Published online Apr 28, 2012. doi: 10.3748/wjg.v18.i16.1892
Revised: June 24, 2011
Accepted: April 1, 2012
Published online: April 28, 2012
AIM: To investigate the relationship between over-expression of urokinase plasminogen activator (uPA) and hepatitis B virus (HBV) related liver diseases in a transgenic mouse model.
METHODS: Albumin-tetracycline reverse transcriptional activator and tetO-uPA transgenic mice were generated respectively through pronuclear injection and crossed to produce the double transgenic in-alb-uPA mice, for which doxycycline (Dox)-inducible and liver-specific over-expression of uPA can be achieved. Hydrodynamic transfection of plasmid adeno-associated virus (AAV)-1.3HBV was performed through the tail veins of the Dox-induced in-alb-uPA mice. Expression of uPA and HBV antigens were analyzed through double-staining immunohistochemical assay. Cytokine production was detected by enzyme linked immunosorbent assay and α-fetoprotein (AFP) mRNA level was evaluated through real-time quantitative polymerase chain reaction.
RESULTS: Plasmid AAV-1.3HBV hydrodynamic transfection in Dox-induced transgenic mice not only resulted in severe liver injury with hepatocarcinoma-like histological changes and hepatic AFP production, but also showed an increased serum level of HBV antigens and cytokines like interleukin-6 and tumor necrosis factor-α, compared with the control group.
CONCLUSION: Over-expression of uPA plays a synergistic role in the development of liver injury, inflammation and regeneration during acute HBV infection.
- Citation: Zhou XJ, Sun SH, Wang P, Yu H, Hu JY, Shang SC, Zhou YS. Over-expression of uPA increases risk of liver injury in pAAV-HBV transfected mice. World J Gastroenterol 2012; 18(16): 1892-1902
- URL: https://www.wjgnet.com/1007-9327/full/v18/i16/1892.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i16.1892
Hepatitis B virus (HBV) infection causes a necroinflammatory liver disease of variable duration and severity, with a high risk of developing cirrhosis and hepatocellular carcinoma. The immune response to HBV-encoded antigens is responsible both for viral clearance and for disease pathogenesis during HBV infection[1]. However, the roles of urokinase plasminogen activator (uPA)/uPA’s receptor (uPAR) systems as important inflammatory mediators have not yet been well investigated in acute and chronic hepatitis B, a common inflammatory disease in China[2]. Clinical studies almost focused on the correlation of uPA levels with the liver disease severity in hepatitis B patients. And the role of uPA in the HBV-induced liver injury, especially in the early stage, is less investigated.
uPA is one kind of plasminogen activator that catalyzes the conversion of plasminogen to plasmin. Together with uPAR, uPA participate in fibrinolysis, innate and adaptive immunity, and pathology[3]. In cancer cells, the effects of uPA and uPAR were thought to be related to cell migration[4], metastasis[5], and a more recent role of uPA in cancer growth has emerged[6]. The levels of uPA and uPAR have been found to be increased in tissues, plasma and other body fluids of cancer patients and to be markers of cancer development and metastasis, such as in patients with colon adenocarcinoma[7], lymphomas and leukemia[8].
The tetracycline (Tet)-inducible expression system is one of the most prominent and widely-used systems, which allows relatively stringent, reversible, and quantitative regulation of transgene expression in a wide range of cells in culture as well as in transgenic animals[9,10]. It consists of two parts: the ligand-dependent transactivator tetracycline reverse transcriptional activator (rtTA) as the effector and a tetO-cytomegalovirus (CMV) minimal promoter cassette regulating the expression of the transgene as the responder[11]. When doxycycline (Dox) is present, rtTA binds to the tetO-sequence and induces expression of the target gene[12].Together with a tissue-specific promoter, it can result in transgene expression in a temporally and spatially defined fashion.
In this study, an effective inducible and liver specific uPA expression mouse model was constructed in which the murine uPA expression was controlled by rtTA which is regulated by murine albumin enhancer/promoter.Through administration of Dox, the inducible expression of uPA specifically in mouse liver can be achieved with lower mortality. Then hydrodynamic injection of pAAV-1.3HBV, which contained inverted terminal repeat elements of adeno-associated virus (AAV) and 1.3 copies of HBV genome(ayw subtype), was performed to mimic the acute HBV infection. The mouse liver showed specific and inducible expression of uPA. Plasmid AAV-1.3HBV transfection in Dox-induced transgenic mice resulted in severer liver injury, higher HBV antigen and cytokine expression compared to the control group. These data further indicated for the first time in mice that the over-expression of uPA may have accelerative role in the development of liver injury, inflammation and liver regeneration during acute HBV infection.
For liver-specific expression of rtTA, the transgenic construct albumin-rtTA was generated, which has rtTA gene under the control of the liver-specific albumin promoter and was based the plasmid pTet-on (Clontech Lab, Inc). To introduce appropriate restriction sites in pTet-on, linker sequences were designed as follows, Tet-on-linker-F: 5’-CTAGGATATCACTAGTGGTACCGGGCCCGCGGCCGCG-3’and Tet-on-linker-R: 5’-AATTCGCGGCCGCGGGCCCGGTACCACTAGTGATATC-3’. The linkers were annealed at 95 °C for 10 min and then were digested with EcoR I and Spe I and ligated to pTet-on digested with the same restriction enzymes, and the construct was named pTet-on-link. The albumin promoter fragment and enhancer fragment (Genbank accession no. AC140220.4) were separately amplified by polymerase chain reaction (PCR) using genomic DNA extracted from C57BL/6 mouse liver as the template. The primers for albumin enhancer were Alb-En-FP: 5’-GCCGAGCTCCTGCCGGCTAGCTTCCTTAGCATG-3’ and Alb-En-RP: 5’-GGGTTAAGGATCCCAAG CTGGAG-3’. The primers for albumin promoter were Alb-Pro-FP: 5’-CGGGATCCACAGCTCCAGATGGCAAACATAC-3’and Alb-Pro-RP: 5’-TTTGCCAGAGGCTAGTGGG GTTG-3’.
