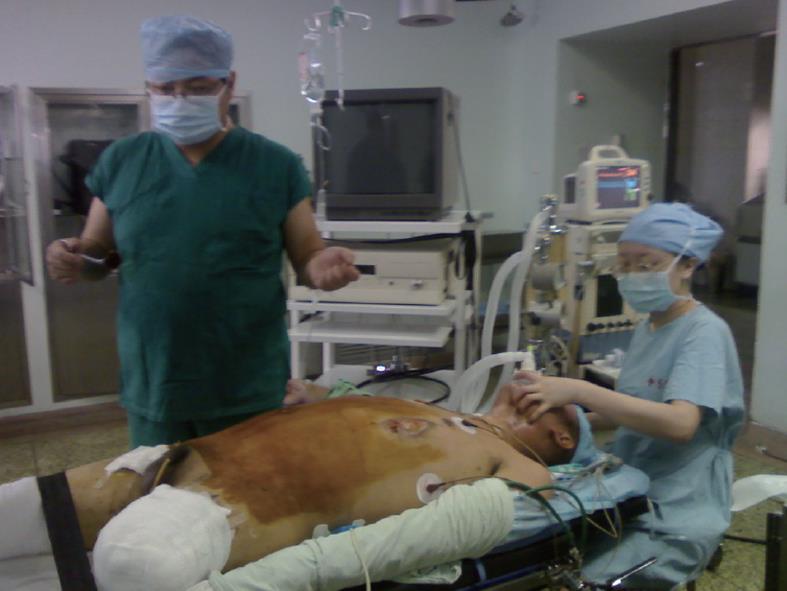
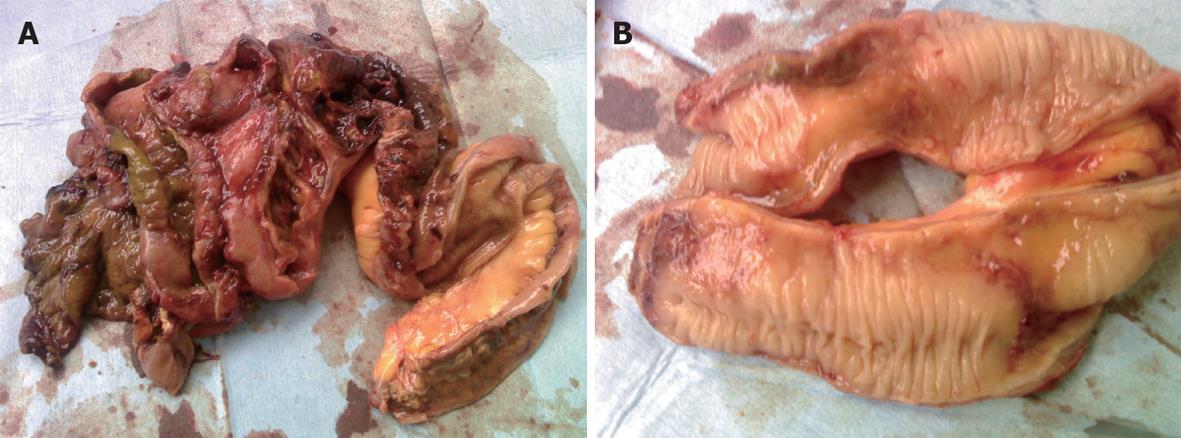
A 27-year-old healthy male patient with a relatively healthy medical history had multiple body injuries due to high-force impacts from a building collapse in the Wenchuan earthquake on May 12, 2008. He was rescued after being trapped in building debris for 22 h. He was admitted to our hospital on May 14. He remained conscious and presented with multiple contusions on the head and both lower extremities at the time of admission. Neither of his legs could be moved freely, and both were significantly swollen. The pulse of the bilateral dorsalis pedis was absent. The patient’s toes presented as dark purple with poor blood circulation. A physical examination showed no signs of abdominal trauma. At the time of admission, emergency decompression surgery was performed on the osteofascial compartment of the left lower extremities under local anesthesia. We suspected that the patient had acute renal failure due to a lack of urination, and hemodialysis was administrated. On May 16, the patient underwent emergency amputation of both legs at the mid-thigh. On May 20, the patient experienced abdominal distension with diarrhea; blood appeared in a stool sample. On May 24, the patient developed worse abdominal distention, mild tenderness around the belly button and no rebound tenderness or muscle tension. Bowel sounds were absent. An ultrasound test revealed intestinal expansion and a small amount of peritoneal fluid. On May 26, jejunum drainage was placed under gastroscopy. The drainage output was 1000 mL, and the abdominal distension was significantly relieved. On May 30, the patient developed infections in right lower extremitie. A second amputation operation was performed on his right thigh. On June 3, thoracentesis was performed on his left chest cavity due to significantly increased pleural effusion. On June 7, the patient developed dark bloody stool that occurred 3-4 times per day. The patient’s urine output gradually returned to normal by intermittent hemodialysis. On June 19, the patient developed worsening abdominal pain and tenderness, and a mass was noted around the middle and lower abdomen. Peritoneal irritation was obviously present. On emergency abdominal exploration (Figure 1), significant intestinal adhesion was noted. The intestine approximately 210 cm below the Treitz ligament and 50 cm above the ileocecal valve was expanded, thickened and twisted into a mass, with a large amount of inflammatory exudate. Intestinal adhesion, necrosis and perforation with fistula were noted (Figure 2). The proximal jejunum was expanded and thickening. The patient underwent small bowel resection, which revealed scattered erosion with hemorrhage in the intestinal mucosa. The postoperative pathology report included hemorrhagic enteritis and intestinal adhesion with fistula formation (Figure 3). The patient experienced dark bloody or tarry stools 2 wk after surgery (approximately 200-800 g/d). Antihemorrhagic, antacid therapy and nutritional support were administrated. Eventually, the patient was discharged and made a full recovery.
