Published online Apr 21, 2012. doi: 10.3748/wjg.v18.i15.1814
Revised: December 6, 2011
Accepted: March 10, 2012
Published online: April 21, 2012
AIM: To compare the endomicroscopic image quality of integrated confocal laser endomicroscopy (iCLE) and sedation efficacy of propofol vs midazolam plus fentanyl (M/F).
METHODS: Consecutive outpatients undergoing iCLE were prospectively recruited and randomized to the propofol group (P group) or M/F group. The patient, performing endoscopist and endoscopic assistant were blinded to the randomization. The quality of endomicroscopic images and anesthetic efficacy outcomes were blindly evaluated after iCLE examination.
RESULTS: There were significantly more good quality endomicroscopic images in the propofol group than in the M/F group (72.75% vs 52.89%, P < 0.001). The diagnostic accuracy for upper gastrointestinal mucosal lesions using confocal laser endomicroscopy favors the P group, although this did not reach statistical significance. Adverse events and patient assessment were not significantly different for M/F vs propofol except for more frequent intraprocedural recall with M/F. Procedure duration and sedation times were significantly longer in the M/F group, while the scores of endoscopist, anesthetist and assistant assessment were all significantly better in the P group.
CONCLUSION: Sedation with propofol might increase the proportion of good quality endomicroscopic images, and may result in improved procedural efficacy and diagnostic accuracy during iCLE examination.
- Citation: Zuo XL, Li Z, Liu XP, Li CQ, Ji R, Wang P, Zhou CJ, Liu H, Li YQ. Propofol vs midazolam plus fentanyl for upper gastrointestinal endomicroscopy: A randomized trial. World J Gastroenterol 2012; 18(15): 1814-1821
- URL: https://www.wjgnet.com/1007-9327/full/v18/i15/1814.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i15.1814
Confocal laser endomicroscopy (CLE) is a novel technique for gastrointestinal (GI) endoscopy. It enables high-resolution analysis of cellular structure during endoscopy. Clinical applications of CLE have been validated in various GI diseases, such as Barrett’s esophagus, gastric cancer, colorectal cancer, ulcerative colitis and celiac disease[1-5]. Recent studies have expanded its application for in vivo molecular imaging of GI cancer[6]. However, integrated CLE (iCLE) is more cumbersome than a standard gastroscope because iCLE has a larger outer diameter (12.8 mm) and a longer rigid tip (43 mm) which contains the scanning head (tip angulations: up/down 130 degrees). In addition, since endomicroscopic imaging can only be achieved by placing the confocal imaging window directly onto the area of interest, patients may suffer from more discomfort, especially when the lesion is located at the pylorus or gastroesophageal junction. Moreover, motion artifacts, which are the most common cause of endomicroscopic image artifacts, can often be caused by patients’ movement and unstable endoscope positions. Thus, compared with conventional esophagogastroduodenoscopy (EGD), iCLE might require more patients’ cooperation and better sedation to get images of good quality and make an accurate diagnosis.
Conscious sedation is routinely used during endoscopic examination because it can provide adequate anxiolysis, acceptance, and amnesia for most patients vs no sedation, and is safer than deep sedation[7,8]. The combined use of a benzodiazepine (e.g., midazolam) and narcotics (e.g., fentanyl) is the most widely applied sedative regimen for GI endoscopy[9]. Recent data suggest that the use of propofol for sedation is increasing[10]. In some endoscopic centers, benzodiazepines, narcotics or propofol have been administered during iCLE[11-13]. However, the most effective and satisfactory sedation agent for iCLE examination has not yet been investigated.
Recently, propofol has been advocated as an alternative to the commonly used combination of midazolam and narcotic regimen (fentanyl, meperidine)[7,14-19]. Compared with midazolam, propofol is a short-acting sedative-hypnotic agent with a faster recovery profile, and its application is associated with some additional advantages, such as being easy to maintain an appropriate sedation level and satisfactory amnestic effect[7,14,15,18,19]. Several studies have reported the effect of sedation of propofol vs midazolam on the quality of upper and lower GI endoscopy by randomized trials[16,17,20], however, no investigation has compared propofol with midazolam plus fentanyl (M/F) as sedatives for iCLE. Therefore, the aim of the present study was to compare the quality of endomicroscopic images and sedation efficacy outcomes between propofol and M/F as sedatives for iCLE.
Consecutive outpatients who underwent iCLE were recruited prospectively from the endoscopy clinic of Qilu Hospital, Shandong University, from February to May 2010. The exclusion criteria: < 18 years of age, known or suspected strictures or stenosis, coagulopathy, acute upper digestive tract bleeding, pregnancy or breast feeding, allergy to propofol, fentanyl, midazolam or fluorescein sodium, contraindications to sedation, mental disorders or did not provide written informed consent. Informed consent was obtained from all patients who underwent endoscopic examination in this study. The study was approved by the Ethical committee of Qilu Hospital and was conducted in accordance with the revised Declaration of Helsinki (1989). This trial was registered at http://www.clinicaltrials.gov, ID number NCT01053871.
The sample size was calculated to achieve a statistical power of 0.8 at an alpha value of 0.05. For patients sedated with midazolam and fentanyl, the rate of good quality endomicroscopic images was estimated to be 66% according to previously reported data[13]. We defined that sedative iCLE examination using propofol increases the rate of good quality endomicroscopic images by 21% as compared with the administration of midazolam and fentanyl. This resulted in a calculated sample size of 100 patients (50 per group). Therefore, we proposed recruiting 104 eligible patients to allow an attrition rate of 4%.
Patients were randomized at a 1:1 ratio into a propofol group (P group) or an M/F group using a computer-generated list. The respective randomization results were kept in sealed envelopes that were opened before the endoscopy by the anesthetist. Because the apparent difference in the color of the sedative agents in this study, the anesthetist was not blinded to the study agents. However, in order to maintain the patients, the endoscopist and the other investigators blinded about the study group, an opaque curtain was placed upon the patient’s infusion arm during the following procedure.
CLE is an advanced method which allows living tissue to be viewed in situ, providing real-time histology during endoscopy. The confocal microscope integrated into the distal tip of a conventional video endoscope can collect images with an adjustable depth of scanning ranging from 0 to 250 μm, a field of view of 475 μm × 475 μm, an optical slice thickness of 7 μm, and a lateral resolution of 0.7 μm. The plane depth was controlled using two additional buttons on the back of the handpiece.
After routine preparations for gastroscopy, intravenous access was established for both groups of patients. Patients in P group received a bolus of 0.8-1.0 mg/kg of 1% propofol before the start of endoscopy. Further bolus of 0.5 mg/kg of 1% propofol was evaluated by an anesthetist, and would be given if the sedation was judged as insufficient by the endoscopist. Patients in M/F group received a bolus of 0.05 mg fentanyl, followed by 3-4 mg midazolam before the start of endoscopy. Further bolus of 1-2 mg midazolam was administered by the anesthetist at certain intervals or when the sedation was judged as inadequate by the endoscopist. A reversal agent of midazolam (flumazenil) was administered after iCLE examination in the M/F group if needed. Endoscopic intubation commenced once the patient showed spontaneous eye closure, but responsive to name called.
All patients received supplemental oxygen (2-4 L/min) by nasal cannula. Their oxygen saturation, pulse rate and arterial blood pressure were continuously monitored and recorded every 5 min by pulse oxymetry and sphygmomanometry. Sedation was performed in accordance with the guidelines for conscious sedation and monitored by a professional anesthetist (Liu XP)[8].
Patients in both groups received standard white-light endoscopic and endomicroscopic examinations using a Pentax EC-3870K confocal laser endomicroscope (Pentax, Tokyo, Japan). All endoscopic procedures were performed by one experienced endoscopist (Zuo XL), who had performed more than 300 iCLE procedures before the present study. After successful intubation of the endoscope into the duodenum, 5 mL fluorescein was administered intravenously to facilitate the endomicroscopic imaging. Endoscopic mucosal lesions (such as mucosal color changes, elevation, depression, ruggedness) and 9 standard locations (duodenal bulb, lesser and greater curvature of the antrum and gastric body, incisura angularis, fundus, gastric cardia and esophagus) were sequentially examined using iCLE. Serial endomicroscopic images were obtained from each examined area using the “movie mode” on the iCLE displaying screen and stored in separate files for further analysis of image quality. Image collection was started when the performing endoscopist activated the endomicroscopic scanning by pressing a control button on the handpiece of the endoscope, and it was stopped when the endoscopist pressed twice on the same button. Real-time endoscopic and endomicroscopic diagnoses were made during the procedure by the performing endoscopist and targeted biopsy specimens were obtained for histopathological assessment.
One endoscopic assistant (Zhen L) was responsible for the data collection, and not involved in patient selection or the randomization. The demographic data, history of alcohol or smoking, and the American Society of Anesthesiologists status were recorded for both groups of patients[21].
Assessment of endomicroscopic image quality: Endomicroscopic images of each patient were reevaluated after the procedure by one investigator (Rui J), who was blinded to the patients’ data and endoscopic findings. Good quality endomicroscopic images were defined as “no moving artifacts, and single cells can be differentiated”. And the number of good quality endomicroscopic images was counted for each examined area[1].
Sedation-related outcomes: The procedure duration was recorded (from the first injection of the sedatives to the moment of the withdrawal of the endoscope), and the time required for sedation (start of the sedation to passage of the larynx). In addition, patient monitoring/complications, including oxygen de-saturation (< 90%), hypotension (SBP < 80 mmHg) and bradycardia (< 40 b/min) were also noted.
Patient assessment: After the endoscopic procedure, patients were transferred to a separate recovery area when vital signs were stable as judged by the anesthesiologist responsible for the sedation. As the patients awoke, a brief questionnaire was asked and collected by a blinded endoscopic assistant (Zhen L). Patient assessment of the procedure involved 4 parameters, including satisfaction (scores ranging from 1 to 10: 1 for “poor” and 10 for “excellent”), pain or discomfort (scores ranging from 0 to 10: 0 for “none” and 10 for “severe”) and intraprocedure recall (scores ranging from 0 to 10: 0 for “none” and 10 for “complete”). Additionally, the patients were also asked whether they would prefer lighter, deeper or the same level sedation for their next EGD.
Endoscopist assessment: The endoscopist’s assessment of the procedure had 4 parameters, including satisfaction with sedation (scores ranging from 1 to 10:1 for “poor” and 10 for “excellent”), level of sedation (apparently inadequate, inadequate, adequate, oversedated), patient cooperation and quality of endoscopy (a scale ranging from 1 to 4: 1 for “very poor”; 2 for “poor”; 3 for “fair”; and 4 for “good”).
In addition, the endoscopic assistant and anesthetist also scored their satisfaction of sedation at the end of each procedure independently using a 10-point scale: 1 (poor) to 10 (excellent).
Continuous outcomes were compared using the independent sample t test for normally distributed data and the Mann-Whitney U test for nonparametric data. The χ2 test and the Fisher exact test were applied for the comparison of categorical variables between the two groups. A P value less than 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 13.0 statistical software package (SPSS, Chicago, IL, United States). The study was reported in accordance with the Consolidated Standards of Reporting Trials[22].
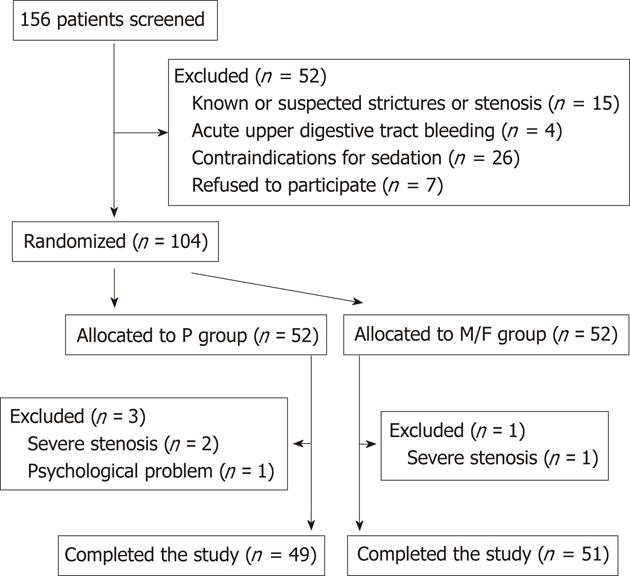
Over the 3-mo study period, 156 subjects who required for sedated iCLE examination were screened for possible enrollment. In the end, 52 patients were excluded according to predefined exclusion criteria, including 15 cases of known or suspected strictures or stenosis, 4 cases of acute bleeding, 26 cases of contraindications to sedation, and 7 cases refused to participate. A total of 100 patients completed the study and were eligible for data analysis (49 in P group and 51 in M/F group) (Figure 1). The patient characteristics for both groups are summarized in Table 1. The mean dosage of sedation used was 194 mg for propofol (range 50-380 mg) and 5.4 mg for midazolam (range 3-8 mg).
| Patient characteristics | P group | M/F group | P value |
| Patients, n | 49 | 51 | |
| Gender (male/female), n | 24/25 | 23/28 | NS |
| Mean age, yr (range) | 53 (27-77) | 55 (32-78) | NS |
| Body weight, kg (mean ± SD) | 64.14 ± 10.21 | 63.84 ± 9.48 | NS |
| Habit, cases (n) | |||
| Alcohol consumption | NS | ||
| Daily drinker | 8 | 5 | |
| Social drinker | 10 | 5 | |
| None-drinker | 31 | 41 | |
| Tobacco | NS | ||
| ≥ 1 PD | 5 | 5 | |
| < 1 PD | 3 | 5 | |
| Quit smoking | 4 | 1 | |
| None-smoker | 37 | 40 | |
| ASA I | 34 | 37 | NS |
| ASA II | 15 | 14 | NS |
Endoscopic mucosal lesions of the duodenum, stomach and esophagus were examined by iCLE. In addition, if multiple lesions, such as multiple polyps of the stomach, were detected, the endomicroscopic images obtained from lesions in the same anatomical compartment (e.g., antrum, incisura angularis, gastric body/fundus and cardia) were poorly analyzed for image quality. The proportion of good quality endomicroscopic images in each examined area is shown in Table 2. Propofol showed superiority to midazolam plus fentanyl in obtaining good quality endomicroscopic images (72.75% vs 52.89%, P < 0.001). χ2 test revealed significant differences in the proportion of good quality endomicroscopic images between the two groups for each predefined area and endoscopic mucosal lesions (P < 0.001).
| P group | M/F group | P value | |
| Duodenal bulb | 72.17 (760/1053) | 50.22 (577/1149) | < 0.001 |
| Lesser curvature of antrum | 66.44 (778/1171) | 45.73 (562/1229) | < 0.001 |
| Greater curvature of antrum | 80.49 (916/1138) | 64.99 (776/1194) | < 0.001 |
| Incisura angularis | 83.72 (581/694) | 50.17 (438/873) | < 0.001 |
| Lesser curvature of gastric body | 71.41 (602/843) | 48.07 (448/932) | < 0.001 |
| Greater curvature of gastric body | 81.85 (857/1047) | 66.46 (757/1139) | < 0.001 |
| Fundus | 67.39 (217/322) | 46.00 (236/513) | < 0.001 |
| Cardia | 71.83 (2068/2879) | 49.84 (1395/2799) | < 0.001 |
| Esophagus | 67.94 (284/418) | 56.04 (297/530) | < 0.001 |
| Lesions | 67.28 (1285/1910) | 52.53 (1161/2210) | < 0.001 |
| Total | 72.75 (8348/11475) | 52.89 (6647/12568) | < 0.001 |
There were no significant differences between the two groups for the number of endoscopic mucosal lesions, as well as their locations and corresponding histopathology (Table 3). According to prior published CLE diagnostic criteria[1,2,13,23-27], the sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) of the two groups were calculated respectively (Table 4). The PLR of the P group for diagnosing neoplasia was significantly higher than that of the M/F group. The NLR of the P group for diagnosing intestinal metaplasia and neoplasia was significantly lower than that of the M/F group. The diagnostic sensitivity and specificity of the P group were higher than that of the M/F group, but the differences were not significant (Table 4). The assessment of intestinal metaplasia included only gastric mucosal lesions and the metaplastic esophageal mucosal lesions.
| P group | M/F group | P value | |
| Number of lesions | 36 | 38 | NS |
| Locations | NS | ||
| Duodenum | 1 | 2 | |
| Antrum | 15 | 14 | |
| Incisure angularis | 9 | 6 | |
| Gastric body/fundus | 3 | 3 | |
| Cardia | 3 | 5 | |
| Esophagus | 5 | 8 | |
| Histopathology | NS | ||
| Inflammation | 22 | 21 | |
| Intestinal metaplasia | 10 | 10 | |
| Neoplasia | 4 | 7 |
| Inflammation | Intestinal metaplasia | Neoplasia | |||||||
| P group | M/F group | P value | P group | M/F group | P value | P group | M/F group | P value | |
| Sensitivity (%) | 90.48 (71.09-97.35) | 89.47 (68.61-97.06) | NS | 90.00 (59.58-98.21) | 80.00 (49.02-94.33) | NS | 100(51.01-1) | 85.71 (48.69-97.43) | NS |
| Specificity (%) | 92.86 (68.53-98.73) | 94.12 (73.02-98.95) | NS | 96.00 (80.46-99.29) | 95.65 (79.01-99.23) | NS | 96.77 (83.81-99.43) | 89.66 (73.61-96.42) | NS |
| PLR | 12.67 | 15.21 | NS | 22.50 | 18.40 | NS | 31 | 8.29 | 0.015 |
| NLR | 0.10 | 0.11 | NS | 0.10 | 0.21 | 0.014 | 0 | 0.16 | < 0.001 |
Patients in M-group required significantly more time to achieve sedation (4.47 ± 2.40 min) than P group (3.22 ± 1.70 min). Procedure duration was also longer in M/F group (28.45 ± 8.04 min) than in P group (25.00 ± 6.51 min). Three patients in the P group and one patient in the M-group experienced a decrease in systolic blood pressure below 80 mmHg which were successfully rectified by intravenous fluid administration. There was no case of de-saturation < 90% or bradycardia during or after the procedure. χ2 analysis showed that there were no statistical differences between the two groups in terms of the above-mentioned parameters (P = 0.339) (Table 5). In addition, the hemodynamic parameters, including the mean values of heart rate, hemoglobin oxygen saturation, and mean arterial pressure were all similar in both groups (P = 0.087, P = 0.903, P = 0.244).
| P group | M/F group | P value | |
| Sedation time (min) | 3.22 ± 1.70 | 4.47 ± 2.40 | 0.002 |
| Procedure time (min) | 25.00 ± 6.51 | 28.45 ± 8.04 | 0.028 |
| Adverse events | 0.339 | ||
| Hypoxemia | 0 | 0 | |
| Hypotension | 3 | 1 | |
| Bradycardia | 0 | 0 | |
| Patient assessment | |||
| Satisfaction | 10 (10–10) | 10 (9–10) | 0.105 |
| Pain or discomfort | 0 (0–0) | 0 (0–1) | 0.145 |
| Intraprocedural recall | 0 (0–0) | 0 (0–1) | 0.006 |
| Willingness to repeat (n) | 0.559 | ||
| Lighter | 5 | 4 | |
| Deeper | 4 | 7 | |
| Same level | 40 | 40 | |
| Endoscopist assessment | |||
| Satisfaction with sedation | 10 (9–10) | 9 (8–10) | 0.003 |
| Patient cooperation | 4 (4–4) | 4 (3–4) | 0.002 |
| Quality of endoscopy | 4 (4–4) | 4 (3–4) | 0.018 |
| Level of sedation | 0.014 | ||
| Apparently inadequate | 0 | 3 | |
| Inadequate | 7 | 16 | |
| Adequate | 41 | 31 | |
| Oversedated | 1 | 1 | |
| Assistant satisfaction | 9 (9–10) | 8 (7–10) | 0.001 |
| Anesthetist satisfaction | 9 (9–10) | 7 (5–8) | < 0.001 |
The results of patient assessment for the procedure are shown in Table 5. No significant differences were observed between the two groups in terms of patient satisfaction and pain or discomfort. However, the amnestic effect was significantly better in the P group than in M/F group (P = 0.006). With regard to the patients’ preference of sedation for their next EGD, some patients in the M/F group seemed to prefer deeper sedation and more patients in the P group preferred lighter sedation. The majority of the two groups (40 patients of each group) would like to receive the same level of sedation.
The endoscopists, based on the mean sedation score as judged by the performing endoscopist (Zuo XL), were significantly in favor of the P group vs the M/F group. In addition, the quality of endoscopy and patient cooperation were also rated as significantly superior in the P group. The level of sedation, as estimated by endoscopist immediately after the procedure, was significantly more adequate for the P group than for the M/F group (P = 0.014 comparing “apparently inadequate and inadequate”vs“adequate”). Furthermore, the assistant and anesthetist scores for overall sedation also favored the P group receiving propofol as compared with the M/F group receiving midazolam plus fentanyl (P = 0.001 and P < 0.001) (Table 5).
CLE is a new endoscopic device that can instantly validate tissue pathology via viewing endomicroscopic images during ongoing endoscopy. Good quality endomicroscopic images can be obtained by achieving full vertical contact of the confocal imaging window with the mucosa[28] . The main cause of reduced quality of endomicroscopic images is to the movement artifacts. Therefore, an adequate level of sedation is desirable to make iCLE examination more tolerable to the patient and easier to perform for the endoscopist. So far, several sedative agents, such as midazolam and propofol, have been applied in iCLE examination to achieve conscious sedation. However, no investigation has yet compared the sedation efficacy of propofol with the regimen of benzodiazepines and narcotics during iCLE. Results of this prospective randomized study showed that the proportion of good quality endomicroscopic images increased by propofol (P group) as the sedative agent rather than midazolam plus fentanyl (M/F group).
Based on our results, the proportion of good quality endomicroscopic images is significantly influenced by the regimen of sedation. Propofol showed clear superiority, either for iCLE scanning of the 9 standard locations or endoscopic mucosal lesions of the upper GI tract. The diagnostic sensitivity, specificity, PLR and NLR were mostly better in patients receiving propofol, although these did not reach statistical significance except for PLR in diagnosing neoplasia and NLR in diagnosing intestinal metaplasia and neoplasia. In our opinion, the reason might be that patients under propofol sedation tolerated inflation of the stomach and the attachment of the iCLE onto the tissue to a greater extent than patients under midazolam and fentanyl sedation, who still tend to experience some retching and belching. The more frequent patient movement in the M/F group not only interferes in the full vertical contact of the confocal window on the interested area, but also disturbs the endoscopist’s attention on making a definite judgment.
In addition, our findings suggest that propofol is more efficient compared to the regimen of midazolam plus fentanyl in the sedation of patients undergoing iCLE. The procedure duration and sedation time were all significantly longer in the M/F group. Since the number, endoscopic location and histological spectrum of mucosal lesions were well matched between the two groups, we therefore interpreted the prolonged procedure time in M/F sedation as being a consequence of the necessity for short-term interruptions of the endoscopic procedure due to the time interval required until repeated administrations of midazolam effectively resedated the patients. Adverse event and postprocedure patient assessment were not significantly different except for more frequent intraprocedural recall with midazolam and fentanyl. The endoscopist assessment, assistant satisfaction and anesthetist satisfaction all favor the use of propofol. These were in accordance with previously published data comparing the sedation effect of propofol vs midazolam-based regimen during endoscopy[7,17,18,29]. A prior study reported that propofol caused more pain on administration, thus leadidng to a lower acceptance rate by patients[30]. In this study, propofol was often mixed with lidocaine (50 mg of 2% lidocaine mixed with 200 mg of 1% propofol) at the time of injection, and no patient experienced pain or complained of pain.
Considering the extensive clinical application of the combined use of midazolam and fentanyl, we choose this regimen as a comparison arm to the increasingly advocated anesthetic drug propofol in the present study. Thus the independent role of midazolam compared with propofol in sedative endomicroscopy may not be clear according to the present research. However, previous data showed that the addition of a narcotic to midazolam may result in better patients’ cooperation, easier insertion of the gastroscope, and increased endoscopists’ satisfaction with the procedure[31,32]. Nevertheless, further studies are warranted to explicit the independent role of midazolam in this procedure.
Our study has certain limitations. First, the difference of the proportion of good quality endomicroscopic images between the two groups did not reach the estimated value (19.86% vs 21%) with the current sample size (100 patients), which will certainly weaken the statistical power of this study. However, we did not expand patient recruitment because the predetermined study period has terminated. Anyway, χ2 analyses demonstrated statistical significance either for total number of good quality endomicroscopic images or for each examined area between the two groups. Therefore, the results of this study need to be warranted in further researches with a larger sample size. Second, although the target level of sedation in this study was conscious sedation, it is possible that some patients may move to deeper sedation during the procedure since they were not continuously called or shaken in order to judge their sedation level when being examined. In addition, there have been reports comparing the sedation depth of propofol vs midazolam and meperidine, which demonstrated that propofol was more likely to produce a deeper level of sedation than midazolam and meperidine[17,19]. Given the narrow therapeutic window of propofol, the onset of sedation may be deeper at first, with effect moderating over time. Indeed, the anesthetic agents in both groups were titrated according to patient safety and comfort rather than sedation. Nevertheless, all patients in the present study were monitored with continuous pulse oxymetry and noninvasive arterial blood pressure measured at 5-min intervals,and no severe side effects were observed in either group of patients in this study.
In conclusion, propofol was superior to midazolam and fentanyl for conscious sedation in achieving good quality endomicroscopic images which an accurate endomicroscopic diagnosis is based on. The sedation related outcomes, such as procedure duration, sedation duration, amnesia, endoscopist satisfaction and patient cooperation, also favor the application of propofol. Therefore, conscious sedation using propofol rather than midazolam and fentanyl might be recommended for iCLE examinations. However, the results of the present study need to be further validated with a larger population in multiple centers.
Confocal laser endomicrosopy (CLE) is a novel endoscopic modality which enables real-time visualization of cellular and subcellular structures in vivo. Yet integrated CLE (iCLE) examination might require more patients’ cooperation and better sedation so as to get endomicroscopic images of good quality and make an accurate diagnosis. Although benzodiazepines, narcotics or propofol have been administered during iCLE procedures, the most effective and satisfactory sedation agent for iCLE examination has not yet been investigated.
The clinical applications of iCLE have been validated in various gastrointestinal (GI) diseases, including Barrett’s esophagus, early esophageal and gastric cancer, ulcerative colitis, and colorectal neoplasia. The most widely used sedative combination for GI endoscopy is benzodiazepine and narcotics. Recent data suggest that the use of propofol for sedation is increasing.
This study first validated that sedation with propofol could increase the proportion of good quality endomicroscopic images, and may result in improved procedural efficacy and diagnostic accuracy during iCLE examination.
The results of the present study help make a preferable anesthetic regimen for sedative iCLE examination. Conscious sedation using propofol rather than midazolam and fentanyl might be recommended for iCLE examinations.
CLE is an outgrowth of conventional laboratory confocal microscope. Currently, there are 2 CLE imaging system available in clinical practice: one is the integrated CLE (iCLE) with a miniaturized confocal microscope integrated at the distal tape of a conventional endoscope, the other is a probe-based CLE (pCLE) which is ultrathin and can be passed through the working channel of standard endoscopes.
Sedation is a big issue in endoscopic procedures. The authors evaluated the quality of endomicroscopic images under anesthetic condition. For getting the good quality of endomicroscopic image, extremely sedative condition is required. Therefore, the authors used variable anesthetic medicines. However, the adverse effects of sedatives are sometimes very severe. In this study, the authors found similar side effects and good quality images in propofol group. That is an important study for the future application of sedative endomicroscopy.
Peer reviewers: Tomoyuki Shibata, MD, PhD, Associate Professor, Department of Gastroenterology, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukake-cho,Toyoake, Aichi 470-1192, Japan; Dieter Schilling, MD, PhD, Department of Gastroenterology, Diakonie Hospital Academic Teaching Hospital of the University of Heidelberg, Speyererstrasse 91 - 93, 68163 Mannheim, Germany
S- Editor Shi ZF L- Editor Ma JY E- Editor Xiong L
| 1. | Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 560] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Leong RW, Nguyen NQ, Meredith CG, Al-Sohaily S, Kukic D, Delaney PM, Murr ER, Yong J, Merrett ND, Biankin AV. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology. 2008;135:1870-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Foersch S, Kiesslich R, Waldner MJ, Delaney P, Galle PR, Neurath MF, Goetz M. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010;59:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 8. | Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Arrowsmith JB, Gerstman BB, Fleischer DE, Benjamin SB. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 320] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Faulx AL, Vela S, Das A, Cooper G, Sivak MV, Isenberg G, Chak A. The changing landscape of practice patterns regarding unsedated endoscopy and propofol use: a national Web survey. Gastrointest Endosc. 2005;62:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, Allen T, Neurath MF, Shroyer NF, Montrose MH. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Pech O, Rabenstein T, Manner H, Petrone MC, Pohl J, Vieth M, Stolte M, Ell C. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol. 2008;6:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Safety of propofol for conscious sedation during endoscopic procedures in high-risk patients-a prospective, controlled study. Am J Gastroenterol. 2003;98:1751-1757. [PubMed] [DOI] [Full Text] |
| 15. | Ng JM, Kong CF, Nyam D. Patient-controlled sedation with propofol for colonoscopy. Gastrointest Endosc. 2001;54:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Meining A, Semmler V, Kassem AM, Sander R, Frankenberger U, Burzin M, Reichenberger J, Bajbouj M, Prinz C, Schmid RM. The effect of sedation on the quality of upper gastrointestinal endoscopy: an investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy. 2007;39:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Koshy G, Nair S, Norkus EP, Hertan HI, Pitchumoni CS. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol. 2000;95:1476-1479. [PubMed] [DOI] [Full Text] |
| 18. | Vargo JJ, Zuccaro G, Dumot JA, Shermock KM, Morrow JB, Conwell DL, Trolli PA, Maurer WG. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology. 2002;123:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc. 2002;55:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK. Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003;1:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 513] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, Liu H. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Li Z, Yu T, Zuo XL, Gu XM, Zhou CJ, Ji R, Li CQ, Wang P, Zhang TG, Ho KY. Confocal laser endomicroscopy for in vivo diagnosis of gastric intraepithelial neoplasia: a feasibility study. Gastrointest Endosc. 2010;72:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zuo XL, Li CQ, Zhang JN, Guo YT, Zhang TG. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Goetz M, Dunbar KB, Canto MI. Examination technique of confocal laser endomicroscopy. Atlas of endomicroscopy. Berlin: Springer-Heidelberg 2008; 37. |
| 29. | Krugliak P, Ziff B, Rusabrov Y, Rosenthal A, Fich A, Gurman GM. Propofol versus midazolam for conscious sedation guided by processed EEG during endoscopic retrograde cholangiopancreatography: a prospective, randomized, double-blind study. Endoscopy. 2000;32:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Patterson KW, Casey PB, Murray JP, O'Boyle CA, Cunningham AJ. Propofol sedation for outpatient upper gastrointestinal endoscopy: comparison with midazolam. Br J Anaesth. 1991;67:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Chin KW, Tan PK, Chin MK. Sedation for gastroscopy: a comparison between midazolam and midazolam with nalbuphine. Ann Acad Med Singapore. 1994;23:330-332. [PubMed] |
| 32. | Laluna L, Allen ML, Dimarino AJ. The comparison of midazolam and topical lidocaine spray versus the combination of midazolam, meperidine, and topical lidocaine spray to sedate patients for upper endoscopy. Gastrointest Endosc. 2001;53:289-293. [PubMed] [DOI] [Full Text] |