INTRODUCTION
The ATP-sensitive potassium (KATP) channel was identified in cardiac muscle[1]. Later, a similar channel was described in liver[2], brain[3,4] and skeletal muscle mitochondria[5]. The primary function of this channel is to allow K+ transport into the mitochondrial matrix and this phenomenon could be involved in maintaining mitochondrial volume homeostasis[6]. KATP channels that exist in the inner membrane of mitochondria[2] have been implicated to mediate the protective effects of ischemic preconditioning (IPC) in ischemic heart[7,8]. Diazoxide (DZ) is a selective mitochondria ATP-sensitive potassium (mitoKATP) channel opener, which has been reported to have a protective effect on the heart[9,10], brain[11,12] and spinal cord[13] following ischemia/reperfusion (I/R) injury. Several recent studies have suggested that mitoKATP channels may be only a trigger, but not a mediator of protection[14]. The exact mechanisms have not been fully clarified.
IPC is a well-known phenomenon in which brief episodes of ischemia and reperfusion confer a state of protection against subsequent sustained long-term I/R injury[15,16]. Although the exact underlying mechanisms of IPC are unknown, activation of mitoKATP channels has been proposed to play a pivotal role in preconditioning[17]. More studies have indicated that pretreatment with mitoKATP channel openers induces IPC-like protective effects[18], and that IPC-induced protection is antagonized by mitoKATP channel inhibitors[19,20]. These findings support the hypothesis that IPC is mediated through the opening of mitoKATP channels.
Heme oxygenase-1 (HO-1) is the rate-limiting step in the oxidative degradation of heme. Overexpression of HO-1 exerts a cytoprotective function in a number of I/R injury and liver transplant models[21-23]. Recent studies have suggested that IPC may protect against systemic inflammatory response via enhanced HO-1 overexpression[24-27]. The purpose of our study was to investigate the protective action of DZ on I/R injury and expression of HO-1 after liver transplantation in rats. We explored the potential molecular mechanisms for DZ to reduce I/R injury. Using HO-1 small interfering RNA (siRNA), we further observed the roles of HO-1 in DZ-induced action.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (S-D) rats (200-250 g) (Kunming Medical College Laboratory Animal Center, China) were used. Rats were maintained on standard rodent chow and water ad libitum in a room according to the local animal welfare guidelines. All experiments were approved by the ethics committee for the use of experimental animals at Kunming Medical College.
HO-1 siRNA design
The design of HO-1 siRNA was based on the characterization by Zhang et al[28]. Additionally, a scrambled sequence was designed as a negative control siRNA. The targeted sequence of rat HO-1 siRNA was 5’-AAGCCACACAGCACUAUGUdTdT-3’ (sense) and 5’-ACAUAGUGCUGUGUGGCUUdTdT-3’ (antisense). siRNA duplexes were chemically synthesized by Guangzhou Rui Bo Biological Technology Co (Guangzhou, China).
Experimental protocol
S-D rats underwent ether anesthesia. The basic techniques of liver harvesting and orthotopic transplantation without hepatic arterial reconstruction were according to the method described previously by Kamada et al[29]. After organ harvest, liver grafts were stored for 4 h in cold University of Wisconsin solution at 4 °C. Subsequently, syngeneic orthotopic liver transplantation (OLT) was performed and the anhepatic phase was estimated to be 11-13 min for all recipients. All transplant experiments in this study were performed by a single person. Separate groups of rats were killed at 6 h after their vessels were unclamped, then samples of blood and liver tissue were taken for further analysis.