The albumin enhancer PCR product was digested with BamH I and cloned into pGEM-7ZF, then the albumin promoter sequence was inserted behind the enhancer at the site of BamH I, and the plasmid was named p7ZF-Albumin, which was confirmed by restriction enzyme digestion analysis and DNA sequence analysis. Then p7ZF-Albumin was digested by Sac I and Kpn I, and the released 2233bp fragment was ligased to pTet-on-link digested by EcoR V and Kpn I, to yield the recombinant construct named pTet-on-Albumin.
For rtTA responsive expression of uPA, the transgenic construct pTRE2-uPA was generated which is based on the plasmid pTRE2 containing tetO. The uPA cDNA and uPA exon 11 was amplified by reverse transcription polymerase chain reaction (RT-PCR) and PCR from the total RNA and genomic DNA extracted from the kidney of C57BL/6 mouse, respectively. For uPA DNA, the primers were uPA-cDNA-F: 5’-CGGGATCC ATGAAAGTCTGGCTGGCGAGCCTG-3’ and uPA-cDNA-R: 5’-CGGTCGACCATCAGAAGGCCAGACCTTTCTCTTC-3’. The RT-PCR product was ligated to pMD18T and the construct pMD18T-uPA cDNA was confirmed by sequence analysis. The uPA exon 11 was amplified by PCR from the genomic DNA template, the primers were uPA-3’-F: 5’-CGGTCGACGCCCTCAGGTAGCTGAGGGAAG-3’ and uPA-3’-R: 5’-CGGTCGACGTGAAACCGACATTTAGTGCTAGTC-3’. The PCR product was ligated to the Sal I site of pMD18T-uPA cDNA to yield the construct pMD18T-uPA and sequence was confirmed. Then the uPA (cDNA + exon 11) fragment was subcloned into the Pvu II and Xba I sites of pTRE2 to yield pTRE2-uPA.
The albumin-rtTA and tetO-uPA transgenic mice were generated in C57BL/6 × CBA F1 zygotes using standard pronuclear injection, which was performed by Shanghai Research Center for Biomodel Organisms. For microinjection, the 6034 bp fragment of transgene albumin-rtTA and the 5739 bp fragment of transgene tetO-uPA were excised from the vector backbone of pTet-on-albumin by Xho I digestion and pTRE2-uPA by Pvu I digestion, respectively, isolated and purified using QIA quick gel extraction kit (Qiagen), and then microinjected into the pronuclei of one cell-stage fertilized embryos. The DNA injected fertilized eggs were implanted into the oviducts of 12 pseudopregant recipient mice. All together 9 positive albumin-rtTA transgenic mice and 5 tetO-uPA positive ones were confirmed by PCR. One upstream pair and one downstream pair of primers, which were designed to amplify the sequences between vector and inserted fragment were designed for albumin-rtTA as follows, 1-up-F: 5’-GTGCAGCTTGGCTTGAACTCGTTC-3’; 1-up-R: 5’-GAGTATGGTGCCTATCTAACATCTC-3’; 1-down-F: 5’-GACGCGCTAGACGATTTCGATCTG-3’; 1-down-R: 5’-ACCTTGCACAGATAGCGTGGTC-3’. By the same way, one upstream pair and one downstream pair of primers were designed for tetO-uPA as follows 2-up-F: 5’-GTTTAGTGAACCGTCAGATCGCCTG-3’; 2-up-R: 5’-CTAGGCTAATAGCATCAGGTCTG-3’; 2-down-F: 5’-GGTAGCTTG AGGAGTAGAGACACT-3’; 2-down-R: 5’-GACAATGGTTGTCAACAGAGTAG-3’. The PCR conditions were the same for each of the primer pairs: 34 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s. Genomic DNA from wild-type mice was amplified as negative control. The PCR positive mice were the transgenic founders.
At 6-8 wk of age, founder mice were backcrossed with wild-type C57BL/6J mice to generate F1. Genomic DNA were isolated from tail biopsy samples of F1 mice at 4 wk and analyzed by PCR, for which the protocols were mentioned above.
The isolation of total RNA from different tissues of 6-8 wk old F1 PCR-positive and negative offsprings of the founders was performed using the RNeasy Mini Kit (Qiagen) following the instructions. Purified RNA was eluted in 40 μL DNA-free water. 400 ng of total RNA reverse transcribed with the Takara RNA LA PCR Kit (AMV)Ver1.1 (TaKaRa), the reaction condition was 30 °C for 10 min, 42 °C for 30 min, 99 °C for 5 min, 5 °C for 5 min. The oligonucleotide primers used for RT-PCR were rtTA-F: 5’-GACGCGCTAGACGATTTCGATCTG-3’; rtTA-R: 5’-ACCTTGCACAGATAGCGTGGTC-3’, the PCR reaction condition was 34 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal control, the primers were GAPDH-F: 5’-TTCACCACCATGGAGAAGGC-3’ and GAPDH-R: 5’-CCT CAGTGTAGCCCAAGATGC-3’, PCR reaction condition was 34 cycles of 94 °C for 30 s, 48 °C for 30 s, 72 °C for 30 s.
The total protein was isolated from different tissues of 6-8 wk old F1 PCR-positive and negative offsprings of the founders by using the Tissue Lysis Buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.2 mmol/L sodium orthovanadate, 1% Triton X-100, 1% sodium deoxycholate, 1% sodium dodecyl sulfate) supplemented with aprotinin (2 μg/mL), pepstatin A (0.7 μg/mL), leupeptin (0.5 μg/mL), phenylmethanesulfonyl fluoride (PMSF) (1 mmol/L). Aprotinin, Pepstatin A, Leupeptin, PMSF were purchased from Amresco. For Western blotting analysis, 25 μg of the total protein was used for each loading; the primary antibody for rtTA was TetR monoclonal antibody (Clontech) (used in 1:1000 dilution), and the primary antibody for GAPDH was an anti-GAPDH polyclonal antibody (Sigma) (1:10000 dilution); and the second antibodies were HRP-labeled goat anti-mouse IgG and goat anti-rabbit IgG (both in 1:5000 dilution), respectively. For imaging results, the SuperSignal WestDura Trial Kit (Pierce) was used following the instructions.
Double transgenic in-alb-uPA and wild type female offspring were generated from a cross between the albumin-rtTA F1 transgenic positive mice and the tetO-uPA F1 positive mice. 20 d after born, these mice were given two intramuscular injection of 2 mg Dox in 0.2 mL 0.9% NaCl-solution each week for a period of 3 wk. Another group of each type of mice was maintained off doxycycline administration.
After 3 weeks’ induction, a 20 μg pAAV-HBV1.3 DNA was injected hydrodynamically into the tail veins of the in-alb-uPA mice within 5 seconds. A control group of in-alb-uPA mice was injected with pAAV-internal ribosome entry site (IRES). At 20 d post transfection, mice were sacrificed and the livers were fixed with 4% (v/v) phosphate-buffered formalin, and paraffin-embedded liver sections were prepared and stained with hematoxylin and eosin. Semi-quantitative assessment of liver injury in each group was evaluated by the area of liver necrosis on the whole slide in each group. NP for no necrosis; P1 for < 10% area of necrosis; P2 for 10%-30% area of necrosis; P3 for > 30% area of necrosis. All the evaluation of liver damage was conducted by two independent observers. The average score of three mice in each group was taken as score for that group.
For uPA and HBV antigens detection, the expression of uPA protein and hepatitis B core antigen (HBcAg) were identified by double-staining with a polyclonal rabbit anti-rodent urokinase (uPA) antibody (American diagnostica Inc) and monoclonal anti-HBcAg antibody (Thermo Scientific). Diamino-benzidine and alkaline phosphatase substance (ZhongShan Goldenbridge biotech, Beijing, China) were used to visualize the uPA and HBV antigens.
At 10 d and 20 d post transfection, mouse serum samples from different groups were harvested. The HBeAg and HBsAg enzyme linked immunosorbent assay (ELISA) kit (Wantai Biotech, Beijing) were used for the detection of the serum HBV antigens respectively. And interleukin (IL)-6 and tumor necrosis factor (TNF)-α ELISA kit (Dakewei Biotech, Beijing) were used for the quantitation of the serum cytokines. Serum ALT were measured with an Olympus Model 640 automated analyzer.
The isolation of total RNA from livers of the AAV-HBV transfected in-alb-uPA mice was performed using the RNeasy Mini Kit (Qiagen) following the instructions. Purified RNA was eluted in 40 μL DNA-free water and 400 ng of total RNA in a 10 μL reaction mixture was reverse transcribed with the Takara RNA LA PCR Kit (AMV) Ver1.1 (TaKaRa). Relative quantization of the α-fetoprotein (AFP) mRNA level was performed using RNA Master SYBR Green1 (Roche Diagnostics) by Eppendorf Replex. The primers used for amplification were AFP-real-F: 5’-TCTGCTGGCACGCAAGAAG-3’ and AFP-real-R: 5’-TCGGCAGGTTCTGGAAACTG-3’. GAPDH serves as a control and the primers were GAPDH-real-F: 5’-TCACCACCATGGAGAAGGC-3’ and GAPDH-real-R: 5’-GCTAAGCAGTTGGTGGTGCA-3’. The amplification conditions included initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s, extension at 68 °C for 30 s.
Results are expressed as mean ± SE. Statistical analysis was performed using Student’s t test.
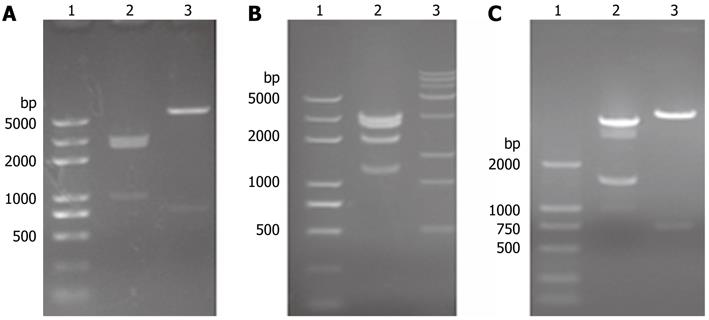
For inserting the albumin enhancer and promoter sequence into pTet-on in place of the CMV promoter, a linker as designed and the following restriction sites were introduced: Spe I, EcoR V, Spe I, Kpn I, Apa I, Not I, EcoR I. pTet-on-link was identified by EcoR V/Sal I and EcoR V/BamH I double digestion respectively (Figure 1A), and results showed that the linker was introduced into pTet-on. pTet-on-Albumin was identified by BamHIdigestion (Figure 1B), the expected 5022 bp, 2689 bp and 1284 bp fragment can be observed. pTRE2-uPA was identified by pvu II and Sal I respectively (Figure 1C). In addition, DNA sequence analysis of the albumin enhance/promoter and uPA sequence shows complete accordance with those in the National Center for Biotechnology Information database (data not shown).
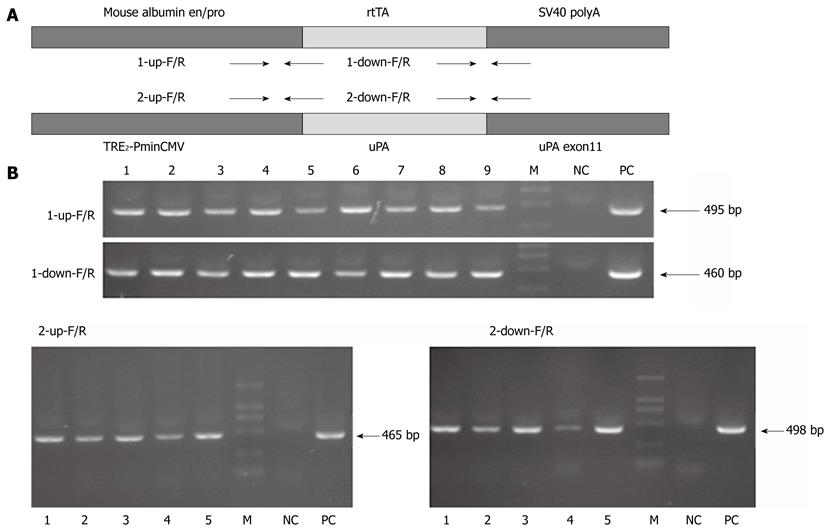
The albumin-rtTA expression unit contains the mouse albumin enhancer/promoter, rtTA coding sequence and SV40 polyA, and the tetO-uPA expression unit contains the TRE2-PminCMV, uPA cDNA and uPA exon11 (Figure 2A). In the end, 9 albumin-rtTA transgenic founder mice and 5 tetO-uPA transgenic founder mice were confirmed positive by PCR for both the upstream and downstream primers (Figure 2B).
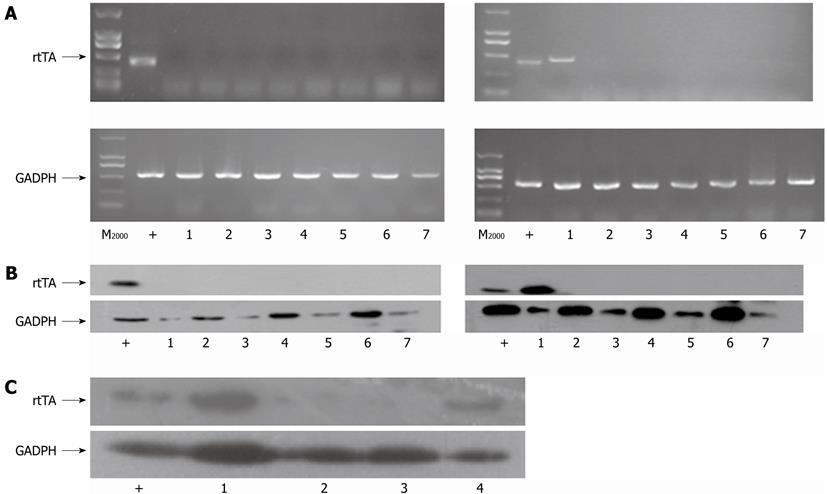
To identify the liver-specific expression of uPA in the livers of transgenic mice, RT-PCR and Western blotting analysis was performed. The data showed that rtTA mRNA expressed specifically in the livers of F1 albumin-rtTA transgenic positive mice (Figure 3A, right image), while there was no rtTA mRNA expression in all the tissues of the albumin-rtTA transgenic negative mouse (Figure 3A, left image). GAPDH mRNA was expressed equally in different tissues of these mice (Figure 3A). Results from Western blotting analysis (Figure 3B) were in accordance with those from RT-PCR analysis. By Western blotting analysis, rtTA expression was also confirmed specifically in the livers of in-alb-uPA transgenic mice and albumin-rtTA transgenic mice (Figure 3C). The cell extract from Huh7 transfected with pTet-on was used as positive control while tetO-uPA and WT mice served as negative control.
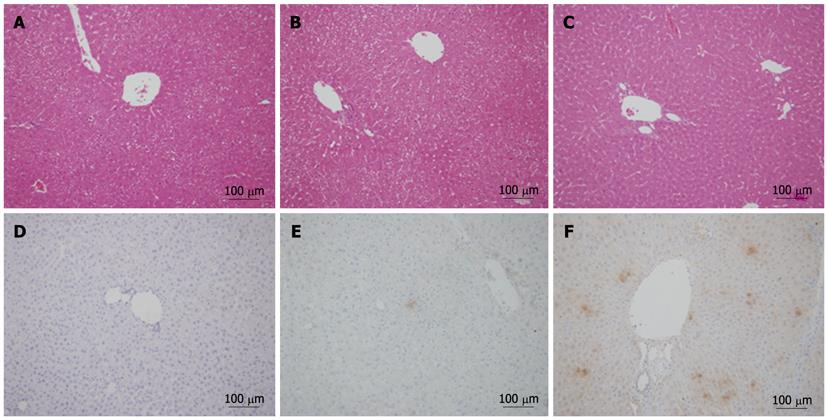
To confirm the expression of uPA in liver and its role on the hepatocytes, uPA expression and the histological changes of liver was analyzed with immunohistochemistry and HE staining respectively. The results showed light degeneration of hepatocytes and mild inflammation in the livers with in-alb-uPA double transgenic mice after 3 wk of Dox induction when compared to that of double transgenic mice without Dox or the control group mice (Figure 4A-C), which was coincident with that of uPA expression with immunohistochemistry in the livers of in-alb-uPA double transgenic mice after Dox induction while almost no expression of uPA detected in double transgenic mice without Dox. The data showed that the specific expression of uPA after Dox induction induced slight histological changes in the liver of this in-alb-uPA double transgenic mice.
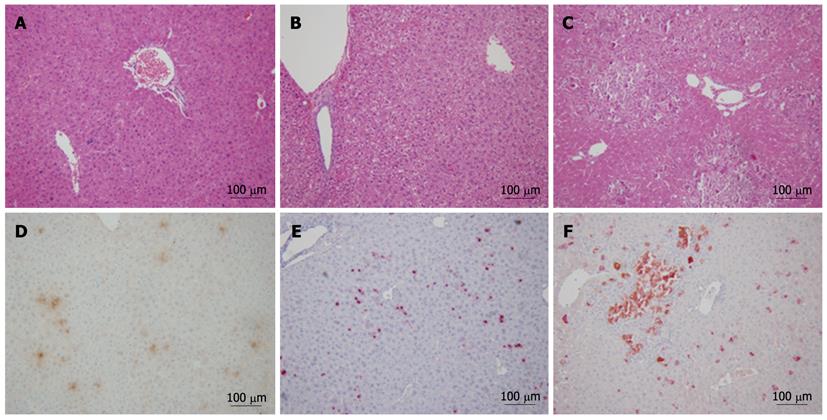
Although uPA plays critical role in hepatic repair via proteolysis of matrix elements and clearance of cellular debris from the field of injury, clinical data showed that the levels of uPA and uPAR in patients with acute and chronic hepatitis B significantly higher than that in healthy controls, which indicated that uPA level was closely related to the degree and period of inflammation and liver injury[13]. To confirm if the coexisting uPA expression and HBV replication induced serious acute liver injury, the in-alb-uPA transgenic mice were transfected with pAAV-1.3HBV, a plasmid which could mediated the production of replicative HBV virus[14] in vivo. Large area necrosis was observed 20 d later in the liver of Dox-induced in-alb-uPA double transgenic mice that were transfected with pAAV-1.3HBV (Figure 5C), compared with that of non-induced in-alb-uPA mice transfected with pAAV-1.3HBV (Figure 5B) or with the control plasmid pAAV-IRES (Figure 5A). Double-staining immunohistochemical analysis confirmed both uPA expression (in brown) and HBcAg expression (in red) in the AAV-HBV transfected Dox-induced in-alb-uPA mice (Figure 5F).Interestingly, the coexpression of uPA and HBcAg exist in the most of the necrosis areas and the hepatocytes with HBcAg expression alone were morphological intact. The severe liver damage in the mice after HBV transfection indicated that the expression of uPA accelerated the liver injury. The result was confirmed by a statistics analysis that about 86.7% of the AAV-HBV transfected Dox-induced in-alb-uPA mice experienced severe liver pathogenic changes compared with the 20% AAV-HBV transfected non-induced in-alb-uPA mice, which could be explained by the leaky expression of uPA due to the tet-on inducible system. And about 40% of the AAV-HBV transfected Dox-induced in-alb-uPA mice experienced severe liver injury (Table 1).
| Group | 1 | 2 | 3 |
| NP | 8 (100) | 8 (80) | 2 (13.3) |
| P1 | 0 | 2 (20) | 2 (13.3) |
| P2 | 0 | 0 | 5 (33.3) |
| P3 | 0 | 0 | 6 (40) |
| Total | 8 | 10 | 15 |
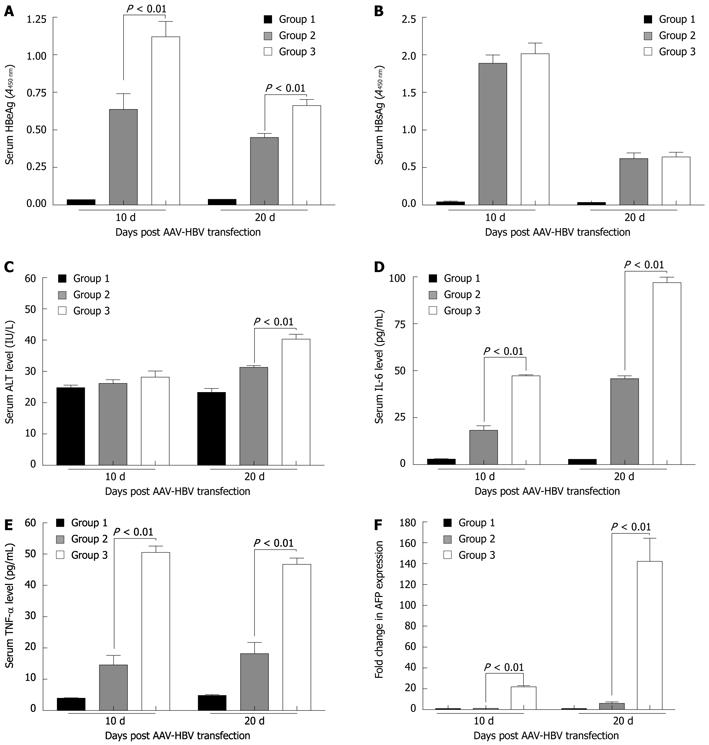
Previous reports have shown that HBV infection is associated with the production of a broad range of proinflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8, IL-12, TNF-α and IFN-γ[15-17], among which IL-6 and TNF-α are important components of the early signaling pathway that lead to liver regeneration[18]. In this study, results also confirmed the elevation of serum IL-6 and TNF-α levels in the AAV-HBV transfected in-alb-uPA mice (Figure 6). 10 d and 20 d after AAV-HBV transfection, the serum IL-6 level for the Dox-induced in-alb-uPA mice was 47.28 ± 0.57 and 96.97 ± 2.91 (pg/mL), while the level for the non-induced in-alb-uPA mice was 18.32 ± 2.38 (pg/mL) and 45.83 ± 1.50 (pg/mL) (P < 0.01) (Figure 6D). The serum TNF-α level for the Dox-induced in-alb-uPA mice was 50.55 ± 2.01 (pg/mL) and 46.72 ± 2.01 (pg/mL), while the level for the non-induced in-alb-uPA mice was 14.58 ± 3.05 (pg/mL) and 18.17 ± 3.63 (pg/mL) (P < 0.01) (Figure 6E). Compared with the non-induced in-alb-uPA mice, the average serum HBeAg level of the Dox-induced in-alb-uPA mice was significantly higher both at 10 d and 20 d after AAV-HBV transfection (P < 0.01)) (Figure 6A), while there was no significant difference between the average serum HBsAg level of the Dox-induced and non-induced mice (Figure 6B). Compared with the non-induced in-alb-uPA mice, the average serum ALT level of the Dox-induced in-alb-uPA mice was slightly higher at 20 d after AAV-HBV transfection (P < 0.01)) (Figure 6C).
It has been reported that AFP level in vivo decreases abruptly soon after bith and remains at a low level throughout life. And reactivation of AFP production occurs during liver regeneration[19]. In this study, we found that, compared with the non-induced in-alb-uPA mice that were transfected by AAV-HBV, the AFP mRNA level for the Dox-induced in-alb-uPA mice that were transfected by AAV-HBV was greatly higher. 10 d after AAV-HBV transfection, the average level of the AFP mRNA for those induced in-alb-uPA mice increased about 21.8 times,while the fold change further increased to 142.1 times at 20 d after AAV-HBV transfection (Figure 6F). The data further confirmed our hypothesis that uPA expression and HBV infection have close relations and HBV infection further accelerated liver injury and regeneration when uPA was overexpressed.
Tet-inducible expression system is one of the most suitable inducible systems which could be used to investigate the function of a given gene in vivo, which including the tTA (Tet-off) system[20] and rtTA (Tet-on) system[21], and facilitates not only the understanding of gene function in development and pathogenesis, but also in transgenic mouse modeling[22-24]. On the other hand, tissue-specific expression of a target gene relies on tissue-specific promoters. Tissue-specific expression is vital for gene function research in organism development and can reduce immunological response and side effects in gene therapy applications. Many liver-specific promoters have been identified so far, such as the AFP promoter[25], the albumin promoter, mouse major urinary protein promoter[26], hSAP promoter (human serum amyloid P component promoter)[27], and apoE promoter (human apolipoprotein E promoter)[28], which have been applied in liver-specific expression of target genes. In addition, elements like enhancers influence the transcriptional activity of the these tissue-specific promoters[29].
The urokinase plasminogen activator (uPA) is a serine protease that can activate the plasminogen into plasmin, and perform multiple functions in fibrinolysis, immunity and pathology[3]. Previous studies showed uPA diverse functions in tissue remodeling, angiogenesis,wound healing and protective effects in liver diseases[30]. The levels of uPA have been found to be increased in tissues, plasma and other body fluids of cancer patients and to be markers of cancer development and metastasis. And in human immunodeficiency virus (HIV)-infected patients, the serum levels of uPA have been found to be increased[2]. Also, the abnormal levels of plasma uPA in the patients with acute or chronic hepatitis B were observed, and it seems that the plasma levels of uPA are closely related to the degree and period of inflammation for these patients[13]. Although the clinical significance of uPA in viral chronic hepatitis B, hepatitis induced liver cirrhosis and HCC has been evaluated, the role of uPA in the process is less well understood, especially in the early stage.
In 1990, an Alb-uPA transgenic mouse which carried the mouse uPA gene under the control of the mouse albumin enhancer/promoter, was developed by Dr. Brinster’s team to study the pathophysiology of plasminogen hyperactivation[31]. The over-expression of the uPA gene in the liver resulted in high plasma uPA levels and hypofibrinogenemia, which led to severe and sometimes abdominal bleeding soon after birth. And the high mortality also increases the difficulty for the generation of human liver chimeric mice[32,33] and the study of hepatitis C virus infections in vivo[34-36]. In this study, we established an uPA inducible double transgenic mouse in-alb-uPA, in which uPA can be expressed specifically in the liver only after Dox induction. Hypofibrinogenemia and neonatal hemorrhaging were not observed in the Dox-induced in-alb-uPA mice, which greatly brought down the mortality rate. Also the inducible expression of uPA makes it possible for us to study and illuminate the relations of uPA over-expression and HBV infection clinically.
Hydrodynamic transfection method was suitable for the AAV-mediated delivery of HBV genome in vivo[37]. To investigate the risk of HBV induced liver injury in the case of uPA over-expression, the hydrodynamic transfection of pAAV-HBV1.3, which could mediated the production of replicative HBV virus in vivo, was performed. In the Dox-induced in-alb-uPA mice that were hydrodynamically transfected by AAV-1.3HBV, severe liver histological changes were observed in the liver (Figure 5). Also uPA over-expression in the liver resulted in higher HBV antigen expression, higher IL-6 and TNF-α production and slight elevation of serum ALT level (Figure 6). Our results also found a significant increase of the AFP mRNA level in the AAV-HBV transfected Dox-induced in-alb-uPA mice (Figure 6). Produced by the embryonic yolk sac and tetal liver, the AFP level decreases abruptly soon after bith and remains at a low level throughout life. And reactivation of AFP production occurs during liver regeneration[19]. As IL-6 and TNF-α are proinflammatory cytokines that lead to liver regeneration, we came to the conclusion that the uPA over-expression in AAV-HBV transfected mice increased the liver necrosis injury, inflammation and liver regeneration, which reflects a process that may eventually lead to hepatocellular carcinoma.
It is generally considered that cell-mediated immunity and inflammation are the main mediators of the hepatic pathology induced by HBV infection. In this study, we found that HBV infection further accelerated liver injury and regeneration when uPA was overexpressed, indicating a close relation between uPA expression and HBV infection. Also as clinical data showed that the increased level of uPA in HIV infected patients, this study may in part explain the increased risk of liver disease during HIV and HBV coinfection.
Hepatitis B virus (HBV) infection causes a high risk of developing liver diseases, such as cirrhosis and hepatocellular carcinoma (HCC). The immune response to HBV-encoded antigens is responsible both for viral clearance and for disease pathogenesis during HBV infection. The urokinase plasminogen activator (uPA) is a serine protease that can activate the plasminogen into plasmin, and perform multiple functions in fibrinolysis, immunity and pathology. However, the roles of uPA/uPA’s receptor (uPAR) systems as important inflammatory mediators have not yet been well investigated in acute and chronic hepatitis B, a common inflammatory disease in China. Clinical studies almost focused on the correlation of uPA levels with the liver disease severity in hepatitis B patients. And the role of uPA in the HBV-induced liver injury, especially in the early stage, is less investigated.
Various researchers have found the levels of uPA to be increased in tissues, plasma and other body fluids of cancer patients and to be markers of cancer development and metastasis. And in human immunodeficiency virus (HIV)-infected patients, the serum levels of uPA have been found to be increased. Also, the abnormal levels of plasma uPA in the patients with acute or chronic hepatitis B were observed, and it seems that the plasma levels of uPA are closely related to the degree and period of inflammation for these patients. Although the clinical significance of uPA in viral chronic hepatitis B, hepatitis induced liver cirrhosis and HCC has been evaluated, the role of uPA in the process is less well understood, especially in the early stage.
In this study, an inducible liver-specific uPA transgenic mice model was developed. Plasmid adeno-associated virus-1.3HBV transfection in doxycycline (Dox)-induced transgenic mice resulted in severer liver injury, higher HBV antigen and cytokine expression compared to the control group. These data further indicated for the first time in mice that the over-expression of uPA may have accelerative role in the development of liver injury, inflammation and liver regeneration during acute HBV infection Also as clinical data showed that the increased level of uPA in HIV infected patients, this study may in part explain the increased risk of liver disease during HIV and HBV coinfection.
This study deepens our knowledge of uPA function in HBV-induced liver diseases, which may not only facilitate the elucidation of the molecular mechanism of HBV pathogenesis, but also provide a basis for the uPA-targeted anti-HBV therapies.
uPA is one kind of plasminogen activator that catalyzes the conversion of plasminogen to plasmin. Together with uPAR, uPA participate in fibrinolysis, innate and adaptive immunity, and pathology. Tetracycline (Tet)-inducible expression system consists of two parts: the ligand-dependent transactivator rtTA as the effector and a tetO-CMV minimal promoter cassette regulating the expression of the transgene as the responder. When Dox is present, rtTA binds to the tetO-sequence and induces expression of the target gene. Together with a tissue-specific promoter, it can result in transgene expression in a temporally and spatially defined fashion.
The authors studied the role of uPA and found that the over-expression of uPA may have a synergistic role in the development of liver injury, inflammation and liver regeneration during acute HBV infection. They added as such new information to the field on the knowledge about uPA function in HBV-induced liver diseases.
Peer reviewers: Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia; Ezio Laconi, MD, PhD, Professor of General Pathology, Department of Sciences and Biomedical Technologies, Unit of Experimental Pathology, University of Cagliari, Via Porcell, 4, IV Piano, 09125 Cagliari, Italy
S- Editor Shi ZF L- Editor A E- Editor Zhang DN
| 1. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1201] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 2. | Sidenius N, Sier CF, Ullum H, Pedersen BK, Lepri AC, Blasi F, Eugen-Olsen J. Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood. 2000;96:4091-4095. [PubMed] |
| 3. | Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 353] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Ossowski L, Clunie G, Masucci MT, Blasi F. In vivo paracrine interaction between urokinase and its receptor: effect on tumor cell invasion. J Cell Biol. 1991;115:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 167] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Lund AH, Turner G, Trubetskoy A, Verhoeven E, Wientjens E, Hulsman D, Russell R, DePinho RA, Lenz J, van Lohuizen M. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat Genet. 2002;32:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Stephens RW, Nielsen HJ, Christensen IJ, Thorlacius-Ussing O, Sørensen S, Danø K, Brünner N. Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J Natl Cancer Inst. 1999;91:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Mustjoki S, Sidenius N, Sier CF, Blasi F, Elonen E, Alitalo R, Vaheri A. Soluble urokinase receptor levels correlate with number of circulating tumor cells in acute myeloid leukemia and decrease rapidly during chemotherapy. Cancer Res. 2000;60:7126-7132. [PubMed] |
| 9. | Dobrovolsky VN, Heflich RH. On the use of the T-REx tetracycline-inducible gene expression system in vivo. Biotechnol Bioeng. 2007;98:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Stieger K, Belbellaa B, Le Guiner C, Moullier P, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933-10938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 592] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Lamartina S, Roscilli G, Rinaudo CD, Sporeno E, Silvi L, Hillen W, Bujard H, Cortese R, Ciliberto G, Toniatti C. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum Gene Ther. 2002;13:199-210. [PubMed] |
| 13. | Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103:17862-17867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Bidgoli SA, Zavarhei MD, Mohagheghil MH, Yazdanmehr B, Daryani NE, Ardalan FA. Differential expression of uPA in chronic hepatitis B and C, liver cirrhosis and hepatocellular carcinoma: comparison with normal liver tissues and liver metastatic tumors. Int J Cancer Res. 2007;3:25-32. |
| 15. | Zhou H, Wu X, Lu X, Chen G, Ye X, Huang J. Evaluation of plasma urokinase-type plasminogen activator and urokinase-type plasminogen-activator receptor in patients with acute and chronic hepatitis B. Thromb Res. 2009;123:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927-933. [PubMed] |
| 17. | Barth H, Robinet E, Liang TJ, Baumert TF. Mouse models for the study of HCV infection and virus-host interactions. J Hepatol. 2008;49:134-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ernst E, Schönig K, Bugert JJ, Bläker H, Pfaff E, Stremmel W, Encke J. Generation of inducible hepatitis C virus transgenic mouse lines. J Med Virol. 2007;79:1103-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Katoh M, Tateno C, Yoshizato K, Yokoi T. Chimeric mice with humanized liver. Toxicology. 2008;246:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Suemizu H, Hasegawa M, Kawai K, Taniguchi K, Monnai M, Wakui M, Suematsu M, Ito M, Peltz G, Nakamura M. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem Biophys Res Commun. 2008;377:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547-5551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3634] [Cited by in RCA: 3795] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 23. | Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1863] [Cited by in RCA: 1854] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 24. | Albanese C, Hulit J, Sakamaki T, Pestell RG. Recent advances in inducible expression in transgenic mice. Semin Cell Dev Biol. 2002;13:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115-132. [PubMed] |
| 26. | van der Weyden L, Adams DJ, Bradley A. Tools for targeted manipulation of the mouse genome. Physiol Genomics. 2002;11:133-164. [PubMed] |
| 27. | Hirano T, Kaneko S, Kaneda Y, Saito I, Tamaoki T, Furuyama J, Tamaoki T, Kobayashi K, Ueki T, Fujimoto J. HVJ-liposome-mediated transfection of HSVtk gene driven by AFP promoter inhibits hepatic tumor growth of hepatocellular carcinoma in SCID mice. Gene Ther. 2001;8:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Shi Y, Son HJ, Shahan K, Rodriguez M, Costantini F, Derman E. Silent genes in the mouse major urinary protein gene family. Proc Natl Acad Sci USA. 1989;86:4584-4588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Zhao X, Araki K, Miyazaki J, Yamamura K. Developmental and liver-specific expression directed by the serum amyloid P component promoter in transgenic mice. J Biochem. 1992;111:736-738. [PubMed] |
| 30. | Thome J, Gewirtz JC, Sakai N, Zachariou V, Retz-Junginger P, Retz W, Duman RS, Rösler M. Polymorphisms of the human apolipoprotein E promoter and bleomycin hydrolase gene: risk factors for Alzheimer's dementia? Neurosci Lett. 1999;274:37-40. [PubMed] |
| 31. | Pinkert CA, Ornitz DM, Brinster RL, Palmiter RD. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987;1:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 359] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1387] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 33. | Takikawa Y, Suzuki K. Is AFP a new reliable marker of liver regeneration in acute hepatic failure? J Gastroenterol. 2002;37:681-682. [PubMed] |
| 34. | al-Wabel A, al-Janadi M, Raziuddin S. Cytokine profile of viral and autoimmune chronic active hepatitis. J Allergy Clin Immunol. 1993;92:902-908. [PubMed] |
| 35. | Geneva-Popova M, Murdjeva M. Study on proinflammatory cytokines (IL-1 beta, IL-6, TNF-alpha) and IL-2 in patients with acute hepatitis B. Folia Med (. Plovdiv). 1999;41:78-81. [PubMed] |
| 36. | Hsu HY, Chang MH, Ni YH, Lee PI. Cytokine release of peripheral blood mononuclear cells in children with chronic hepatitis B virus infection. J Pediatr Gastroenterol Nutr. 1999;29:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |