Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1664
Revised: January 16, 2012
Accepted: February 8, 2012
Published online: April 14, 2012
AIM: To develop a prognostic approach for gastrointestinal stromal tumors (GISTs) using a cluster of indicators and follow-up information.
METHODS: One hundred and four GISTs that had not been subjected to targeted therapies were collected and classified by NIH risk assessment and anatomic location. By immunohistochemistry, the expressions of PTEN, Ki-67, CD44s matrix metalloproteinase (MMP)-9 and TIMP-1 were detected on tissue microarray. Univariate and multimarker survival analyses were performed and then a COX hazard proportion model was constructed to evaluate a cluster of predictors of GIST.
RESULTS: Our data showed small intestinal GIST are more aggressive than gastric GIST. The NIH risk assessment correlated with disease-free survival for either gastric GIST or small intestinal GIST. Immunohistochemical analysis revealed that Ki-67 labeling indexes (LIs) < 5% predicted higher disease-specific survival (DSS) in gastric and small intestinal GIST. CD44s positivity and PTEN LIs ≥ 50% correlated with higher DSS in gastric GIST. MMP-9 and TIMP-1 had no correlation with survival. Multimarker analysis revealed that the expression pattern of PTEN LIs ≥ 50% combined with Ki-67 LIs < 5% and CD44s positivity reliably predicted favorable outcomes for gastric GIST (P = 0.009), as did the combination of PTEN LIs ≥ 50% and Ki-67 LIs < 5% for small intestinal GIST (P = 0.011). Authors also found that high NIH risk grade was correlated with DSS in patients with gastric GIST and disease-free survival in patients with small intestinal GIST.
CONCLUSION: PTEN LIs ≥ 50%, Ki-67 LIs < 5% and CD44s positivity provides an accurate, favorable prognosis for gastric GIST. PTEN LIs ≥ 50% and Ki-67 LIs < 5% does the same for small intestinal GIST. Ki-67 LIs enhances the NIH assessment.
- Citation: Liang YM, Li XH, Li WM, Lu YY. Prognostic significance of PTEN, Ki-67 and CD44s expression patterns in gastrointestinal stromal tumors. World J Gastroenterol 2012; 18(14): 1664-1671
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1664
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the alimentary tract. The principle pathogenesis of GIST has been identified as the continuous activation of KIT, caused by gain-of-function mutation in c-kit[1] or platelet derived growth factor α (PDGFRA)[2]. Based on this finding, the molecular targeted therapy of imatinib[3] or sunitinib[4,5] has achieved great success.
Clinicians are trying to improve the survival rate of GIST patients by prophylactic interference of imatinib or sunitinib, based on the prognosis. Because of the expense of the targeted therapy, an accurate prognosis is very important, but prognoses of GIST are highly variable. Although the NIH risk assessment is currently used as a standard guideline for localized tumors[6], many researchers consider that evaluation of large cohorts is necessary for reliable data. Some modifications have been made to the NIH assessment, to enhance prediction accuracy[7,8]. Current researchers have also found some molecular indicators, such as p16INK4a[9], PTEN[10], p53[11,12], p27[13,14], CD44s[15,16] and some other cell-cycle regulators[17], but their utility is debated. Despite the mixed opinion, there are limitations in using one marker for a prognosis. A tumor is resulted from the accumulation of numerous molecular incidents, and one prognostic marker may only be applicable to a minority of patients. Our objective was to find a multi-marker indicator to improve prognoses for all patients. PI3K/Akt has been found to be a major signal transduction pathway in GIST[18]. Accordingly, we selected PTEN, the inhibitor of PI3K/Akt, for analysis. The markers related to adhesion and metastasis, such as CD44s, matrix metalloproteinase (MMP)-9 and TIMP-1, were also selected. Ki-67 was chosen to analyze the prognostic value of cell proliferation. All five markers were detected on tissue microarray of 104 GISTs. Follow-up survival data was collected. The prognostic values of these five markers were analyzed and compared to the NIH risk assessment.
A total of 155 gastric and small intestinal GISTs were collected from the archive of the Pathology Department of Chinese PLA General Hospital. All the tumors were reassessed by the immunohistochemical panel of CD117, CD34, SMA and S-100 protein. The cases with CD117 positivity were diagnosed as GIST. One hundred and four cases were completely followed-up. One hundred and twenty one samples were obtained from these 104 cases. These tumor samples included primary, recurrent and metastatic GIST. All the tumor paraffin blocks were used to construct the tissue microarray.