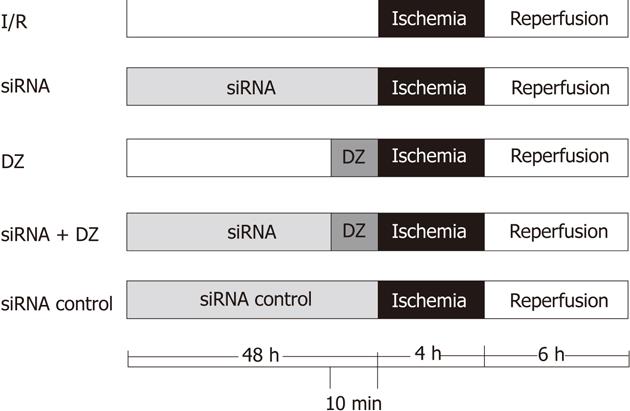
Eighty rats (16 for every group) were divided randomly into five groups (Figure 1): (1) I/R group: OLT was performed according to method described previously; (2) siRNA group: same treatment as I/R group, but donors were injected with HO-1 siRNA (200 nmol/kg body weight) via the portal vein 48 h before liver harvest; (3) DZ group: same as I/R group, but donors were injected with DZ (5 mg/kg body weight), a mitoKATP channel opener, via the portal vein 10 min before liver harvest; (4) siRNA + DZ group: same treatment as I/R group, donors were treated with HO-1siRNA and DZ; and (5) siRNA control group: same as I/R group, donors were injected with negative control siRNA (200 nmol/kg body weight). DZ was purchased from Sigma Chemical Co. (St Louis, MO, United States) and dissolved in dimethyl sulfoxide (DMSO) (Sigma, United States) before addition to experimental solutions. The final concentration of DMSO in the solution was less than 0.05%.
Figure 1 Experimental protocol.
DZ: Diazoxide; siRNA: Small interfering RNA.
Serum enzymes
At 6 h following liver reperfusion, blood was collected via the abdominal aorta. After centrifugation of whole blood (3000 rpm, 15 min), serum was extracted and stored at -70 °C until analysis. Alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were measured using a clinical chemistry system (7060 automatic analyzer, Hitachi, Japan).
Reverse-transcriptase polymerase chain reaction
Animals were sacrificed 6 h after transplantation. Liver samples were obtained, shock-frozen in liquid nitrogen and stored at -80 °C for further RNA isolation. Total RNA was extracted from 100 mg liver tissues with Trizol reagents (Invitrogen, United States) according to the manufacturer's guidelines and quantified by UV absorption. Following DNase I (Invitrogen, United States) digestion, 1 μg total RNA was subjected to reverse transcription polymerase chain reaction (RT-PCR) with M-MLV reverse transcriptase (Promega, United States), using oligo-dT as primers. The mixture was inactivated at 42 °C for 60 min, and then the reverse transcriptase was inactivated by heating at 70 °C for 10 min. Specific primers for rat HO-1 and β-actin (an internal standard) were designed by Primer Premier 5.0. For HO-1, the primers were 5’-TGGAAGAGGAGATAGAGCGA-3’ (sense) and 5’-TGTTGAGCAGGAAGGCGGTC-3’ (antisense), generating a 451-bp fragment. For β-actin, the primers used were 5’-CACGATGGAGGGGCCGGACTCATC3’ (sense) and 5’-TAAAGACCTCTATGCCAACACAGT-3’ (antisense), generating a 240-bp fragment. PCR constituents were as follows: 2 μL cDNA mixture was subjected to amplification in 20 μL final volume, 94 °C, 2 min; then 94 °C, 1 min; 60 °C, 1 min; 72 °C, 2 min, for 40 cycles; and 72 °C, 5 min to end the reaction. The PCR products were subjected to electrophoresis on 2% agarose gel containing ethidium bromide and visualized by UV illumination. This semi- quantitative measure for HO-1 was expressed as ratios to β-actin.
Western blotting analysis
Protein extracts were isolated from liver tissue with radioimmunoprecipitation containing phenylmethyl sulfonylfluoride. Protein concentration was determined by a bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, United States). Proteins (20 μg/sample) in SDS-loading buffer were heated to 100 °C for 5 min, and separated by 12% SDS-PAGE and transferred to nitrocellulose membranes using an electroblotting apparatus. The membrane was blocked overnight at 4 °C in 5% nonfat dry milk and TBST buffer to block nonspecific binding sites. Western blots were probed with primary antibodies (anti-HO-1, 1:200 dilution; Millipore, United States) at room temperature (RT) for 2 h. After being washed, blots were incubated with horseradish-peroxidase-labeled goat anti-rabbit IgG antibodies (dilution, 1:50 000; Pierce) for 2 h at RT. Thereafter, the proteins were visualized by ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ, United States) and analyzed by the Quantity One Analysis Software (Bio-Rad, United States). β-actin was used as protein loading control.
Histology and electron microscopic examination
Livers were excised rapidly and fixed in conventional fixing solutions (10% neutral-buffered formalin) after 6 h reperfusion. Livers were paraffin embedded and sectioned into 3-μm-thick slices with a tissue chopper. The sections were examined by HE staining. The histological severity of I/R injury was graded according to Suzuki’s classification[30]. All slides were judged by the same investigator who had been blinded to the corresponding study group. The excised liver samples were cut into small pieces, and immersed in excessive volumes of 2.5% glutaraldehyde with 0.1 mol phosphate buffer (pH 7.2). Following fixation for 24 h, samples were further immersed in 2% osmium tetroxide (OsO4) in 0.1 mol cacodylate buffer (pH 7.2) for 2 h at 4 °C, dehydrated, and embedded in epoxide resin (EPON 812). Ultrathin sections were stained with lead citrate and uranyl acetate, and examined using a JEM-1200EX transmission electron microscope.
Enzyme-linked immunosorbent assay
Serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels of different groups were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R and D System, United States) according to the test protocols. All samples, including standard and control solution, were assayed in duplicate. Values were expressed as pg/mL.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 for Windows (SPSS, Inc, Chicago, IL, United States). Values are expressed as means ± SD. Difference between experimental groups were analyzed by one-way analysis of variance. P < 0.05 was considered statistically significant.
RESULTS
Hepatic transaminases
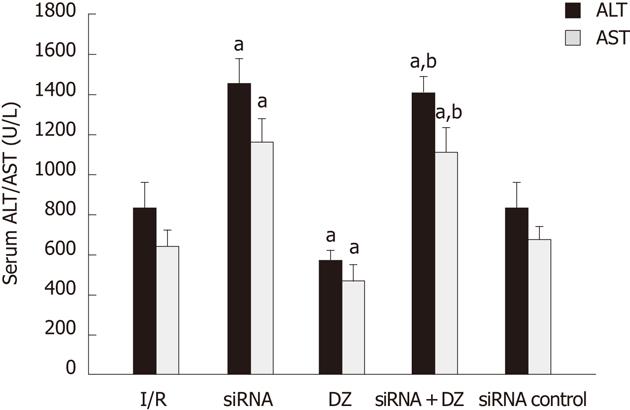
The hepatocellular damage was measured with the determination of ALT and AST levels. Rats treated with siRNA showed a significant increase in ALT and AST compared with I/R rats. Liver function measurement in the DZ group was significantly lower than that in the I/R group. There was no significant difference between the siRNA and siRNA + DZ groups (P > 0.05) (Figure 2).
Figure 2 Serum alanine aminotransferase and aspartate transaminase levels after reperfusion.
aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. ALT: Alanine aminotransferase; AST: Aspartate transaminase.
HO-1 mRNA and protein expression levels
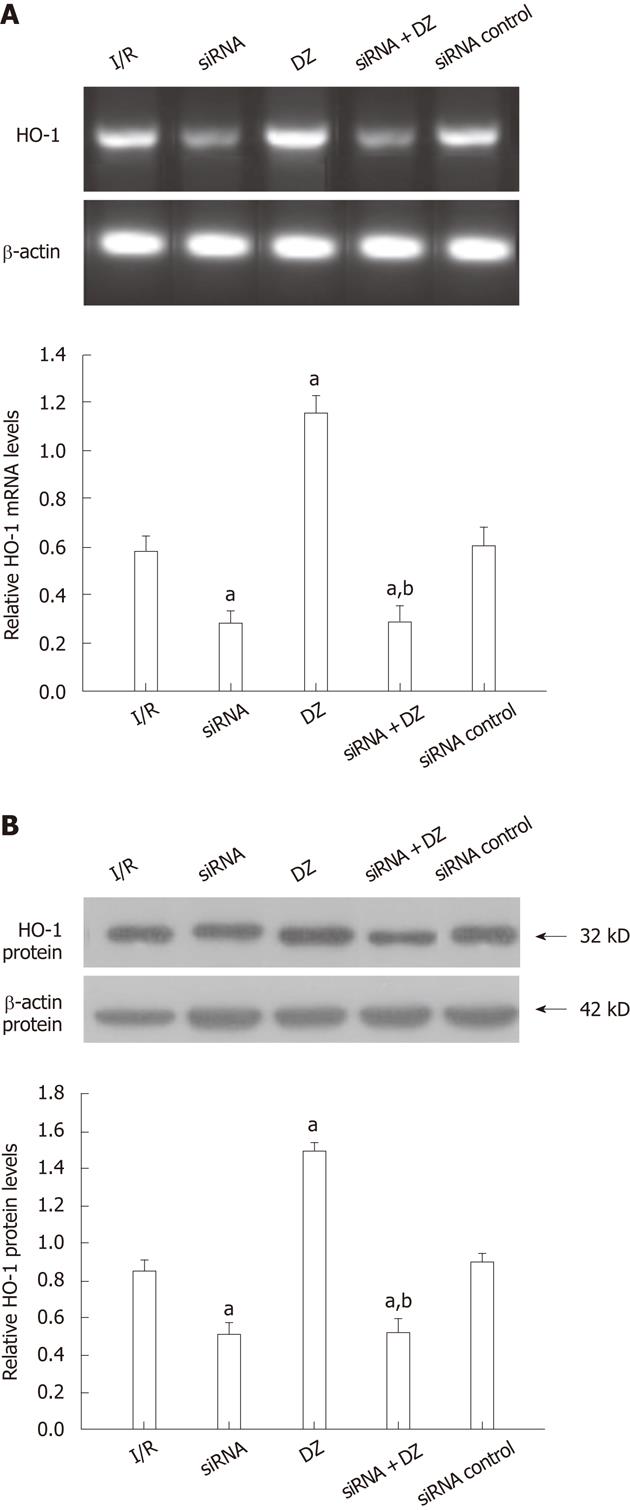
The PCR products were separated on agarose gel and the relative expression of HO-1 were shown. As shown in Figure 3A, HO-1 mRNA expression was decreased in both siRNA and siRNA + DZ groups compared with the I/R group at 6 h after I/R injury. In contrast, DZ treatment significantly enhanced the hepatic HO-1 mRNA level. No significant difference was observed between the siRNA and siRNA + DZ groups. HO-1 protein level was determined by western blotting. The results were almost the same as those obtained in the RT-PCR analyses. Compared with the I/R group, HO-1 protein expression was markedly downregulated in both siRNA and siRNA + DZ groups. The level of HO-1 was significantly higher in the DZ group than all other groups. Moreover, the difference between siRNA and siRNA + DZ groups did not attain statistical significance (Figure 3B).
Figure 3 Liver heme oxygenase-1 mRNA and protein levels at 6 h after transplantation.
aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. A: Liver heme oxygenase-1 (HO-1) mRNA expression; B: Liver HO-1 protein levels.
Histology
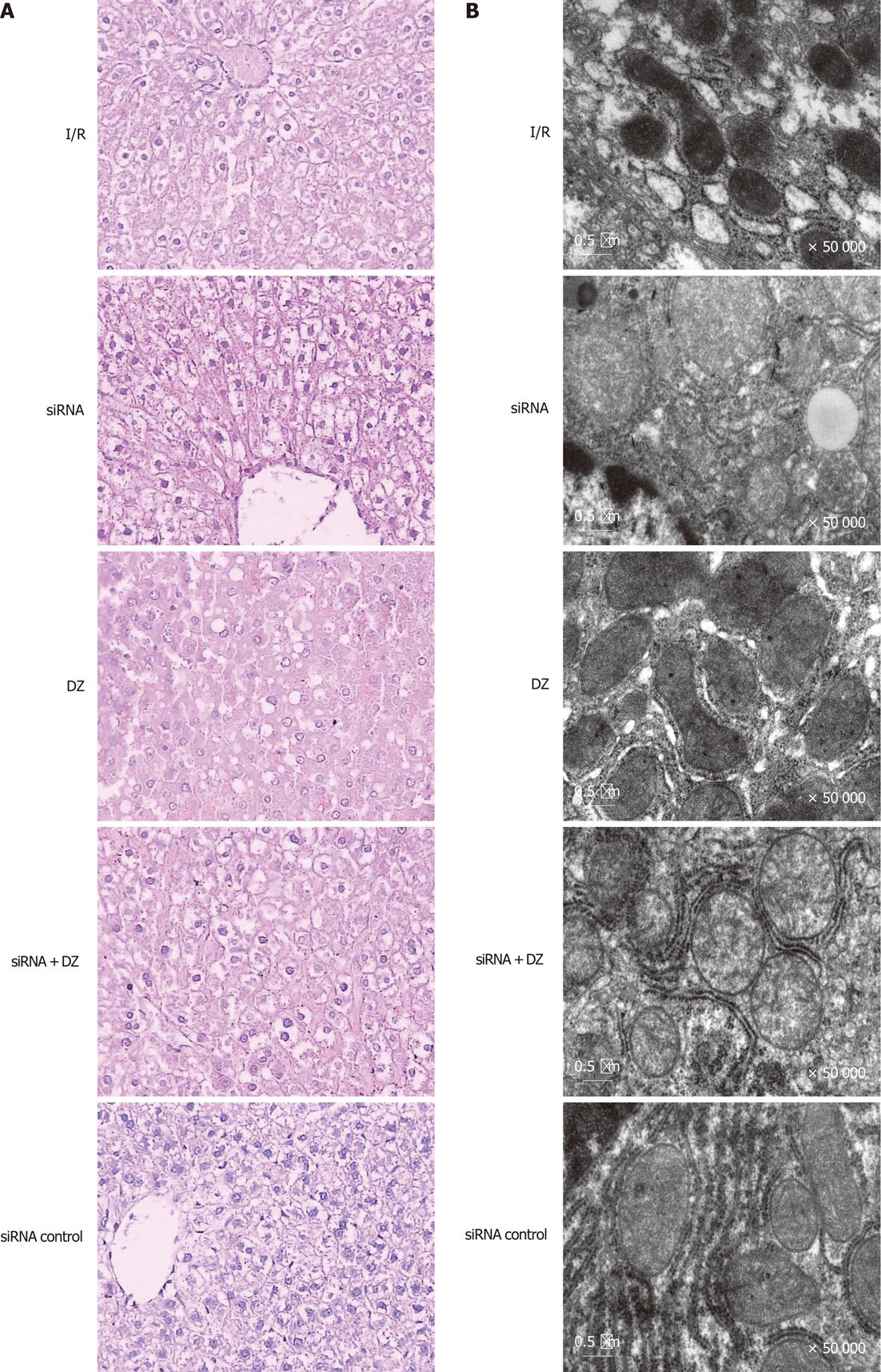
Necrosis and liver damage were assessed with HE-stained liver tissue 6 h after reperfusion. Liver specimens from rats subjected to I/R showed macrovesicular fatty changes, nuclear fragmentation, and sinusoidal congestion and congested with many red blood cells. In siRNA and siRNA + DZ groups, histological tissue damage was significantly aggravated. Moreover, multiple and extensive ballooning/hepatocellular necrosis and massive infiltration of neutrophils were observed. However, these pathological changes were significantly decreased in the DZ group (data not shown). There was no significant difference between the I/R and siRNA control groups (Figure 4A).
Figure 4 Liver histology and ultrastructural changes of hepatocytes.
A: Ischemia/reperfusion (I/R) treated livers showed vacuolization, nuclear fragmentation, sinusoidal congestion, and hepatocyte necrosis. However, histological tissue damage was aggravated in the small interfering RNA (siRNA) and siRNA + diazoxide (DZ) groups. In contrast, DZ treatment relieved liver damage (HE, × 400); B: The mitochondria and the smooth endoplasmic reticulum were dilated and even destroyed, and the mitochondrial cristae were disorganized in I/R treated hepatocytes.
Transmission electron microscopy
To confirm hepatocyte ultrastructural alterations, we examined the liver grafts during cold ischemia and reperfusion, by electron microscopy. In the siRNA group, the nuclei of the hepatocytes were irregular in shape and contained damaged nuclear chromatin in the nucleoplasm. The hepatocytes appeared largely intact vacuolar structures with lipid droplets present in the cytoplasm. The mitochondria and the smooth endoplasmic reticulum were dilated and even destroyed, and the mitochondrial cristae were disorganized. By contrast, mitochondria from rats treated with DZ appeared markedly better with less mitochondrial membrane damage and swelling in the rat hepatocytes after I/R. However, siRNA + DZ group treatment had markedly aggravated these damages as compared with DZ group. The I/R and siRNA control groups revealed less hepatocyte damage than the siRNA group (Figure 4B).
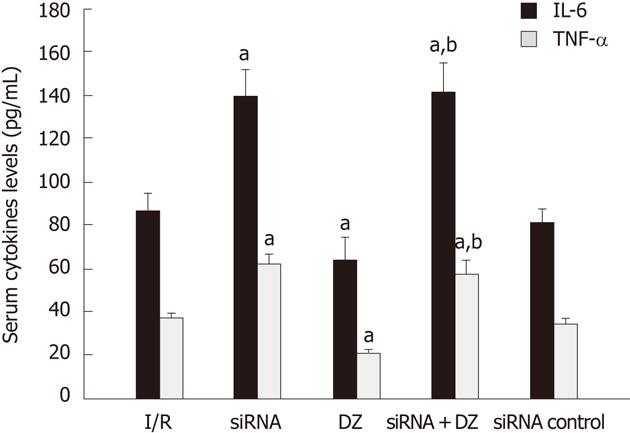
Cytokines assay
Serum cytokine levels (IL-6/TNF-α) were determined by ELISA. The IL-6/TNF-α levels revealed a substantial increase in the siRNA group as compared with the I/R group. Groups pretreated with DZ had a marked decrease in these products. Moreover, the cytokine levels (IL-6/TNF-α) in the siRNA + DZ group were significantly higher, when compared to the DZ group. There was no statistical difference between the I/R and siRNA control groups (P > 0.05) (Figure 5).
Figure 5 Serum cytokine levels (interleuki-6/ tumor necrosis factor-α) were measured.
aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α.
DISCUSSION
HO-1 is up-regulated in response to oxidative stress and catalyzes the degradation of pro-oxidant heme to carbon monoxide (CO), iron and bilirubin[31]. Three HO isoforms have been identified to date: HO-1, HO-2 and HO-3. HO-1 is inducible and distributed ubiquitously in mammalian tissue[32], while HO-2 is expressed constitutively and found mainly in the central nervous system[33]. HO-3 is only described in the rat brain and has no activity. HO-1 expression is an adaptive and protective response to oxidative stress in a wide variety of cells[34,35]. HO-1 is induced by a variety of stimuli, including nonheme inducers and various agents that cause oxidative stress, and prevents the oxidative DNA damage caused by heat shock and reactive oxygen species (ROS).
MitoKATP channel opener is responsible for more effective oxidative phosphorylation[36] and regulation of ROS generation[37,38] in IPC. Prevention of mitochondrial failure improves cellular survival after an ischemic event. Pharmacological agents that maintain mitoKATP channels can prevent mitochondrial failure in a manner similar to that induced by IPC[13,39]. MitoKATP is shown to have protective effect on the heart, kidney and brain following I/R injury[40,41]. However, the detailed effect of mitoKATP in liver I/R injury has not been fully studied. DZ is mainly used as a vasodilator in the treatment of clinical hypertension. However, in our study, we used DZ, the putative mitoKATP channel opener to evaluate the effects of mitoKATP channels in liver I/R injury in OLT.
In the present investigation, rats pretreated with DZ results in enhanced resistance to liver cold I/R injury and increased HO-1 expression levels. To examine whether HO-1 was responsible for the protective effect of DZ in liver I/R injury, we used HO-1 siRNA, which interferes with the enzymatic expression of HO-1 to examine its possible effect. Inhibition of the expression of HO-1 almost completely blocked the protection afforded by DZ. These results strongly indicated that the protection of DZ was mediated through upregulation of HO-1. We speculate that DZ induces an increase in HO-1 levels that protect liver I/R injury. The K+ channel-independent effects of DZ to inhibit succinate oxidation[42] and succinate dehydrogenase[43] or inhibit opening of the mitochondrial membrane pore[44] may be responsible for its protective effects. When we inhibited the expression of HO-1 and the above effects of DZ remained, the protection of DZ against hepatic I/R injury in rats almost disappeared. It is possible that DZ, through opening KATP channels, activated an unknown pathway, which directly or indirectly upregulated the expression of HO-1. HO-1 plays a critical role in protection against liver I/R injury in rat OLT, thus, inhibition of HO-1 activity may exaggerate I/R injury.
We measured serum cytokine levels (IL-6/TNF-α) by ELISA to determine the effect on anti-inflammatory activity. Rats pretreated with DZ significantly decreased serum IL-6/TNF-α levels. However, they were markedly elevated in the siRNA + DZ group than those in the DZ group. Xu et al[45] showed that DZ inhibits the production of the proinflammatory cytokines TNF-α and IL-6 by peripheral blood mononuclear cells in normal pregnancy. However, our study showed that DZ was able to reduce the release of cytokines. However, when the expression of HO-1 was inhibited, the release of cytokines was not reduced. HO-1 has been shown to be expressed principally in Kupffer cells[21,46,47]. Our study suggests that the potential utility of HO-1 overexpression in preventing I/R injury results from inhibition of Kupffer cells activation[48]. Activated Kupffer cells release a large amount of proinflammatory cytokines[49-51], which lead to aggravation of I/R injury. Devey et al[52,53] reported that depletion of Kupffer cells using liposomal clodronate led to loss of hepatic HO-1 expression and much more severe injury. It has been demonstrated that overexpression of HO-1 plays an important role in a number of I/R injury and liver transplant models. Therefore, we could think that the protective effect of DZ was by increasing HO-1 expression in our study.
In summary, DZ can attenuate hepatic I/R injury in rat liver transplantation and induce hepatic HO-1 expression. In addition, by inhibiting HO-1 expression with a specific siRNA, this protection pretreated with DZ was abolished. Thus, the mechanism of the protective effects of DZ is dependent on HO-1. The upregulation of HO-1 results in alleviation of hepatic I/R injury, as well as a decrease of the production of inflammatory cytokine such as IL-6 and TNF-α. Our results provide an insight into induction of HO-1, and suggest that DZ may have the potential to be developed as a hepatic HO-1 inducer for therapeutic purposes. Our work also raises the possibility that HO-1 can be induced by DZ to stimulate protective process that attenuates I/R injury.
COMMENTS
Background
Diazoxide (DZ) is a selective mitochondria ATP-sensitive potassium channel opener, which has been reported to have a protective effect on the heart, brain and spinal cord following ischemia/reperfusion (I/R) injury. However, the protective action of DZ on liver I/R injury is unknown and the exact underlying mechanisms are poorly understood.
Research frontiers
Heme oxygenase-1 (HO-1) is the rate-limiting step in the oxidative degradation of heme. Overexpression of HO-1 exerts a cytoprotective function in a number of I/R injury and liver transplant model. HO-1 expression is an adaptive and protective response to oxidative stress in a wide variety of cells.
Innovations and breakthroughs
The data represent a major advance in our understanding of ATP-sensitive potassium channel. DZ, can attenuate hepatic I/R injury in rat liver transplantation and induce hepatic HO-1 overexpression. In addition, the authors clearly demonstrated a direct correlation between expression of HO-1 and DZ. Thus, DZ may have the potential to be developed as a hepatic HO-1 inducer for therapeutic purposes.
Applications
The study results suggest that the mechanism of the protective effects of DZ is dependent on HO-1. Further studies are needed to evaluate the safety and efficiency of DZ used in clinical.
Peer review
This was a well designed study with excellent results. It was well written. It seemed almost too perfect. However, as long as they have a history of academic integrity.