Two patients from this study died of respiratory failure on the first and second postoperative day (post-traumatic days 22 and 12, respectively). One patient survived. Our patients had the following common characteristics: (1) Multiple severe trauma events with no direct penetrating gastrointestinal injury; (2) Multiple surgeries with impaired renal function and intermittent hemodialysis treatment; (3) Progressive abdominal pain and tenderness, and peritoneal irritation was present on post-traumatic days 10-38; (4) Abdominal operations confirmed segment ulcer, necrosis of the small intestine, hyperplasia and stiffness of the intestinal wall; and (5) Pathological examinations suggested submucosal hemorrhage, necrosis, fibrosis and hyalinization of the vascular wall.
How did we make the diagnosis of intestinal necrosis in these patients? We reviewed the relevant literature and found no related cases. However, cases of stress-induced intestinal injury were relatively common[9-11]. Lu et al[10] studied the stress response in rats under heat conditions. They found severe intestinal mucosal damage associated with changes in gene expression that were related to stress-induced immune regulation in the rat small intestine. Smith et al[11] studied the stress response to early weaning in porcine intestines and found that early weaning in pigs can induce stress and lead to impaired mucosal barrier function. The response to trauma, as a comprehensive stress reaction, can cause stress-induced damage to multiple organs in the body[12,13]. However, stress-induced intestinal necrosis has not been reported previously. There are many reports of gastrointestinal feeding tubes leading to partial necrosis of the small intestine, which were thought to be the result of bacterial infections[14,15].
In the 3 patients in our study, no gastrointestinal feeding tubes were placed. After careful consideration, we made the diagnosis of “stress-induced intestine necrosis”. The differential diagnosis of “acute intestinal necrosis” includes the following: (1) “Stress-induced intestine necrosis” occurs in severe trauma or multiple surgeries, while “acute intestinal necrosis” occurs for unknown reasons and may be related to contaminated food[16]; (2) “Stress-induced intestinal necrosis” has a relatively slow onset, as the 3 patients in this study had signs of peritoneal irritation on post-trauma days 10-38; “acute intestinal necrosis” develops early, usually within 5 d; (3) “Stress-induced intestinal necrosis” has no obvious abdominal pain and mainly presents as abdominal distention with no signs of peritoneal irritation, while “acute intestinal necrosis” presents with acute abdominal pain and unbearable and obvious signs of peritoneal irritation; (4) With regard to intraoperative findings, “stress-induced intestinal necrosis” usually has intestinal epithelial hyperplasia, segmental ulceration induced necrosis and fistula formation, while “acute intestinal necrosis” has segmental necrosis without intestinal wall thickening; and (5) With regard to pathological results, “stress-induced intestinal necrosis” has submucosal necrosis, hyperplasia, fibrosis and a large number of macrophages and cells with hemosiderin within the tissue, while “acute intestinal necrosis” has fibrin deposition within the small arteries, intestinal hemorrhage and necrosis[17].
We believe that intestinal necrosis is associated with a continuous high level of stress due to primary turuma and traumatic complications. Under stress: (1) Intestinal mucosal permeability increases, which results in increases in peritoneal inflammatory exudate[18]; (2) Intestinal bacteria can translocate peritoneally and worsen peritoneal infections[19-21]; (3) The intracellular space within the endothelium of mesenteric vessels increases, allowing blood cells and plasma to leak through the connective tissue and vessel lamina, leading to vascular hyalinization, sclerosis and fibrosis; (4) As the response to stress continues, the sympathetic adrenergic medullary system is highly excited, which can lead to intestinal vasoconstriction and a reduction in the blood supply[22]; (5) As arteriosclerosis and contraction of the mesenteric vessels continues, the blood supply continues to decrease, resulting in hypoxia of the mucosal tissue, acidosis, mucosal necrosis and sloughing, and ulcers; and (6) Another explanation is that stress induces vagal inactivation and consequently suppresses the “cholinergic anti-inflammatory pathway” which might lead to intestinal injury ,even intestinal necrosis[23].