Formalin fixed, paraffin embedded, HE stained slides were reviewed by two experienced pathologists. The mitotic index was determined by counting 50 adjacent high-power fields in the most active areas. Using the NIH risk assessment[6], all the tumors were classified into four grades of risk: very low, low, intermediate and high.
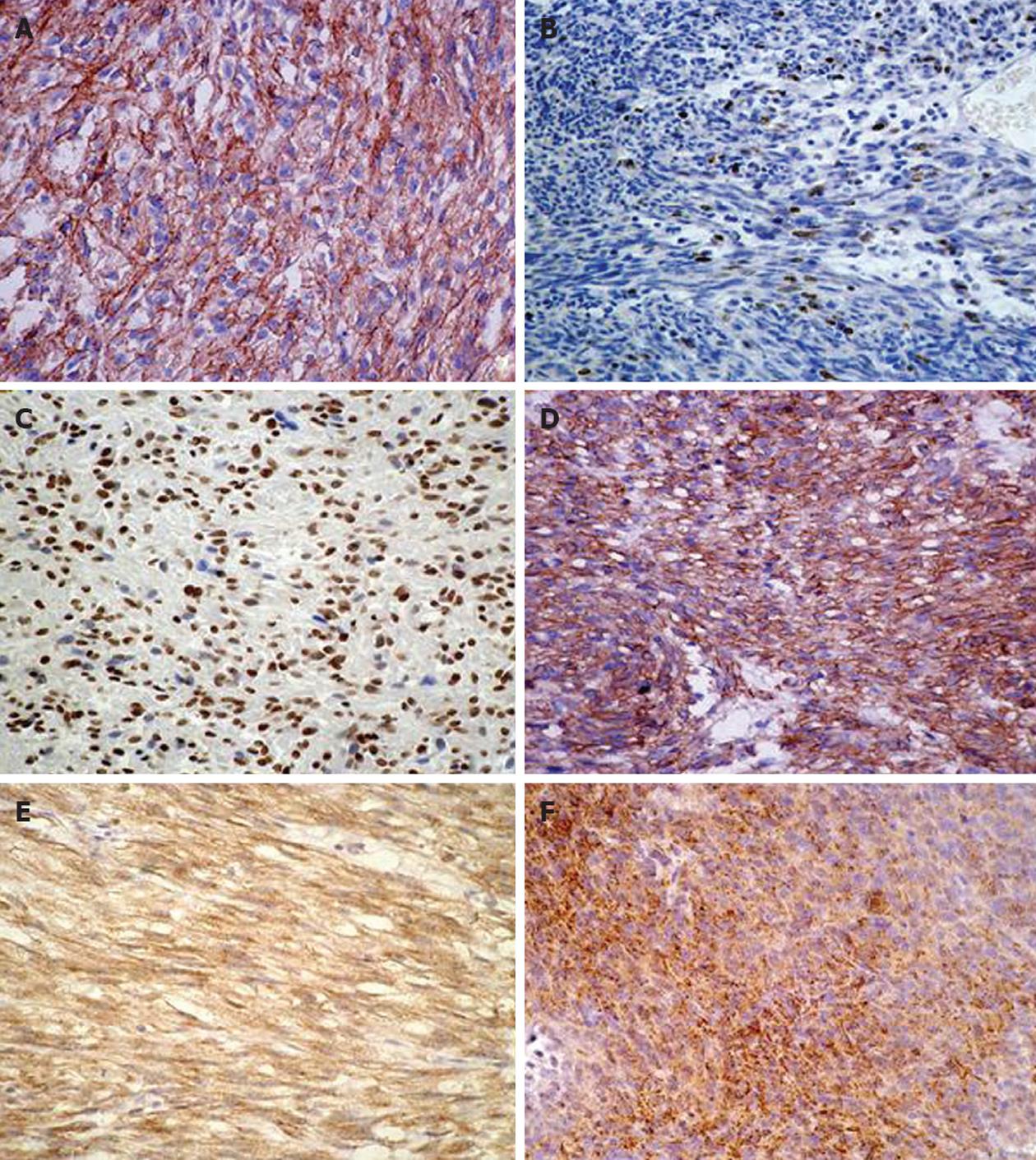
Three tissue cores, 1 mm in diameter, were sampled from each tumor specimen to construct the tissue microarray (TMA). The TMA blocks were sectioned at 4 μm and stained with hematoxylin-eosin sequentially. Antigen retrieval was carried out with EDTA (pH 8.0; Santa Cruz Biochemistry, Calif) for 15 min by microwave. The primary antibody was incubated for 1 h at room temperature and subsequently detected using a Two-step PicTure™ kit (PV6000, Invitrogen Co. Carlsbad California United States). The primary antibodies, sources, and dilutions were as follows: CD117, A4502, polyclonal, Dako A/S Co. Ltd., Denmark, 1:200; SMA, clone 1A4, Invitrogen Co. Carlsbad California, United States, 1:100; S-100, clone 4C4.9, Invitrogen Co. Carlsbad California, United States, 1:50; Ki-67, clone K-2, Invitrogen Co. Carlsbad California, United States, 1:50; PTEN, clone 28H6, Invitrogen Co. Carlsbad California, United States 1:100; CD44s, clone 156-3c11, Novocastra Laboratories Ltd. Newcastle, United Kingdom, 1:50; MMP-9, clone 15W2, Novocastra Laboratories Ltd. Newcastle, United Kingdom, 1:25; TIMP-1, clone 6F6a, Novocastra Laboratories Ltd. Newcastle, United Kingdom, 1:50.
The labeling indexes (LIs, %) of PTEN and Ki-67 were determined by counting 1000 cells in the most active area. For CD117, SMA, S-100, CD44s, MMP-9 and TIMP-1, staining of more than 10% of the tumor cells was considered positive.
All the patients were followed up after the resection of the tumor. The death of patients with GIST, untreated with imatinab, was selected as the end point. We analyzed follow-up data using Stata Statistical Software (Intercooled Stata 7.0, Stata Co., College Station, TX). The χ2 test was used to analyze the expression status of the selected markers. Survival analysis was performed using Kaplan-Meier plots and the log-rank test to reveal the prognostic usefulness of the selected markers. Univariate and multivariate COX proportional hazard models with both backward and forward elimination of variables were also constructed to find the most significant factor for prognosis. Statistical significance was set at P < 0.05.
In a total of 155 patients, having a male-to-female ratio of 2.5:1 (111 vs 44), there were 83 gastric and 72 small intestinal GISTs. A total of 104 cases had complete follow-up data. The median follow-up time was 33 mo, within the range of 3 to 230 mo. Fifty one cases without follow-up data were excluded from the subsequent survival analysis.
In 83 gastric patients, the male to female ratio was 2.1:1 (55 vs 26). The age ranged from 13 to 82 years (mean: 55.4 years; median: 57 years). The 62 followed-up cases included 42 males and 20 females. Thirteen patients died of GIST, four were alive with GIST and 45 were disease-free. The survival time of the 13 died patients ranged from 6 to 132 mo. The 3-year disease-specific survival rate (DSS) was 80.77% ± 11.5% and the 5-year DSS was 66.51% ± 17.06%.
In 72 small intestinal patients, the male to female ratio was 3.3:1 (55 vs 17). The age ranged from 20 to 77 years (mean: 50.6 years; median: 51.5 years). Forty-two followed-up cases included 31 males and 11 females. Fifteen patients died of GIST, four were alive with GIST and 23 were event-free. The survival time of the died patients ranged from 3 to 230 mo. The 3-year DSS was 73.65% ± 14.24% and the 5-year DSS was 61.76% ± 18.30%. There was no significant difference between the DSS of patients with gastric and small intestinal GIST (P = 0.274).
There were 23 patients that suffered recurrence, nine with gastric GIST and 14 with small intestinal GIST. Most of them were noted to have intra- abdominal spreading. A total of 16 patients developed metastasis, eight with gastric GIST and eight with small intestinal GIST. Small intestinal GIST presented a higher liability to recurrence and metastasis than gastric GIST (22/42 vs 17/60, P = 0.013). GIST metastasized to the liver in 15 cases, indicating that the liver was another common metastatic site. One of these 15 patients suffered multi-organic metastases to the bone, brain and lung at the same time. Beside liver metastasis and abdominal spread, one patient also suffered metastasis to subcutaneous tissue. Detailed information on the 155 GISTs is provided in Table 1.
| In general | Gastric GIST | Small intestinal GIST | |
| Total case | 153 | 81 | 72 |
| Followed-up cases | 104 | 62 | 42 |
| Age (yr) | |||
| < 50 | 38 | 14 | 24 |
| ≥ 50 | 64 | 46 | 18 |
| Gender | |||
| Male | 72 | 41 | 31 |
| Female | 30 | 19 | 11 |
| Tumor size (cm) | |||
| < 2 | 1 | 1 | 0 |
| 2.1-5 | 31 | 20 | 11 |
| 5.1-10 | 46 | 29 | 17 |
| > 10 | 24 | 10 | 14 |
| Mitotic index | |||
| ≤ 5/50HPF | 54 | 31 | 23 |
| 5-10/50HPF | 15 | 9 | 6 |
| > 10/50HPF | 33 | 20 | 13 |
| Risk grade | |||
| Very low risk | 2 | 2 | 0 |
| Low risk | 23 | 14 | 9 |
| Intermediate Risk | 28 | 17 | 11 |
| High risk | 49 | 27 | 22 |
| Recurrence | 22 | 8 | 14 |
| Metastasis | 15 | 7 | 8 |
| Survival rate (%) | |||
| 3-yr disease specific | 76.64 ± 9.38 | 80.77 ± 11.50 | 73.65 ± 14.24 |
| 5-yr disease specific | 69.05 ± 11.18 | 66.51 ± 17.06 | 61.76 ± 18.30 |
Five patients suffered a secondary malignant tumor. Two patients with gastric GIST had early and advanced gastric adenocarcinoma, respectively. Three small intestinal GIST patients were found to have early gastric carcinoma, sigmoid colonal adenocarcinoma and bile duct adenocarcinoma, respectively.
Sixty-one gastric and 42 small intestinal GIST patients were included in the analysis of survival data based on the expressions of selected immunohistochemical markers. These results are summarized in Table 2 and displayed in Figure 1. All 103 GISTs were CD117 positive. Among these 103 cases, 83 were positive for CD34, 32 for SMA and four for S-100. The expressions of CD44s and MMP-9 were statistically predominant in small intestinal GISTs. TIMP-1 and Ki-67 had no significant difference between the gastric and the small intestinal GIST. The expression of Ki-67 was negatively correlated to PTEN (P = 0.027) and CD44s (P = 0.02). In patients that died of gastric GIST, the expressions of MMP-9 and Ki-67 were statistically higher than in those who survived. In contrast, CD44s and PTEN were significantly lower. The difference in TIMP-1 was not significant.
| Protein | Gastric GISTs | Small intestinal GISTs | ||||
| Positive (%) | P value | Positive (%) | P value | |||
| DOD (n = 13) | Alive (n = 48) | DOD (n = 15) | Alive (n = 27) | |||
| CD44s | 5 (38.4) | 35 (72.9) | 0.020 | 15 (100) | 23 (85.2) | 0.397 |
| MMP-9 | 11 (84.6) | 23 (47.9) | 0.018 | 11 (73.3) | 22 (81.5) | 0.339 |
| TIMP-1 | 7 (53.8) | 22 (45.8) | 0.608 | 3 (20.0) | 14 (51.9) | 0.085 |
| PTEN | ||||||
| < 50% | 5 (38.5) | 8 (8.3) | 0.014 | 11 (73.3) | 6 (22.2) | 0.003 |
| ≥ 50% | 8 (61.5) | 44 (91.7) | 4 (26.7) | 21 (77.8) | ||
| Ki-67 | ||||||
| < 5% | 7 (53.8) | 41 (85.4) | 0.022 | 5 (33.3) | 24 (88.9) | 0.0001 |
| ≥ 5% | 6 (46.2) | 7 (14.6) | 10 (66.7) | 3 (11.1) | ||
In patients that died of small intestinal GIST, Ki-67 LIs were much higher than in those who survived, while PTEN was significantly lower. The other markers, including CD44s, MMP-9, and TIMP-1, showed no significant difference.
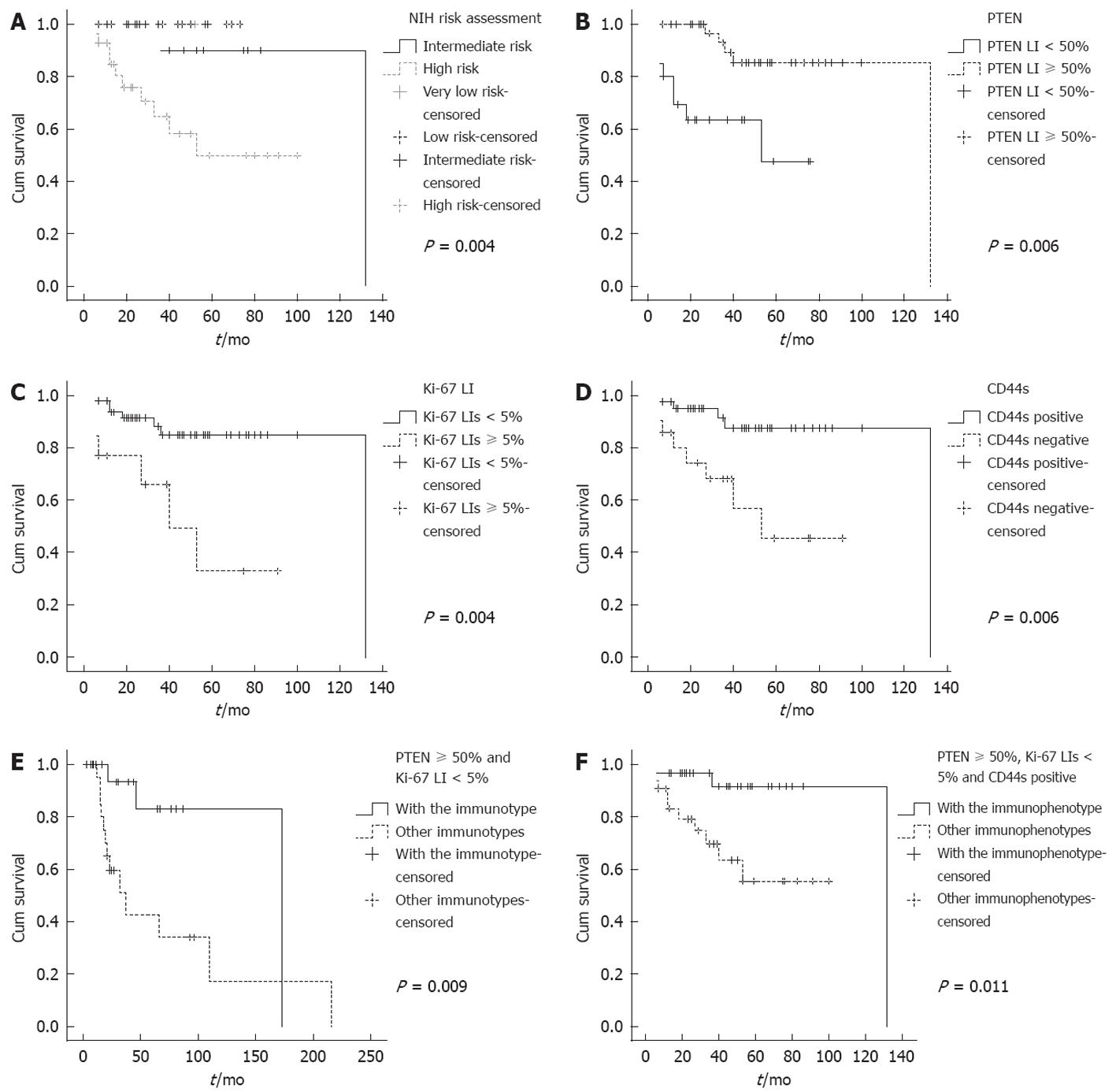
For gastric GISTs, PTEN LIs < 50% was significantly correlated with lower specific survival rate (P = 0.006, Figure 2B), as was Ki-67 LIs ≥ 5% (P = 0.004, Figure 2C) and CD44s negativity (P = 0.006, Figure 2D). In a total of 13 patients who died of GIST, there were five with PTEN LIs < 50%, six with Ki-67 LIs ≥ 5% and eight patients who lost the expression of CD44s. For small intestinal GISTs, Ki-67 LIs ≥ 5% significantly correlated with worse outcomes. Multivariate analysis did not reveal an independent factor for gastric or small intestinal GISTs. In a total of 15 patients who died of GIST, there were 10 with Ki-67 LIs ≥ 5%.
No statistically significant difference was observed in disease specific survival for the patients grouped by the expressions of MMP-9 or TIMP-1. The balance between MMP-9 and TIMP-1 also did not correlate with disease specific survival. We failed to find an independent prognosis indicator after constructing the univariate and multivariate COX hazard proportional model (Table 3).
| Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Gastric GIST | ||||
| PTEN | 0.23 (0.07-0.74) | 0.013 | 0.35 (0.10-1.20) | 0.100 |
| Ki-67 | 4.54 (1.45-14.16) | 0.009 | 2.69 (0.73-9.87) | 0.130 |
| CD44s | 0.21 (0.07-0.73) | 0.013 | 0.43 (0.83-9.98) | 0.320 |
| PTEN, Ki-67, CD44s | 5.92 (1.29-27.08) | 0.022 | 1.35 (0.15-11.80) | 0.780 |
| MMP-9 | 4.22 (1.92-19.31) | 0.042 | ||
| TIMP-1 | 0.90 (0.29-2.82) | 0.862 | ||
| MMP-9/TIMP-1 | 0.72 (0.22-2.38) | 0.586 | ||
| Small intestinal GIST | ||||
| Ki-67 | 3.29 (1.14-9.46) | 0.019 | ||
| PTEN, Ki-67 | 4.02 (1.25-16.09) | 0.021 | ||
Combined analysis revealed that for gastric GISTs, the survival rate of the patients with the expression pattern of PTEN LIs ≥ 50%, Ki-67 LIs < 5% and CD44s positivity was significantly higher than those with other immunophenotypes (P = 0.009, Figure 2E). In the 13 patients who died of gastric GIST, 11 did not show the above immunophenotype. Of the two patients with the phenotype, one survived over 10 years after the resection of GIST and the other survived 53 mo.
For patients with small intestinal GIST, the survival rate was significantly higher in those with the combined expressions of PTEN LIs ≥ 50% and Ki-67 LIs < 5% relative to those with other immunophenotypes (P = 0.011, Figure 2F). In the 15 patients who died of small intestinal GIST, only two showed this favorable immunophenotype.
The NIH risk assessment showed excellent correlation with disease-specific survival. The patients were classified as higher risk with worse prognosis (P = 0.001). GIST arising from gastric and small intestine showed different recurrent potential; we subsequently analyzed the prognostic value of risk assessment in these two anatomic sites. For gastric GIST, high risk stratification was correlated with significantly worse prognoses (P = 0.004, Figure 2A). But for small intestinal GIST, the risk stratification had no significant correlation with DSS (P = 0.205). Considering the disease-free survival rate, the risk stratification was significantly correlated with survival not only in small intestinal GISTs (P = 0.011), but also in gastric GISTs (P = 0.044).
In our series, 29 gastric patients who were graded as high risk had shorter survival time than the other three grades (61.31 ± 17.28 mo vs 121.64 ± 17.06 mo). Fourteen gastric patients with Ki-67 LIs ≥ 5% had shorter survival time than those with Ki-67 LIs < 5% (49.75 ± 10.84 mo vs 111.46 ± 15.26 mo). Ten of these 14 cases were classified as high risk grade, four were not in this group but suffered relapse or metastasis.
For small intestinal GISTs, two cases with Ki-67 LIs ≥ 5% suffered relapse and one died of the GIST in 32 mo. Neither of these two cases were classified as high risk grade by NIH risk assessment.
In this study based on follow-up data, we developed a cluster of immunohistochemical markers that can facilitate the assessment of GIST prognosis in Chinese patients. The expression patterns of PTEN, Ki-67 and CD44s can help clinicians evaluate the clinical outcome of the patients with gastric GIST, as can the combination of PTEN and Ki-67 for those with small intestinal GIST.
Although PTEN, Ki-67 and CD44s can individually assist the prognosis, there are still apparent limitations because each marker just focuses on some of the patients. When tested by follow-up data, although eight patients expressed PTEN LIs ≥ 50%, seven of them died of GIST within 5 years after the surgery resection. Such a conflict phenomenon is also very common in patients with gastric GIST when the prediction is based only on Ki-67 or CD44s. This also occurs for Ki-67 or PTEN in patients with small intestinal GIST. Combining the expressions of PTEN, Ki-67 and CD44s can improve the specificity and accuracy of the prognosis.
Our results showed that Ki-67 LIs is negatively correlated with PTEN and CD44s. PTEN and Ki-67 are both involved in cell proliferation. PTEN can upregulate p27kip1 resulting in apoptosis and cell cycle arrest, suppression of cell proliferation by a mechanism independent of Akt activity in nuclei[19]. Also, PTEN upregulates p53 activity by maintaining the high acetylation of p53[20]. In our study, PTEN was exclusively located in nuclei, and PTEN LIs < 50% was statistically correlated with a worse outcome of gastric GIST. A similar result was also reported by Ricci, regarding the correlation of decreased PTEN expression with worse outcomes[10].
Ki-67 is widely used to predict the proliferation potential of malignant tumors. Many reports have confirmed the prognostic value of Ki-67 in GIST[21-23]. The differences in these reports are the cut-off value of the Ki-67 index, which varied from 4.5% to 10%. Whether Ki-67 is one of best predictors is debatable. Nakamura proposed that Ki-67 LIs and risk assessment were useful for predicting GIST outcome[17], while Wong considered that mitotic count[24], not Ki-67 LIs, remained the best predictor of gastric GIST. Our results revealed that higher Ki-67 LIs were associated with worse outcomes. Interestingly, there were four cases of low risk gastric GISTs with high Ki-67 LIs that suffered worse outcomes. Another two cases of low risk intestinal GISTs that had high Ki-67 LIs also resulted in worse outcomes. This indicates that Ki-67 may enhance the NIH consensus criteria, especially when applied to patients of low risk. Though the absolute cut-off of Ki-67 LIs is difficult to define, Ki-67 may become one of the most robust indicators of GIST.
CD44 is now widely accepted as a stem cell marker in many kinds of carcinomas, including gastric cancer, colorectal cancer[25], and breast cancer. While only a minority of the cancer cells have the capability of carcinogenesis, this portion of the cell population is stem cells; in Du’s series, no more than 5% of the cells were positive for CD44. In GISTs, once CD44 is positive, it is diffusely expressed by nearly all the cells. Based on this finding, CD44 may not be a suitable stem cell marker for GIST. CD117 and CD34 are stem cell markers for the haematopoietic stem cells. In GIST, CD117 is the crucial diagnostic marker; CD34 is one of the most important differential diagnostic markers. The expression of these stem cell markers in GIST may support the hypothesis that GIST originates from mesenchymal stem cells. The suitable robust stem cell markers for GIST needs further investigation. CD44 and its associated partner proteins monitor changes in the extra-cellular matrix that influences cell growth, survival and differentiation. It can be a molecular switch between growth and arrest depending on the extra-cellular conditions. As reported for CD44-deficient fibroblasts, the recruited CD44s suppress metastasis and proliferation[26]. Furthermore, CD44 can promote tumor invasion by recruiting MMP-9[27]. In small intestinal GIST, the expressions of CD44s and MMP-9 are significantly higher than in gastric GISTs. The co-expression of CD44s and MMP-9 may be responsible for the high metastatic liability of small intestinal GIST.
The anatomic site of GIST is now believed to be a prognostic factor. Some experts believe that when GIST is considered as a location-specific entity, multiple clinicopathologic parameters can be used together to define the biological behavior. In the revised version of NIH risk stratification, anatomic location was considered to be an important factor in the assessment of moderate and high risk[6]. In our series, the small intestinal GIST was more aggressive than gastric GIST. Of the patients with small intestinal GIST, 52.38% suffered recurrence or metastasis, while only 25% of gastric GIST patients did. The molecular makers expressed in gastric and small intestinal GIST were also different, as were the prognostic indicators. CD44 had no impact on the prognoses for patients with small intestinal GIST, and a similar result has been reported for rectal GIST[28]. The dissimilar anatomic locations may be responsible for these difference. Based on these facts, we believe that GIST should be sub-grouped by anatomic location first and then assessed by other factors.
In conclusion, the expression patterns of PTEN, Ki-67 and CD44s are useful for the prognosis of patients with gastric GIST, and PTEN and Ki-67 are valuable outcome indicators for patients with small intestinal GISTs, Ki-67 LIs can enhance the NIH consensus criteria.
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the digestive tract. Because the targeted therapy of imatinib and sunitinib has achieved great success, accurate prediction of GIST outcomes is becoming more and more important. The biological behavior of GIST is highly variable. NIH risk assessment is now widely used as a prognostic indicator, but further investigation of the prognostic factors is still needed.
NIH risk assessment is widely used for GIST risk stratification; the revised version is based on tumor location, diameter and mitotic index. Many investigators have analyzed the prognostic value of other factors including stage, grade, KIT mutation and immunohistochemical markers.
Based on follow-up information on patients without targeted therapy, we comprehensively analyzed anatomic location, NIH risk assessment and a number of immunohistochemical markers including PTEN, Ki-67, CD44s, matrix metalloproteinase 9 and TIMP-1.
This study provides a new cluster of prognostic markers for GIST. PTEN LIs ≥ 50%, Ki-67 LIs < 5% and CD44s positivity correlates with favorable outcomes for gastric GISTs, as does PTEN LIs ≥ 50% and Ki-67 LIs < 5% for small intestinal GISTs. Anatomic location is a prognostic indicator of GISTs; depending on location, GISTs may have different biological features. The intrinsic nature needs further investigation.
GIST is the most common mesenchymal tumor of digestive tract. Gain-of-function mutation of in c-kit or platelet derived growth factor α are hypothesized as the principle molecular pathogenesis. Based on this point, the targeted therapy of imatinib or sunitinib has achieved great success. But the biological behavior of GIST is highly variable; many experts are investigating improvements in prognostic markers.
This is an interesting, well done and well written paper adding useful insights in the field.
Peer reviewer: Vittorio Ricci, Professor, Physiology, Human Physiology Section, University of Pavia Medical School, Human Physiology Sect, Via Forlanini 6, 27100 Pavia, Italy
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 2. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 3. | Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 751] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1918] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 5. | Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [PubMed] |
| 7. | Yokoi K, Tanaka N, Shoji K, Ishikawa N, Seya T, Horiba K, Kanazawa Y, Yamashita K, Ohaki Y, Tajiri T. A study of histopathological assessment criteria for assessing malignancy of gastrointestinal stromal tumor, from a clinical standpoint. J Gastroenterol. 2005;40:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Huang HY, Li CF, Huang WW, Hu TH, Lin CN, Uen YH, Hsiung CY, Lu D. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Schneider-Stock R, Boltze C, Lasota J, Peters B, Corless CL, Ruemmele P, Terracciano L, Pross M, Insabato L, Di Vizio D. Loss of p16 protein defines high-risk patients with gastrointestinal stromal tumors: a tissue microarray study. Clin Cancer Res. 2005;11:638-645. [PubMed] |
| 10. | Ricci R, Maggiano N, Castri F, Rinelli A, Murazio M, Pacelli F, Potenza AE, Vecchio FM, Larocca LM. Role of PTEN in gastrointestinal stromal tumor progression. Arch Pathol Lab Med. 2004;128:421-425. [PubMed] |
| 11. | Chou YP, Lin JW, Wang CC, Chiu YC, Huang CC, Chuah SK, Tai MH, Yi LN, Lee CM, Changchien CS. The abnormalities in the p53/p21WAF1 pathway have a significant role in the pathogenesis and progression of gastrointestinal stromal tumors. Oncol Rep. 2008;19:49-56. [PubMed] |
| 12. | Ryu MH, Kang YK, Jang SJ, Kim TW, Lee H, Kim JS, Park YH, Lee SS, Ryoo BY, Chang HM. Prognostic significance of p53 gene mutations and protein overexpression in localized gastrointestinal stromal tumours. Histopathology. 2007;51:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Shirin H, Kravtsov V, Shahmurov M, Shabat VS, Krinshpon I, Alin A, Avinoach I, Avni Y. The cyclin-dependent kinase inhibitor, p27, has no correlation with the malignant potential of GIST. Digestion. 2007;75:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gelen T, Elpek GO, Aksoy NH, Ogüş M, Keleş N. p27 Labeling index and proliferation in gastrointestinal stromal tumors: correlations with clinicopathologic factors and recurrence. Jpn J Clin Oncol. 2003;33:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Montgomery E, Abraham SC, Fisher C, Deasel MR, Amr SS, Sheikh SS, House M, Lilliemoe K, Choti M, Brock M. CD44 loss in gastric stromal tumors as a prognostic marker. Am J Surg Pathol. 2004;28:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hsu KH, Tsai HW, Shan YS, Lin PW. Significance of CD44 expression in gastrointestinal stromal tumors in relation to disease progression and survival. World J Surg. 2007;31:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560-7568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211-6224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Li AG, Piluso LG, Cai X, Wei G, Sellers WR, Liu X. Mechanistic insights into maintenance of high p53 acetylation by PTEN. Mol Cell. 2006;23:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Gumurdulu D, Erdogan S, Kayaselcuk F, Seydaoglu G, Parsak CK, Demircan O, Tuncer I. Expression of COX-2, PCNA, Ki-67 and p53 in gastrointestinal stromal tumors and its relationship with histopathological parameters. World J Gastroenterol. 2007;13:426-431. [PubMed] |
| 22. | Huang HY, Huang WW, Lin CN, Eng HL, Li SH, Li CF, Lu D, Yu SC, Hsiung CY. Immunohistochemical expression of p16INK4A, Ki-67, and Mcm2 proteins in gastrointestinal stromal tumors: prognostic implications and correlations with risk stratification of NIH consensus criteria. Ann Surg Oncol. 2006;13:1633-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Filiz G, Yalçinkaya O, Gürel S, Yerci O, Memik F. The relationship between MIB-1 proliferative activity and mitotic index in gastrointestinal stromal tumors. Hepatogastroenterology. 2007;54:438-441. [PubMed] |
| 24. | Wong NA, Young R, Malcomson RD, Nayar AG, Jamieson LA, Save VE, Carey FA, Brewster DH, Han C, Al-Nafussi A. Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology. 2003;43:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751-6760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 26. | Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 1808] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 27. | Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 529] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Changchien CR, Wu MC, Tasi WS, Tang R, Chiang JM, Chen JS, Huang SF, Wang JY, Yeh CY. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Dis Colon Rectum. 2004;47:1922-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |