Published online Apr 21, 2012. doi: 10.3748/wjg.v18.i15.1793
Revised: September 20, 2011
Accepted: February 27, 2012
Published online: April 21, 2012
AIM: To correlate cyclooxygenase-2 (COX-2) expression profile with clinical and pathological variables to assess their prognostic/predictive value in colorectal carcinoma (CRC).
METHODS: Archival tumor samples were analyzed using immunohistochemistry for COX-2 expression in 94 patients with CRC. Patients were diagnosed and treated at the Departments of Surgery and Oncology, King Abdulaziz University Hospital, Saudi Arabia.
RESULTS: Fifty-six percent of the tumors showed positive cytoplasmic COX-2 expression, whereas 44% of cases were completely COX-2-negative. There were no significant correlations between COX-2 expression and sex, age, grade or tumor location. However, COX-2 expression revealed a significant correlation with tumor stage (P = 0.01) and distant metastasis (P = 0.02), and a borderline association with lymph node involvement (P = 0.07). Tumors with high COX-2 expression showed a higher recurrence rate than tumors with no expression (P < 0.009). In univariate Kaplan-Meier survival analysis, there was a significant (P = 0.026) difference in disease-free survival between COX-2-positive and negative tumors in favor of the latter. COX-2 expression did not significantly predict disease-specific survival, which was much shorter for COX-2-positive tumors. In multivariate (COX) models, COX-2 did not appear among the independent predictors of disease-free survival or disease-specific survival.
CONCLUSION: COX-2 expression seems to provide useful prognostic information in CRC, while predicting the patients at high risk for recurrent disease.
- Citation: Al-Maghrabi J, Buhmeida A, Emam E, Syrjänen K, Sibiany A, Al-Qahtani M, Al-Ahwal M. Cyclooxygenase-2 expression as a predictor of outcome in colorectal carcinoma. World J Gastroenterol 2012; 18(15): 1793-1799
- URL: https://www.wjgnet.com/1007-9327/full/v18/i15/1793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i15.1793
Numerous epidemiological and clinical studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) can reduce the number and size of adenomas in patients with familial adenomatous polyposis and decrease the incidence of colorectal cancer (CRC)[1,2]. Recent studies have shown that regular use of aspirin after the diagnosis of CRC is associated with a lower risk of mortality, particularly when the tumors overexpress cyclooxygenase-2 (COX-2)[3,4]. It is suggested that this effect is mediated through inhibition of COX-2, an enzyme involved in prostaglandin metabolism, inflammation and carcinogenesis[4,5]. Elevated levels of COX-2 have been detected in 85%-95% of CRC samples, and in 40%-50% of adenomas, and elevated mRNA levels of COX-2 in 86% of CRC and 43% of adenomas, in contrast to no or weak expression of COX-2 protein or mRNA in normal colorectal mucosa[6,7]. There is some recent evidence that COX-2 is related to apoptosis inhibition, induction of Bcl-2 expression, as well as stimulation of angiogenesis[8-10]. However, the role of COX-2 in colorectal carcinogenesis remains unclear.
Increased COX-2 expression has been reported in several human malignancies including uterine cervix[11], non-small cell lung carcinoma[12], prostate[13], stomach[9] and breast cancer[14], as well as in nonepithelial tumors such as gastrointestinal stromal tumors[15] and Hodgkin’s lymphoma[16]. The aim of the present study was to explore the relationship between COX-2 expression profile and clinicopathological features of CRC as well as its value as a predictor of disease outcome.
The material of the present study consists of a series of 94 CRC specimens, retrospectively collected from the archives of Anatomical Pathology Laboratory in King Abdulaziz University, Jeddah, Saudi Arabia, covering the period from January 2005 to December 2009. Serial sections were cut from paraffin blocks, stained with hematoxylin and eosin for routine histological examination, classification, grading and staging following the American Joint Committee on Cancer staging system[17]. The pertinent clinicopathological data (sex, age, stage, grade and lymph node status), and follow-up results were retrieved from the patients’ records after obtaining the relevant ethical approval (Table 1). The mean age of the patients was 58 years (median: 59 years, range: 24-90 years).
| Features | n (%) | COX-2 expression | P value | |
| Negative | Positive | |||
| Sex | 0.97 | |||
| Male | 48 (51) | 21 | 27 | |
| Female | 46 (49) | 20 | 26 | |
| Age group (yr) | 0.59 | |||
| < 60 | 47 (50) | 22 | 25 | |
| > 60 | 47 (50) | 19 | 28 | |
| LN involvement | 0.07 | |||
| Yes | 60 (64) | 22 | 38 | |
| No | 34 (36) | 19 | 15 | |
| Distant metastasis | 0.02 | |||
| Yes | 33 (35) | 11 | 22 | |
| No | 61 (65) | 30 | 31 | |
| Tumor stage | 0.01 | |||
| I/II | 32 (34) | 20 | 12 | |
| III/IV | 62 (66) | 21 | 41 | |
| Tumor grade | 0.50 | |||
| Well | 25 (27) | 11 | 14 | |
| Moderate | 56 (60) | 26 | 30 | |
| Poor | 11 (13) | 3 | 8 | |
| Tumor location | 0.80 | |||
| Right colon | 38 (40) | 16 | 22 | |
| Left colon | 56 (60) | 25 | 31 | |
| Recurrence | 0.22 | |||
| Yes | 31 (33) | 11 | 20 | |
| No | 38 (40) | 19 | 19 | |
| Unknown | 25 (27) | |||
Four-micrometer-thick tissue sections were cut from the paraffin blocks (containing both tumor and benign tissues), mounted on charged poly-l-lysine-coated slides, and subjected to immunohistochemistry using the Avidin Biotin detection system, following the manufacturer’s instructions. The antibody used was a mouse anti-human COX-2 monoclonal antibody (Dako Cytomation Norden A/S, Glostrup, Denmark; dilution 1:50). Immunohistochemistry was carried out by an automatic immunostainer (Ventana Bench Mark XT; Ventana Inc., Tucson, AZ, United States). In each analysis, positive controls were used consisting of CRC samples previously shown to stain with this antibody. Tris-buffered saline in place of the primary antibody was used as a negative control.
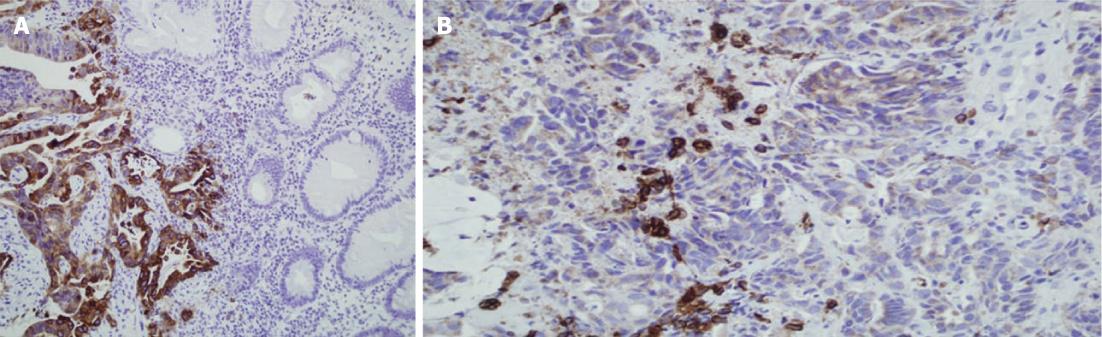
Cells were considered positive for COX-2 when distinct cytoplasmic yellow to brown staining was identified. The extent and intensity of the staining were recorded on a scale from 0 to +++; +++ implied strong staining that was maximally intense throughout the specimen, and 0 implied negative staining. When dichotomized for statistical risk assessment [odds ratio (OR) calculation], negative (-) and weak (+) staining were defined as low expression, whereas moderate (++) and intense (+++) staining were included in the high expression category.
Statistical analyses were performed using SPSS (IBM, New York, NY, United States) and Stata (Stata Corp., College Station, TX, United States) software (PASW Statistics for Windows, version 18.0.2 and STATA/SE version 11.1). Frequency tables were analyzed using the χ2 test, with likelihood ratio or Fischer’s exact test to assess the significance of the correlation between the categorical variables. OR and 95% confidence interval (CI) were calculated when appropriate, using the exact method. Differences in the means of continuous variables were analyzed using nonparametric tests (Mann-Whitney or Kruskal-Wallis) for two and multiple independent samples, respectively. Analysis of variance was only used to derive the mean values (and 95% CI) of each individual stratum. Univariate survival analysis for the outcome measure [disease-specific survival (DSS) and disease-free survival (DFS)] was based on the Kaplan-Meier method, with log-rank (Mantel-Cox) comparison test. To assess the value of COX-2 as an independent predictor, multivariate survival analysis was performed, using the Cox proportional hazards regression model, controlling for the confounding by the following variables: age, sex, tumor localization, tumor stage, grade, (for DFS), and recurrence as additional variable for DSS. In all tests, P < 0.05 was regarded as statistically significant.
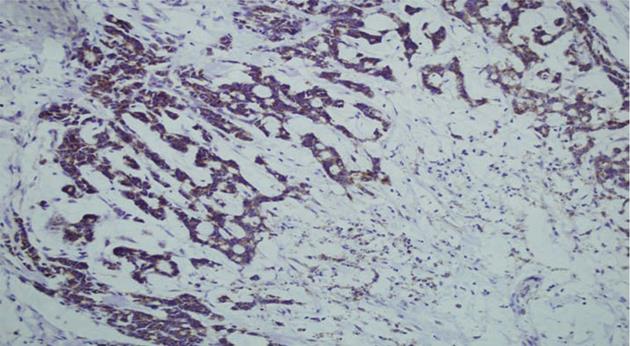
Normal colorectal mucosa showed no or weak COX-2 expression, but weak cytoplasmic expression was detected in a few inflammatory mononuclear cells (Figure 1). In cancer cells, COX-2 expression appeared as yellow-brown staining and was observed mainly in the cytoplasm and occasionally in the nuclear envelope (Figures 1 and 2). Expression was high in 53 (56%) cases, and low in 41 cases (44%).
The associations between COX-2 expression and clinicopathological features are presented in Table 1. Sex, age, grade, or tumor location had no significant relationship with the expression of COX-2. However, the tumor stage and distant metastasis were significantly associated with COX-2 expression, with higher expression being more common in advanced tumors (P < 0.01), and fewer distant metastases being found in tumors with negative expression (P < 0.02). Of particular interest was the borderline association between lymph node (LN) involvement and expression of COX-2 (P < 0.07); 63% of the tumors with LN involvement tested positive for COX-2, whereas only 37% of the cases with no COX-2 expression had LN involvement.
Similarly, COX-2 expression showed a clear association with DFS in that the patients who developed early recurrence (22 mo) had positive COX-2 expression, in contrast to those with no expression of COX-2 who developed recurrence much later (32 mo) (P = 0.009). The same trend was observed in DSS time, with patients with COX-2 negative tumors living significantly longer (P = 0.009).
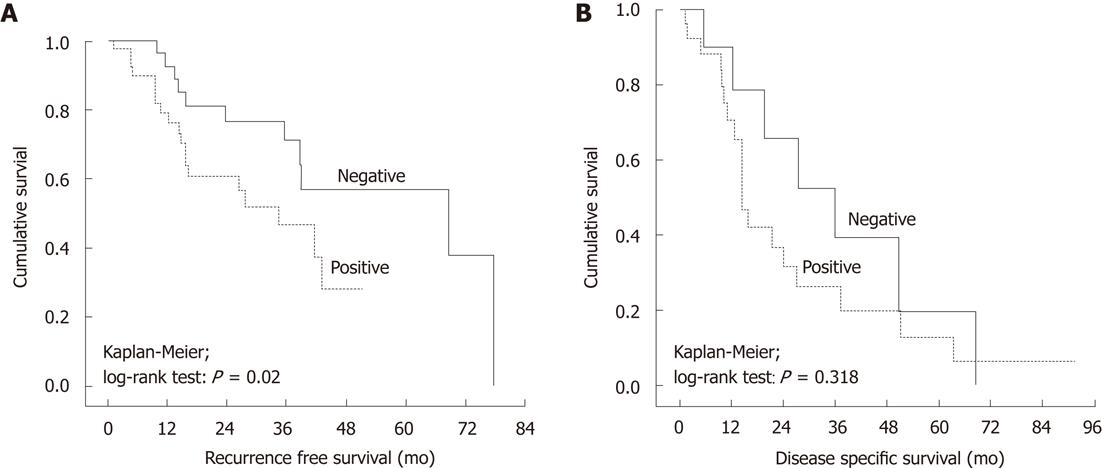
In Kaplan-Meier survival analysis, there was a significant (P = 0.02) difference in DFS between patients with COX-2-negative tumors (longer DFS) and those with COX-2-positive tumors (Figure 3A). As to DSS, COX-2 did not show any predictive power in Kaplan-Meier analysis (P = 0.32) (Figure 3B).
To assess the value of COX-2 as an independent predictor, a multivariate survival analysis was done, using the Cox proportional hazards regression model controlling for confounding by the following covariates: age, sex, tumor localization, T, grade, (for DFS), and recurrence as additional variable for DSS. In the final multivariate model, only tumor grade (P = 0.023) and tumor stage (P = 0.0001) remained significant predictors of DFS, decreasing with progressing dedifferentiation and increasing stage, as expected. In a similar model for DSS (including recurrence as a covariate), none of the included covariates were independent predictors of DSS.
The aims of the present study were to cast further light on the issues related to prognostication of CRC, and assess the value of COX-2 expression profiles as predictive and prognostic markers. We focused on stage II-IV disease, in which molecular and other markers may help to pinpoint a subgroup of patients who would eventually benefit from the use of adjuvant therapy. This important therapeutic decision involves a careful weighing of the risks of drug toxicity and complications against the potential curability of the disease[18]. It is well established that early CRCs can be cured with radical surgical resection alone[19]. Unfortunately, however, some 30% of all patients who undergo curative resection subsequently present with relapse and eventually die of their disease[20]. Prediction of disease outcome in individual patients after curative resection is still far from reliable[21]. However, there is some hope for improvement, and in fact, our results suggest that COX-2 expression assessed by immunohistochemistry might be helpful in this respect. In addition, more rational decisions can be done as soon as we learn more of the markers and other diagnostic tools for accurate prediction of disease outcome in individual patients[22,23].
On the basis of the present results, we do believe that this detection can be improved using the assessment of COX-2 expression in the primary tumors. In this assessment, quantifying the COX-2 expression seems to be important. We compared different methods of grading this expression, and found the dichotomized negative/positive grading to provide the most consistent and meaningful correlations to the clinicopathological variables and outcome. This leads us to suggest that classifying CRCs as COX-2 positive/negative is the clinically most relevant approach.
In our series, 56% of the primary CRCs expressed COX-2, whereas the results in previous studies have show that COX-2 was expressed in 85%-95% of CRCs[6,7]. This implies its important role in cancer progression. The discrepancy of these observations might be explained by differences in the technical aspects of recording COX-2 expression or by differences of interpretation of expression profiling. The possibility that these discrepancies may have a genetic basis is one favored hypothesis. However, the markedly different expression of COX-2 in normal and cancer tissues substantiates the view that paracrine effects in the crosstalk between normal and cancer cells are likely to be involved in the upregulation of COX-2 in the tumor tissue.
In the present cohort, several potentially important observations were made, all implying that the quantitatively measurable COX-2 expression in tumor cells could provide significant prognostic information in CRC. First, positive expression of COX-2 was more common among advanced stage tumors than in early stage tumors. This is in agreement with a study by Lim et al[24] who showed that COX-2 expression was correlated with the depth of invasion and advanced tumor stage. Similarly, we observed a significant correlation between COX-2 expression and distant metastasis, and tumors with more COX-2 expression revealed more distant metastasis than did those with no expression. This observation also substantiates the data of Tomozawa et al[25], who have reported that COX-2 overexpression was significantly associated with tumor recurrence, and especially with hematogenous metastasis. In contrast to these results, however, the study by Fujita et al[26] failed to establish any correlation between COX-2 overexpression and metastasis.
A trend with borderline significance was observed between COX-2 and LN involvement; 63% of the tumors with LN involvement showed positive expression of COX-2, whereas only 37% of the cases not expressing COX-2 had LN involvement. Xiong et al[27] have reported that among 45 cases of CRC with LN metastasis, COX-2 detection rate was 87% in the primary tumors, whereas diffuse cytoplasmic COX-2 staining in the cancer cells was detected in 100% of the LN metastatic lesions. The preferential expression of COX-2 in LN metastases is consistent with the view of clonal selection of tumor cells with COX-2 expression, conferring upon them a growth advantage with a higher potential of metastasis. This could be how COX-2 expression is related to progression of CRC. Altogether, these observations implicate COX-2 as a biological factor that might affect the behavior of tumor cell populations. Some studies have shown that higher expression of COX-2 is found in benign breast tissue adjacent to breast carcinoma[28].
Obviously, one of the most important observations of the present study is that linking COX-2 expression with disease outcome, that is, disease recurrence and length of DFS. This is clinically relevant for several reasons. Some CRC patients at different stages are at high risk of recurrence, thus, it is of paramount importance to develop reliable markers that accurately predict those patients so that they may be considered for adjuvant therapy. In the present series, 37% of the patients eventually developed a recurrent disease within the median follow-up time of 15.6 mo (mean, 22 mo; range: 15.8-28.2 mo). This is a substantially high rate, particularly for a group of LN-negative (stage II) CRC patients. Importantly, our data showed that the patients who developed an early recurrence (mean DFS of 22 mo) had positive COX-2 expression, in contrast to patients with negative expression, who developed recurrence with a significantly longer delay (mean DFS of 32 mo).
As shown in Figure 3, at 5 years follow-up, only 40% of the patients with negative COX-2 expression had recurrence, as compared to 85% of those with COX-2-expressing tumors. This is in agreement with the study by Wan et al[29], who showed that the recurrence and metastasis rates were significantly higher in COX-2-positive patients than in their negative counterparts. Similarly, Tomozawa et al[25] also have found that COX-2 overexpression was correlated with disease recurrence and hematogenous metastasis, with DFS time being significantly shorter for patients with high expression of COX-2 compared with low expression. Furthermore, COX-2 was the only independent predictor of survival time when adjusted for other prognostic factors, including age, histological type, and tumor size and stage. Therefore, COX-2 overexpression is considered to be correlated with recurrence and metastasis of CRC. Tsujii et al[30] also have found that metastatic potential increases with COX-2 overexpression. These important results suggest that selective COX-2 inhibitors might be useful chemopreventive agents, not only in growth of the primary tumor but also for prevention of hematogenous metastasis of CRC. When adjusted for other potential predictors in multivariate Cox regression model, COX-2 expression lost its value as a significant independent predictor of DFS.
As to DSS, COX-2 expression was shown to be high (positive) more often among patients who eventually died of their disease as compared with those staying alive at the completion of the follow-up, although the difference was not significant (P = 0.30). However, the difference in DSS time between COX-2-positive and -negative tumors was significant (P = 0.009); being shorter for the former. However, this difference did not appear significant in univariate (Kaplan-Meier) or multivariate (COX) survival analysis. These observations suggest that CRC tumors with positive COX-2 expression are at high risk for local or distant recurrence and, because of the strong adverse impact of the latter on survival (i.e., none of the patients without recurrence died of disease in our cohort), these patients are also more likely to eventually die of their disease. These patients should be appropriate candidates for close and frequent postoperative follow-up and eventual adjuvant therapy.
Taken together, COX-2 expression did not correlate with sex, age or tumor grade, as confirmed by other studies[27,31]. However, COX-2 expression showed significant associations with tumor stage and metastasis. Furthermore, positive COX-2 staining was related to a higher recurrence rate as compared to COX-2-negative tumors, suggesting a link towards development of a metastatic phenotype. Finally, positive COX-2 expression was associated with shorter DSS, as compared with COX-2-negative CRCs, implying some differences in the inherent malignancy of CRC that become manifest after a reasonable follow-up period. To elucidate this fully, however, we would need a larger cohort and a substantially longer follow-up time to provide the basis for clinical trials that evaluate the contribution of COX-2 inhibitors in therapy and prevention of CRC.
We would like to offer special thanks to Ministry of High Education, Kingdom of Saudi Arabia.
Phospholipase A2 (PLA2) is a key regulatory enzyme in arachidonic acid metabolism, which leads to synthesis of prostaglandins via the cyclo-oxygenase (COX)-1 and COX-2 pathways. Inhibition of COX-2 results in suppression of tumor development and growth, and it has been proposed that prostaglandins may have an important role in tumor growth in colorectal cancer (CRC). Prostaglandin E2 induces bcl-2 gene expression and inhibits apoptosis in human colorectal cell lines, which may partially explain the COX-2-mediated tumor growth. Thus, in addition to COX, functional defects in PLA2 in tumor cells may interfere with the regulatory mechanisms of tumor growth. Therefore, COX-2 represents a potential molecular target in colorectal management and specific COX-2 inhibitors may be useful as chemopreventive as well as therapeutic agent in humans.
It is mandatory to find new prognostic factors capable of identifying high-risk stage II CRC patients for better targeting of treatment options.
COX-2 expression did not correlate with sex, age or tumor grade, as confirmed by other studies. However, COX-2 expression showed significant associations with tumor stage and metastasis. Furthermore, positive COX-2 staining was related to a higher recurrence rate as compared to COX-2-negative tumors, suggesting a link towards development of a metastatic phenotype. Finally, positive COX-2 expression was associated with shorter disease-specific survival, as compared with COX-2-negative CRC, implying some differences in the inherent malignancy of CRC that become manifest after a reasonable follow-up period. To elucidate this fully, however, we would need a larger cohort and a substantially longer follow-up time to provide the basis for clinical trials that evaluate the contribution of COX-2 inhibitors in therapy and prevention of CRC.
Quantification of COX-2 expression seems to provide valuable prognostic information in CRC, particularly in selecting the patients at high risk for recurrent disease who might benefit from adjuvant therapy.
Although this manuscript makes the point that COX-2 expression can predict disease outcomes in CRC, it is clear that COX-2 expression is not an independent predictor, and in fact, in the multivariate analysis, only tumor grade and stage were independent predictors.
Peer reviewer: Robert C Moesinger, MD, FACS, Northern Utah Surgeons, Adjunct Assistant Professor, Department of Surgery, University of Utah, Ogden, UT 84403, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Xiong L
| 1. | Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313-1316. [PubMed] |
| 2. | Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404. [PubMed] |
| 3. | Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29-38. [PubMed] |
| 4. | Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649-658. [PubMed] |
| 5. | Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res. 2005;11:1618-1628. [PubMed] |
| 6. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 7. | Kutchera W, Jones DA, Matsunami N, Groden J, McIntyre TM, Zimmerman GA, White RL, Prescott SM. Prostaglandin H synthase 2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816-4820. [PubMed] |
| 8. | Abbate A, Limana F, Capogrossi MC, Santini D, Biondi-Zoccai GG, Scarpa S, Germani A, Straino S, Severino A, Vasaturo F. Cyclo-oxygenase-2 (COX-2) inhibition reduces apoptosis in acute myocardial infarction. Apoptosis. 2006;11:1061-1063. [PubMed] |
| 9. | Park JH, Kang KH, Kim SH, Lee JH, Cho CM, Kweon YO, Kim SK, Choi YH, Bae HI, Kim MS. Expression of Cyclooxygenase-2 and Bcl-2 in human gastric adenomas. Korean J Intern Med. 2005;20:198-204. [PubMed] |
| 10. | Sahin M, Sahin E, Gümüslü S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology. 2009;60:242-253. [PubMed] |
| 11. | Khunamornpong S, Settakorn J, Sukpan K, Srisomboon J, Ruangvejvorachai P, Thorner PS, Siriaunkgul S. Cyclooxygenase-2 expression in squamous cell carcinoma of the uterine cervix is associated with lymph node metastasis. Gynecol Oncol. 2009;112:241-247. [PubMed] |
| 12. | Laga AC, Zander DS, Cagle PT. Prognostic significance of cyclooxygenase 2 expression in 259 cases of non-small cell lung cancer. Arch Pathol Lab Med. 2005;129:1113-1117. [PubMed] |
| 13. | Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, Levine AC. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671-676. [PubMed] |
| 14. | Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis G, Cohen C. COX-2 expression in invasive breast cancer: correlation with prognostic parameters and outcome. Appl Immunohistochem Mol Morphol. 2007;15:255-259. [PubMed] |
| 15. | Sevinc A, Camci C, Sari I, Kalender ME, Er O, Soyuer I, Dikilitas M, Yilmaz U, Sagol O, Alacacioglu A. Cyclooxygenase-2 expression in gastrointestinal stromal tumours. Asian Pac J Cancer Prev. 2010;11:849-853. [PubMed] |
| 16. | Barisik NO, Bozkurt S, Gumus M, Kaygusuz I, Karadayi N, Bas E, Bayik M, Tecimer T. Expression and prognostic significance of Cox-2 and p-53 in Hodgkin lymphomas: a retrospective study. Diagn Pathol. 2010;5:19. [PubMed] |
| 17. | Greene F, Page D, Fleming I. AJJC cancer staging manual. 6th ed. New York: Springer –Verlag 2002; 113-122. |
| 18. | Haydon A. Adjuvant chemotherapy in colon cancer: what is the evidence? Intern Med J. 2003;33:119-124. [PubMed] |
| 19. | Wilkinson N, Scott-Conner CE. Surgical therapy for colorectal adenocarcinoma. Gastroenterol Clin North Am. 2008;37:253-267, ix. [PubMed] |
| 20. | Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783-1799. [PubMed] |
| 21. | Heimann TM, Cohen RD, Szporn A, Gil J. Correlation of nuclear morphometry and DNA ploidy in rectal cancer. Dis Colon Rectum. 1991;34:449-454. [PubMed] |
| 22. | Barderas R, Babel I, Casal JI. Colorectal cancer proteomics, molecular characterization and biomarker discovery. Proteomics Clin Appl. 2010;4:159-178. [PubMed] |
| 23. | Ross JS, Torres-Mora J, Wagle N, Jennings TA, Jones DM. Biomarker-based prediction of response to therapy for colorectal cancer: current perspective. Am J Clin Pathol. 2010;134:478-490. [PubMed] |
| 24. | Lim SC, Cho H, Lee TB, Choi CH, Min YD, Kim SS, Kim KJ. Impacts of cytosolic phospholipase A2, 15-prostaglandin dehydrogenase, and cyclooxygenase-2 expressions on tumor progression in colorectal cancer. Yonsei Med J. 2010;51:692-699. [PubMed] |
| 25. | Tomozawa S, Tsuno NH, Sunami E, Hatano K, Kitayama J, Osada T, Saito S, Tsuruo T, Shibata Y, Nagawa H. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83:324-328. [PubMed] |
| 26. | Fujita T, Matsui M, Takaku K, Uetake H, Ichikawa W, Taketo MM, Sugihara K. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823-4826. [PubMed] |
| 27. | Xiong B, Sun TJ, Hu WD, Cheng FL, Mao M, Zhou YF. Expression of cyclooxygenase-2 in colorectal cancer and its clinical significance. World J Gastroenterol. 2005;11:1105-1108. [PubMed] |
| 28. | Denkert C, Winzer KJ, Hauptmann S. Prognostic impact of cyclooxygenase-2 in breast cancer. Clin Breast Cancer. 2004;4:428-433. [PubMed] |
| 29. | Wan XB, Pan ZZ, Ren YK, Ding PR, Chen G, Wan DS. [Expression and clinical significance of metastasis-related tumor markers in colorectal cancer]. Ai Zheng. 2009;28:950-954. [PubMed] |
| 30. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [PubMed] |
| 31. | Lim SC, Lee TB, Choi CH, Ryu SY, Min YD, Kim KJ. Prognostic significance of cyclooxygenase-2 expression and nuclear p53 accumulation in patients with colorectal cancer. J Surg Oncol. 2008;97:51-56. [PubMed] |