The 3 patients in this study suffered from long periods of crush injuries followed by several surgeries and intermittent hemodialysis. Their bodies were in a state of continuous stress. The long-term reduction in the mesenteric blood supply led to intestinal necrosis.
Relative to acute intestinal necrosis, stress-induced intestinal necrosis is a slow progressive process that is difficult to identify. In the Wenchuan earthquake, we only made diagnoses of stress-induced intestinal necrosis for 3 cases. Two of these patients died, and 1 survived, indicating the difficulty of treating this condition. Due to the small number of cases analyzed, our treatment experience is limited, but the following recommendations can be made. First, the primary trauma should be controlled as soon as possible. Stress was continuously present in part because the primary injury was not treated directly. In the representative case, after both legs were amputated, the infection of the amputated surface was not well controlled, resulting in a second right leg amputation. In addition, intermittent dialysis after renal failure could cause intestinal ischemia-reperfusion injury. Second, early administration of appropriate vasodilator could improve intestinal microcirculation. Third, vasoconstrictors should be used as little as possible. As the blood pressure drops, the application of a vasoconstrictor can increase the blood supply of vital organs such as the heart and brain, but it can reduce the intestinal blood supply. Fourth, intestinal flora should be adjusted appropriately. Many studies indicate that in cases of trauma or weakened immune systems, the intestinal flora can translocate, leading to infections in other parts of the body. A large amount of inflammatory secretions were found in the abdominal cavity of all 3 patients in this study, which may be directly related to intestinal flora that translocated peritoneally. Fifth, the timing of surgery is a key factor. All 3 patients in this study underwent laparotomies after they showed signs of peritoneal irritation. As the results showed, the optimal timing might be prior to this point. We believe that the late diagnosis of intestinal necrosis occurred for the following reasons: (1) Stress-induced intestinal necrosis is a relatively slow process; (2) Intestinal ischemia will inevitably lead to inflammatory exudate. At the same time, the omentum and surrounding intestine can wrap around the injured intestinal regions; and (3) It is only after multiple necroses occur and inflammatory substances cannot be contained that the peritoneum will be stimulated, and signs of peritoneal irritation will be presented. Therefore, the optimal timing of surgery is before the signs of peritoneal irritation. Frequent abdominal ultrasounds and abdominal biopsies can help in determining the optimal timing of surgery.
During intestinal stress-induced injury, fatty acid binding protein (FABP) is widely recognized as a specific marker of intestinal damage[24,25]. FABP is released by epithelial cells of the intestinal mucosa into the circulation following mucosal damage. Studies have shown that the plasma concentration of FABP gradually increases with the severity of shock. Pathological examinations have suggested that the intestinal mucosal damage was becoming progressively worse[26].
Therefore, we could use FABP as a routine test marker for patients with severe trauma. The increased plasma levels of FABP, combined with other abdominal physical signs and ultrasound results, could aid in early abdominal exploration and facilitate the early diagnosis of this condition.
Stress-induced intestinal injury is generally associated with damage to the intestinal mucosal barrier. This type of injury has been confirmed in many animal experiments, but few studies have reported stress-induced intestinal necrosis.
There are many reports of partial of the small intestinal necrosis, which were thought to be the results of mesenteric thrombosis or bacterial infection. However, few authors have reported stress-induced intestinal necrosis.
In this paper, the authors investigated the possible reasons and suggested therapeutic plan of stress-induced intestinal necrosis resulting from the severe trauma of an earthquake. Conclound: Stress-induced intestinal necrosis is strongly associated with high level of stress from trauma and post-traumatic complications; For the therapeutic strategy, the primary trauma should be controlled as soon as possible, and timely exploratory laparotomy maybe an effective method for preventing and treating stress-induced intestinal necrosis.
The patients suffered stress-induced intestinal necrosis should be rarely identified. However, this disease is very dangerous, and even result in death. This paper will offer an alert and instructions for preventing and treating stress-induced intestinal necrosis due to trauma.
Fatty acid binding protein is widely recognized as a specific marker of intestinal damage, and which is released by epithelial cells of the intestinal mucosa into the circulation following mucosal damage.
In this paper, the authors reported 3 patients who suffered Wenchuan earthquake, had no direct abdominal trauma, and presented small intestinal necrosis diagnosed as “stress-induced intestinal necrosis”. This is an interesting paper since, effectively, few data have been published in this domain. And this paper may offer an alert and instructions for preventing and treating stress-induced intestinal necrosis due to trauma.
Peer reviewer: Bruno Bonaz, MD, PhD, Professor, Clinique Universitaire d’Hépato-Gastroentérologie, Department of Gastroenterology Grenoble Hospital, CHU de Grenoble, BP 217, France
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Vitin AA, Metzner JI. Anesthetic management of acute mesenteric ischemia in elderly patients. Anesthesiol Clin. 2009;27:551-567, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yanar H, Taviloglu K, Ertekin C, Ozcinar B, Yanar F, Guloglu R, Kurtoglu M. Planned second-look laparoscopy in the management of acute mesenteric ischemia. World J Gastroenterol. 2007;13:3350-3353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Gazzalle A, Braun D, Cavazzola LT, Wendt LR, Navarini D, Fauri Mde A, Vitola SP. Late intestinal obstruction due to an intestinal volvulus in a pregnant patient with a previous Roux-en-Y gastric bypass. Obes Surg. 2010;20:1740-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Zachariah SK. Adult necrotizing enterocolitis and non occlusive mesenteric ischemia. J Emerg Trauma Shock. 2011;4:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Luo W, Li M, Luo J, He Y. Clinical Analysis of Patients with Autoimmune Disease Complicated by Mesenteric Vein Thrombosis: A Retrospective Study in a Hospital. Hepatogastroenterology. 2011;59:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | van Minnen LP, Blom M, Timmerman HM, Visser MR, Gooszen HG, Akkermans LM. The use of animal models to study bacterial translocation during acute pancreatitis. J Gastrointest Surg. 2007;11:682-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Guven A, Uysal B, Gundogdu G, Oztas E, Ozturk H, Korkmaz A. Melatonin ameliorates necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2011;46:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Jin W, Wang HD, Hu ZG, Yan W, Chen G, Yin HX. Transcription factor Nrf2 plays a pivotal role in protection against traumatic brain injury-induced acute intestinal mucosal injury in mice. J Surg Res. 2009;157:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Lu A, Wang H, Hou X, Li H, Cheng G, Wang N, Zhu X, Yu J, Luan W, Liu F. Microarray analysis of gene expression profiles of rat small intestine in response to heat stress. J Biomol Screen. 2011;16:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE, Blikslager AT, Moeser AJ. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352-G363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | North CS, Pollio DE, Smith RP, King RV, Pandya A, Surís AM, Hong BA, Dean DJ, Wallace NE, Herman DB. Trauma exposure and posttraumatic stress disorder among employees of New York City companies affected by the September 11, 2001 attacks on the World Trade Center. Disaster Med Public Health Prep. 2011;5 Suppl 2:S205-S213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Karunakar MA, Staples KS. Does stress-induced hyperglycemia increase the risk of perioperative infectious complications in orthopaedic trauma patients? J Orthop Trauma. 2010;24:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Sarap AN, Sarap MD, Childers J. Small bowel necrosis in association with jejunal tube feeding. JAAPA. 2010;23:28, 30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Melis M, Fichera A, Ferguson MK. Bowel necrosis associated with early jejunal tube feeding: A complication of postoperative enteral nutrition. Arch Surg. 2006;141:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Renner P, Kienle K, Dahlke MH, Heiss P, Pfister K, Stroszczynski C, Piso P, Schlitt HJ. Intestinal ischemia: current treatment concepts. Langenbecks Arch Surg. 2011;396:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Gurtner C, Popescu F, Wyder M, Sutter E, Zeeh F, Frey J, von Schubert C, Posthaus H. Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells. Infect Immun. 2010;78:2966-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol. 2010;65:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Ocal K, Avlan D, Cinel I, Unlu A, Ozturk C, Yaylak F, Dirlik M, Camdeviren H, Aydin S. The effect of N-acetylcysteine on oxidative stress in intestine and bacterial translocation after thermal injury. Burns. 2004;30:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, Timmerman HM, Ahmed Ali U, Cirkel GA, Bollen TL. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Chan KL, Wong KF, Luk JM. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World J Gastroenterol. 2009;15:4745-4752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, Tang SG, Yang PC. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One. 2008;3:e2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, van Heurn LW, Heineman E, Buurman WA. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol. 2009;43:727-733. [PubMed] |
| 25. | Besnard P, Niot I, Poirier H, Clément L, Bernard A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol Cell Biochem. 2002;239:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Niewold TA, Meinen M, van der Meulen J. Plasma intestinal fatty acid binding protein (I-FABP) concentrations increase following intestinal ischemia in pigs. Res Vet Sci. 2004;77:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |