Published online Apr 28, 2012. doi: 10.3748/wjg.v18.i16.1946
Revised: October 27, 2011
Accepted: February 16, 2012
Published online: April 28, 2012
AIM: To evaluate long-term clinical course of Budd-Chiari syndrome (BCS) and predictive factors associated with the development of hepatocellular carcinoma (HCC) and survival.
METHODS: We analyzed 67 patients with BCS between June 1988 and May 2008. The diagnosis of BCS was confirmed by hepatic venous outflow obstruction shown on abdominal ultrasound sonography, computed tomography, magnetic resonance imaging, or venography. The median follow-up period was 103 ± 156 [interquartile range (IQR)] mo.
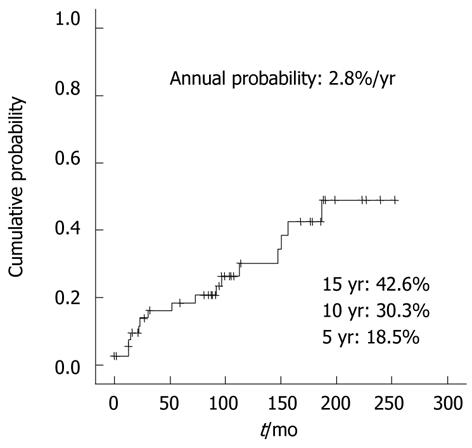
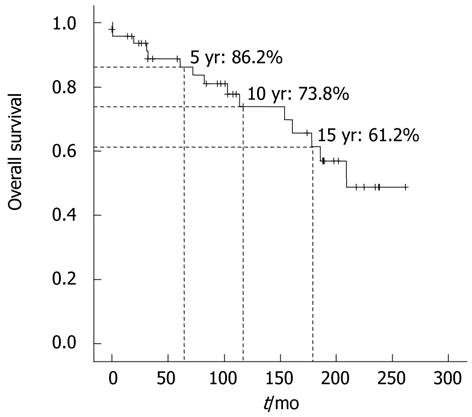
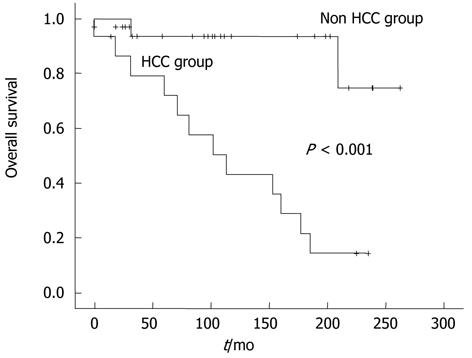
RESULTS: The median age of the patients was 47 ± 16 (IQR) years. At diagnosis, 54 patients had cirrhosis, 25 (37.3%) Child-Pugh class A, 23 (34.3%) Child-Pugh class B, and six (9.0%) patients Child-Pugh class C. During the follow-up period, HCC was developed in 17 patients, and the annual incidence of HCC in patients with BCS was 2.8%. Patients in HCC group (n = 17) had higher hepatic venous pressure gradient (HVPG) than those in non-HCC group (n = 50) (21 ± 12 mmHg vs 14 ± 7 mmHg, P = 0.019). The survival rate of BCS patients was 86.2% for 5 years, 73.8% for 10 years, and 61.2% for 15 years. In patients with BCS and HCC, survival was 79% for 5 years, 43.1% for 10 years, and 21.5% for 15 years.
CONCLUSION: The incidence of HCC in patients with BCS was similar to that in patients with other etiologic cirrhosis in South Korea. The HVPG is expected to provide additional information for predicting HCC development in BCS patients.
- Citation: Park H, Yoon JY, Park KH, Kim DY, Ahn SH, Han KH, Chon CY, Park JY. Hepatocellular carcinoma in Budd-Chiari syndrome: A single center experience with long-term follow-up in South Korea. World J Gastroenterol 2012; 18(16): 1946-1952
- URL: https://www.wjgnet.com/1007-9327/full/v18/i16/1946.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i16.1946
Budd-Chiari syndrome (BCS) is a rare hepatic disease caused by occlusion of the hepatic venous outflow. Thrombogenic conditions among the known causes of BCS are well documented, such as coagulopathy, chronic intake of contraceptives, myeloproliferative diseases, autoimmune diseases, and others[1]. Hepatic venous outflow block can also be caused by malignant tumors[2]. BCS was initially defined as a symptomatic occlusion of the hepatic veins, but with increasing reports on various obstructive cases in the hepatic portion of the inferior vena cava (IVC), it became to include obstructive IVC lesions as well as the major hepatic veins. BCS induces chronic liver congestion so that it causes hepatomegaly, ascites, leg edema, collateral venous dilatation in the body trunk and portal hypertension[3]. Several studies have suggested that hepatic congestion caused by obstruction of hepatic venous outflow can lead to cirrhosis and hepatocellular carcinoma (HCC)[4,5]. The incidence of HCC in patients with BCS has varied according to regions and investigators[3,6-9]. Japan and South Africa showed relatively higher incidences compared to those of United States and France: 6.4%-47.5% vs 4%-20%[5,7,9,10]. Prognosis of HCC in BCS has varied as well[5,6,11].
We followed BCS patients treated at our hospital for more than 20 years, and our long term follow-up data can be helpful to understand the prognosis of BCS patients and natural course of the disease. Thus, we (1) evaluated the incidence and cumulative annual risk of HCC in BCS patients; (2) analyzed the characteristics associated with the development of HCC in BCS patients; and (3) investigated the prognosis of BCS and HCC in BCS patients.
From June 1988 to May 2008, 95 consecutive patients who were diagnosed with BCS at Severance Hospital were studied retrospectively. Among them, 28 patients were excluded based on the criteria as follows: patients with secondary BCS [occlusion of the hepatic venous outflow by an outside structure (HCC, a klatskin tumor, and renal cell carcinoma)], hematologic diseases which could result venous obstructive disease, hepatic septic emboli of colon cancer, or post-operative complication of HCC. Finally 67 patients who were diagnosed with primary BCS were investigated. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and was approved by the institutional review board of our institute.
We retrospectively reviewed patients’ age, gender, and presenting symptoms of decompensate liver cirrhosis such as ascites, encephalopathy and variceal bleeding. We also investigated their history of medication which can induce thrombosis such as oral contraceptive, herbal medication, and steroid. Patients were assessed for other risk factors of cirrhosis and HCC such as alcohol, hepatitis B virus and hepatitis C virus (HCV) infections.
Laboratory tests included complete blood count, prothrombin time, alanine aminotransferase (ALT), bilirubin, albumin, creatinine, viral markers such as hepatitis B virus surface antigen (HBsAg) and anti-HCV antibody. Based on laboratory and physical examination results, Model for End-Stage Liver Disease (MELD) score and Child Pugh score were calculated to evaluate liver function. Every 3-6 mo, imaging studies such as computed tomography (CT), ultrasonography or magnetic resonance imaging (MRI) were performed, and alpha-fetoprotein (AFP) level was checked for HCC surveillance.
The diagnosis of BCS was confirmed by hepatic venous outflow obstruction shown on ultrasound sonography (US), contrast-enhanced CT, MRI, or venography. Patients were confirmed to have HCC according to the American Association for the Study of Liver Disease practice guidelines for the HCC diagnosis[12]. Briefly, patients were diagnosed with HCC if they had a tumor with a maximum diameter of > 2 cm and the typical features of HCC on dynamic CT (defined as enhancement in the arterial phase and early washout in the portal phase), and an AFP > 200 ng/mL[12]. If the maximum diameter of the tumor was 1 cm to 2 cm, dynamic CT and MRI were performed. HCC was diagnosed if coincidental typical features of HCC were noted. If the tumor did not satisfy above criteria, a biopsy was performed. During hospital stay, abdominal ultrasonography was carried out to evaluate the presence of cirrhosis and hepatocellular carcinoma.
The hepatic venous pressure gradient (HVPG) was measured through catheterization during venography or angioplasty. Forty-five patients underwent venography or angioplasty when diagnosed with BCS or during follow up period. Patients were diagnosed with clinical liver cirrhosis if they satisfied one or more of the following 3 conditions: (1) a platelet count < 100 000/μL and ultrasonographic findings suggestive of cirrhosis including a blunted, nodular liver edge accompanied by splenomegaly (> 12 cm)[13,14]; (2) the presence of esophageal or gastric varices; and (3) overt complications of liver cirrhosis, including ascites, variceal bleeding, and hepatic encephalopathy.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 17.0). Descriptive statistics were computed for all variables, including median ± interquartile range (IQR) and percentiles for continuous variables and frequencies for categorical factors. Mann-Whitney U test and χ2-test were used to compare HCC and non-HCC groups. Kaplan-Meier analysis was performed to estimate the cumulative incidence of HCC from the time of BCS diagnosis and survival of all BCS patients and those who developed HCC. To evaluate the factors associated with the development of HCC in patients with BCS, multivariate Cox regression analysis was used. A two-sided P value < 0.05 was considered to indicate a significant difference.
The baseline characteristics of the 67 patients are shown in Table 1. The site of obstruction was IVC in 56 patients (83.6%), hepatic vein in five patients (7.4%), and both IVC and hepatic vein sites in six patients who were classified as “combined”. There was no patient with history of chronic use of medication such as oral contraceptive, herbal medication, and steroid. Patients with heavy alcohol consumption defined as over 80 g/d were 20 (29.9%). HBsAg was positive in 3 patients (4.5%) and there was no patient with positive for anti-HCV antibody. Fifty-four patients had underlying liver cirrhosis at the time of BCS diagnosis. Twenty-five of them (37.3%) were classified as Child-Pugh class A, 23 (34.3%) as class B, and six (9.0%) as class C. The median MELD score was 11 ± 6 (IQR). Twenty three patients (34.3%) had decompensated liver cirrhosis symptoms such as variceal bleeding, uncontrolled ascites or hepatic encephalopathy higher than grade 3. Twenty-seven (40.3%) patients were treated with percutaneous angioplasty, four (5.9%) with shunt operation, three (4.5%) with transjugular intrahepatic portosystemic shunt (TIPS), and one (1.5%) with thrombolysis. Thirty-two (47.7%) patients received only symptomatic medical treatment. The median follow up period was 103 ± 156 (IQR) mo.
| Characteristics | Budd-Chiari syndrome |
| Age (yr) | 47 ± 16 |
| Gender (male) | 34 (50.7) |
| Obstruction site | |
| IVC | 56 (83.6) |
| Hepatic vein | 5 (7.5) |
| Combined | 6 (9.0) |
| Alcohol consumption | |
| None/social/heavy (> 80 g/d) | 32 (47.8)/15 (22.3)/20 (29.9) |
| Positive viral marker | |
| HBsAg/anti-HCV | 3 (4.5)/0 (0) |
| Liver cirrhosis at diagnosis | 54 (80.6) |
| Child-Pugh A/B/C | 25/23/6 (37.3/34.3/9.0) |
| MELD score | 11 ± 6 |
| Decompensate LC symptoms | 23 (34.3) |
| Length of obstruction (cm) | 2.0 ± 4.0 |
| HVPG (mmHg) | 15 ± 10 |
| Laboratory data | |
| ALT (IU/L) | 19 ± 17 |
| Bilirubin (mg/dL) | 1.4 ± 1.3 |
| Albumin (g/dL) | 3.8 ± 0.7 |
| Hemoglobin (g/dL) | 12.3 ± 2.6 |
| Platelet count (k/μL) | 109 ± 78 |
| Creatinine (mg/dL) | 0.9 ± 0.2 |
| PT (%) | 73 ± 27 |
| Treatment modality | |
| Angioplasty | 27 (40.3) |
| Shunt operation | 4 (5.9) |
| TIPS | 3 (4.5) |
| Thrombolysis | 1 (1.5) |
| Symptomatic medical treatment | 32 (47.7) |
| Median follow up period (mo) | 103 ± 156 |
During follow-up periods, HCC was occurred in 17 patients. At the time of diagnosis of HCC, the median age of the patients was 53 ± 12 (IQR) years, and time period between diagnoses of BCS and HCC was 51 ± 115 (IQR) mo (Table 2). HCC was histologically confirmed in 2 patients with hepatic resection. According to the Kaplan-Meier analysis, as shown in Figure 1, the cumulative probability was 18.5% at 5 years, 30.3% at 10 years, and 42.6% at 15 years. The annual occurrence of HCC in BCS patients was 2.8%.
| Variables | HCC |
| Age (yr) | 53 ± 12 |
| Time period from BCS to HCC (mo) | 51 ± 115 |
| Child-Pugh class | |
| A/B/C | 6 (35.3)/8 (47.1)/3 (17.6) |
| Tumor stage (AJCC 6th)1 | |
| I/II/III/IV | 8 (47.1)/6 (35.3)/3 (17.6)/0 (0) |
| Treatment modality | |
| TACE/TACI | 9 (52.9) |
| Intra-arterial chemotherapy | 3 (17.6) |
| Conservative management | 3 (17.6) |
| Operation | 2 (11.9) |
| Prognosis | |
| Alive | 12 (70.5) |
| Death | 3 (17.6) |
| F/U loss | 2 (11.9) |
We compared the baseline characteristics (at the time of diagnosis with BCS) of HCC group (n = 17) with non-HCC group (n = 50). The differences between the two groups are shown in Table 3. There were no significant differences in the comparison of age, gender, obstruction site, alcohol consumption and presence of viral markers between two groups. HVPG was significantly higher in HCC group [21 ± 12 (IQR) mmHg] than in non-HCC group [14 ± 7 (IQR) mmHg] (P = 0.019). However, in the multivariate Cox regression analysis, HVPG showed no significant difference between in HCC group and in non-HCC group (P = 0.452).
| Variables | HCC(n = 17) | Non HCC(n = 50) | P value |
| Age (yr) | 47 ± 11 | 47 ± 18 | 0.863 |
| Gender (male) | 10 (58.8) | 24 (48) | 0.441 |
| Obstructive site | 0.264 | ||
| IVC | 15 (88.2) | 41 (82) | |
| Hepatic vein | 2 (11.8) | 3 (6) | |
| Combined | 0 (0) | 6 (12) | |
| Alcohol consumption | 0.329 | ||
| None | 6 (35.3) | 26 (52.0) | |
| Social | 4 (23.5) | 11 (22.0) | |
| Heavy (> 80 g/d) | 7 (41.2) | 13 (26.0) | |
| Positive for HBsAg | 0 (0) | 3 (6.0) | 0.554 |
| LC at diagnosis | 14 ( 82.4) | 40 (80.0) | 0.832 |
| Decompensate LC symptoms | 8 (47.1) | 15 (28.0) | 0.148 |
| Child Pugh A/B/C | 5/7/2 | 20/16/4 | 0.647 |
| MELD score | 11 ± 6 | 11 ± 6 | 0.778 |
| Follow up period (mo) | 103 ± 146 | 103 ± 160 | 0.648 |
| Length of obstruction (cm) | 1.0 ± 2.5 | 2.0 ± 4.4 | 0.144 |
| HVPG (mmHg) | 21 ± 12 | 14 ± 7 | 0.019 |
| Laboratory data | |||
| ALT (IU/L) | 21 ± 21 | 18 ± 14 | 0.160 |
| Bilirubin (mg/dL) | 1.5 ± 1.9 | 1.3 ± 1.3 | 0.521 |
| Albumin (g/dL) | 3.6 ± 0.85 | 3.8 ± 0.72 | 0.245 |
| Hemoglobin (g/dL) | 12.5 ± 4.1 | 12.3 ± 2.3 | 0.897 |
| Platelet count (k/μL) | 97 ± 59.5 | 113 ± 80.7 | 0.559 |
| Creatinine (mg/dL) | 0.9 ± 0.14 | 0.85 ± 0..24 | 0.798 |
| PT (%) | 71 ± 31 | 74 ± 22 | 0.756 |
| Survival (%) | < 0.001 | ||
| 5 yr | 79 | 93.4 | |
| 10 yr | 43.1 | ||
| 15 yr | 21.5 | 74.7 |
We estimated the overall survival rates of all 67 BCS patients using the Kaplan-Mayer method. The overall survival rate was 86.2% at 5 years, 73.8% at 10 years, and 61.2% at 15 years (Figure 2). During the follow-up periods, three patients among 17 who were diagnosed with HCC died, and two of them died from hepatic failure and the other from massive variceal bleeding. In HCC group, the 5-year survival rate was 79%, 10-year rate 43.1%, and 15-year rate 21.5% (Figure 3). Meanwhile, non-HCC group (n = 50) showed significantly higher survival rates than HCC group: 93.4% for 5 years and 74.7% for 15 years (P < 0.001).
BCS is caused by obstruction of hepatic venous outflow at any level from the small hepatic veins to the junction of the IVC with the right atrium. There are two forms of BCS according to the obstruction site: primary hepatic vein obstruction (classical BCS) and obstruction of the hepatic portion of the inferior vena cava (IVCO). The IVCO form is common in Asia and Africa but rarely reported in Western countries[9,15]. Most of our patients (83.6%) had the IVCO form, which is similar to previous studies which reported the predominance of IVCO form in Asia. The major difference between classical BCS and IVCO is that the former is rarely associated with HCC, while the latter is frequently complicated by HCC[3,8]. In our study, HCC was developed in 17 of 67 patients, and the annual incidence was 2.8%, similar to the incidence in patients with other etiologic cirrhosis in South Korea[16,17].
Until now, the accurate pathogenesis of HCC in BCS has not been elucidated yet. Gwon et al[6] suggested that chronic liver injuries and congestion caused by obstruction of hepatic venous outflow might contribute to a fibrotic process and development of nodular type of HCC. Prolonged congestion can lead to hepatocyte necrosis, and its replacement with fibrous tissue results in fibrosis, which is assumed to be the mechanism of cirrhosis and HCC development[18-20]. This hypothesis is supported by frequent findings of liver parenchymal cirrhotic change adjacent to HCC in BCS context[7].
The HVPG was significantly higher in HCC group than non-HCC group in our study. HVPG has been accepted as the gold standard for assessing the severity of portal hypertension[21]. With the pathogenesis proposed above, the higher pressure gradient means a greater degree of portal hypertension and hepatic congestion; this might have contributed to a higher pressure gradient in HCC group in our study. Our study that showed significantly high HVPG in HCC group is worthy, which supports the hypothesis of development of HCC in patients with BCS. Until now, there were several published reports to analyze the risk factors for HCC in patients with BCS. Although Moucari et al[7] showed that BCS patients with HCC compared with those without HCC presented with IVC obstruction more frequently, there was no report that showed direct differences of pressure gradient as our data presented. For comparison, other published data concerning the prevalence and characteristics of HCC in patients with BCS were reviewed in Table 4.
| Ref. | Matsui et al[3] | Shin et al[24] | Moucari et al[7] | Gwon et al[6] |
| Year, country | 2000, Japan | 2004, South Korea | 2008, France | 2010, South Korea |
| Study design | Retrospective | Retrospective | Retrospective | Retrospective |
| Study period | Apr 1968-Feb 1999 | Mar 1989-Aug 2001 | 1987-2005 | Mar 1990-Nov 2008 |
| No. of patient with BCS | 12 | 73 | 97 | 98 |
| HCC (%) | 3 (25) | 15 (20.5) | 11 (11.3) | 23 (23) |
| Cumulative incidence of HCC | - | - | 4 yr 3% | 1 yr 7.3% |
| 7 yr 6% | 5 yr 13.5% | |||
| 14 yr 12% | 10 yr 31.8% | |||
| Tx. for HCC | Resection (1) | TACE (11) | TACE (7) | TACE (20) |
| TAE (1) | Resection (2) | LT (3) | TACE + LT (3) | |
| iA chemotherapy (1) | Conservative tx. (2) | Conservative tx. (1) | ||
| Survival rate of HCC in patients with BCS | ||||
| Median survival period (mo) | - | 58 (range, 3-59) | - | - |
| Cumulative survival | - | 1 yr 93% | - | 1 yr 90% |
| 2 yr 84% | 2 yr 85% | |||
| 3 yr 72% | 3 yr 61% | |||
| 4 yr 61% | ||||
| 5 yr 46% | ||||
| Risk factors for HCC in patients with BCS | ||||
| Risk factors | Chronic congestion in the liver, caused by an outflow block of hepatic veins | - | Male gender, | Female gender2 |
| Coagulopathy1, | ||||
| IVC obstruction | ||||
| Analysis method | No statistical analysis (mere presumption) | - | Univariate analysis | Multivariate analysis |
Varying survival results of BCS have been reported, and the 5-year survival rate ranges from 69% to 87%[22,23]. In our data, the overall survival rate was 86.2% for 5 years, 73.8% for 10 years, and 61.2% for 15 years. In patients with BCS and HCC, the survival rate was 79% for 5 years, 43.1% for 10 years, and 21.5% for 15 years in our study. This is comparable with the results of other published reports[6,24]. Improvement in availability and techniques of diagnostic tools and development of treatment modalities may allow earlier diagnosis of BCS patients and better prognosis[18-20].
Although our study has an advantage of long-term follow up data of patients with BCS, there are still some limitations. First, this was retrospective and therefore has the limitations of such an investigational design. Another limitation was that the values of HPVG obtained during venography were not checked in all patients. Because of invasiveness of venography, it could not be performed on all patients for HVPG measurement. These days, Doppler US is used to non-invasively assess the HPVG with portal vein velocity. Considering the low incidence of BCS, a multicenter study should be performed to overcome the limitations of patient number and insufficient information. Despite these limitations, this study is worthy to analyze the incidence and prognosis of HCC in BCS with long-term follow-up periods in South Korea.
In conclusion, the annual incidence of HCC in patients with BCS was similar to the incidence in patients with other etiologic cirrhosis in South Korea. Furthermore, the HVPG can be a possible predictive factor of BCS-associated HCC development. Thus, BCS patients who are expected to have high pressure gradient should be actively managed as a high risk group for HCC development. An intervention to decrease the pressure gradient in BCS patients may be helpful to reduce the incidence of HCC. A large-scale study will be necessary to further investigate whether the treatment of congestion decreases the incidence of HCC.
The authors are grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, South Korea) for his help with the figures.
Budd-Chiari syndrome (BCS) is a rare disease caused by obstruction of the hepatic venous outflow. BCS induces chronic liver congestion so that it causes hepatomegaly, ascites, leg edema, collateral venous dilatation in the body trunk, and portal hypertension. Several studies have suggested that hepatic congestion caused by obstruction of hepatic venous outflow can lead to cirrhosis and hepatocellular carcinoma (HCC). However, the incidence of HCC in patients with BCS has varied according to regions and investigators and there has been a lack of reports for long-term prognosis of HCC in patients with BCS.
Although BCS is a relatively rare disease as contrasted with other viral liver disease that can lead to advanced liver disease such as liver cirrhosis or HCC, several studies have reported the association between BCS and HCC. Long-term follow-up data of BCS may help to understand not only the prognosis of BCS but also the process of HCC in patients with BCS.
This study showed that the hepatic venous pressure gradient (HVPG) was significantly higher in HCC group than non-HCC group. Although the accurate pathogenesis of HCC in BCS has not been elucidated, there were several suggestions that chronic liver injuries and congestion caused by obstruction of hepatic venous outflow might contribute to a fibrotic process and development of HCC. The study is worthy because it supports this hypothesis about the development of HCC in patients with BCS.
From the study, it was suggested that the HVPG can be a possible predictive factor of BCS-associated HCC development. Thus, BCS patients who are expected to have a high pressure gradient should be actively managed as a high risk group for HCC development. An intervention to decrease the pressure gradient in BCS patients may be helpful to reduce the incidence of HCC.
This study provides basic and essential data for the clinical care of the patients with BCS.
Peer reviewer: Guang-Wen Cao, MD, PhD, Professor and Chairman, Department of Epidemiology, The Second Military Medical University, 800 Xiangyin Road, Shanghai 200433, China
S- Editor Cheng JX L- Editor A E- Editor Zhang DN
| 1. | Dhiman RK, Saraswat VA, Radhakrishnan S, Parashar A, Agarwal DK, Naik SR. Multiple venous thromboses and membranous obstruction of inferior vena cava in association with hereditary protein C deficiency: a case report. J Gastroenterol Hepatol. 1992;7:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863-877. [PubMed] |
| 3. | Matsui S, Ichida T, Watanabe M, Sugitani S, Suda T, Takahashi T, Asakura H. Clinical features and etiology of hepatocellular carcinoma arising in patients with membranous obstruction of the inferior vena cava: in reference to hepatitis viral infection. J Gastroenterol Hepatol. 2000;15:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Okuda K. Inferior vena cava thrombosis at its hepatic portion (obliterative hepatocavopathy). Semin Liver Dis. 2002;22:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Gwon D, Ko GY, Yoon HK, Sung KB, Kim JH, Lee SS, Lee JM, Ohm JY, Shin JH, Song HY. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Moucari R, Rautou PE, Cazals-Hatem D, Geara A, Bureau C, Consigny Y, Francoz C, Denninger MH, Vilgrain V, Belghiti J, Durand F, Valla D, Plessier A. Hepatocellular carcinoma in Budd-Chiari syndrome: characteristics and risk factors. Gut. 2008;57:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Eapen CE, Mammen T, Moses V, Shyamkumar NK. Changing profile of Budd Chiari syndrome in India. Indian J Gastroenterol. 2007;26:77-81. [PubMed] |
| 9. | Kew MC, McKnight A, Hodkinson J, Bukofzer S, Esser JD. The role of membranous obstruction of the inferior vena cava in the etiology of hepatocellular carcinoma in Southern African blacks. Hepatology. 1989;9:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Rector WG, Xu YH, Goldstein L, Peters RL, Reynolds TB. Membranous obstruction of the inferior vena cava in the United States. Medicine (. Baltimore). 1985;64:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Okuda H, Yamagata H, Obata H, Iwata H, Sasaki R, Imai F, Okudaira M, Ohbu M, Okuda K. Epidemiological and clinical features of Budd-Chiari syndrome in Japan. J Hepatol. 1995;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 144] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 13. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology. 1989;172:389-392. [PubMed] |
| 15. | Parker RG. Occlusion of the hepatic veins in man. Medicine (. Baltimore). 1959;38:369-402. [PubMed] |
| 16. | Jang JW. Current status of liver diseases in Korea: liver cirrhosis. Korean J Hepatol. 2009;15 Suppl 6:S40-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009;15 Suppl 6:S50-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lee BB, Villavicencio L, Kim YW, Do YS, Koh KC, Lim HK, Lim JH, Ahn KW. Primary Budd-Chiari syndrome: outcome of endovascular management for suprahepatic venous obstruction. J Vasc Surg. 2006;43:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Valla D. Hepatic venous outflow tract obstruction etiopathogenesis: Asia versus the West. J Gastroenterol Hepatol. 2004;19:S204-S211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Schepke M, Raab P, Hoppe A, Schiedermaier P, Brensing KA, Sauerbruch T. Comparison of portal vein velocity and the hepatic venous pressure gradient in assessing the acute portal hemodynamic response to propranolol in patients with cirrhosis. Am J Gastroenterol. 2000;95:2905-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Slakey DP, Klein AS, Venbrux AC, Cameron JL. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Shin SH, Chung YH, Suh DD, Shin JW, Jang MK, Ryu SH, Park NH, Lee HC, Lee YS, Suh DJ. Characteristic clinical features of hepatocellular carcinoma associated with Budd-Chiari syndrome: evidence of different carcinogenic process from hepatitis B virus-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004;16:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |