Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1622
Revised: February 20, 2012
Accepted: February 26, 2012
Published online: April 14, 2012
AIM: To investigate risk factors for low bone mineral density (BMD) in celiac disease (CD) patients, focusing on circulating autoantibodies against osteoprotegerin (OPG).
METHODS: Seventy asymptomatic CD adult patients on gluten-free diet (GFD) and harbouring persistent negative CD-related serology were recruited. Conventional risk factors for osteoporosis (e.g., age, sex, menopausal status, history of fractures, smoke, and body mass index) were checked and BMD was assessed by dual energy X ray absorptiometry. Serum calcium and parathyroid hormone (PTH) levels were evaluated. Thirty-eight patients underwent repeat duodenal biopsy. Serum samples from a selected sub-group of 30 patients, who were also typed for human leukocyte antigen (HLA) DQ2 and DQ8 haplotype, were incubated with homodimeric recombinant human OPG and tested by western blotting with an anti-OPG antibody after immunoprecipitation.
RESULTS: Despite persistent negative CD-related serology and strict adherence to GFD, 49 out of the 70 (74%) patients displayed low BMD. Among these patients, 13 (24%) showed osteoporosis and 36 (76%) osteopenia. With the exception of age, conventional risk factors for osteoporosis did not differ between patients with normal and low BMD. Circulating serum calcium and PTH levels were normal in all patients. Duodenal mucosa healing was found in 31 (82%) out of 38 patients who underwent repeat duodenal biopsy with 20 (64%) still displaying low BMD. The remaining 7 patients had an incomplete normalization of duodenal mucosa with 6 (84%) showing low BMD. No evidence of circulating antibodies against OPG was found in the serum of 30 celiac patients who were tested for, independent of BMD, duodenal histology, and HLA status.
CONCLUSION: If any, the role of circulating autoantibodies against OPG in the pathogenesis of bone derangement in patients with CD is not a major one.
- Citation: Larussa T, Suraci E, Nazionale I, Leone I, Montalcini T, Abenavoli L, Imeneo M, Pujia A, Luzza F. No evidence of circulating autoantibodies against osteoprotegerin in patients with celiac disease. World J Gastroenterol 2012; 18(14): 1622-1627
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1622
Celiac disease (CD) is a permanent gluten intolerance in genetically predisposed individuals who display an inflammatory process in the small intestinal mucosa with villous atrophy, crypt hyperplasia, and increased number of lymphocytes[1].
Evidences indicate that a low bone mineral density (BMD) is found in 20%-50% newly diagnosed patients with CD[2]. By means of dual energy X ray absorptiometry (DEXA) it can be now rapidly and easily obtained semi-quantitative values of BMD[3].
Osteoporosis is a quantitative and qualitative alteration in the components of bone tissue, in which the process of demineralization becomes intense and prolonged and minerals are used up more quickly than they can be replaced resulting in bones fragility and increased risk of fractures[4,5]. Individual’s gender, constitution and age as well as variations in endocrine systems associated with factors such as the menopause, and the presence of other pathologies, can all interact with lifestyle factors, including smoking, lack of exercise and low dietary calcium intake, to determine the onset of osteoporosis[6].
Impaired absorption of calcium during CD is thought to result principally from loss of villous in the proximal intestine, where calcium is most actively absorbed, and also from the unabsorbed fatty acids, which bind calcium in the intestinal lumen and may reduce dietary vitamin D absorption[7]. Adherence to a strict gluten-free diet (GFD) will reverse the histological changes in the intestine and also the biochemical evidence of calcium malabsorption[8], resulting in normal BMD in these treated patients[9]. Nevertheless, there may be long-term impairment of bone mineralization in some otherwise healthy CD patients adhering to GFD[10]. Furthermore, osteopenia has been found in treated CD patients who showed improvement or even complete healing of intestinal mucosa[11]. These findings suggest that other mechanisms of bone injury than calcium malabsorption are probably involved in patients with CD[12].
Mediators of inflammatory immune-mediated responses (e.g., cytokines), parathyroid hormone (PTH), estrogens, androgens, corticosteroids and vitamin D are all acknowledged to affect BMD by modulating the receptor activator of nuclear factor B/receptor activator of nuclear factor B-ligand/osteoprotegerin [RANK/RANK-L/osteoprotegerin (OPG)] system[13]. Furthermore, neutralizing auto-antibodies against OPG have been recently shown in the sera of a few patients with CD, leading to the hypothesis that blocking the inhibitory effect of OPG on RANKL may have a role in the development of bone derangement in these patients[14]. Nevertheless, this finding has not yet been confirmed by others, so the role of auto-antibodies against OPG, if any, in the pathogenesis of reduced BMD in patients with CD remains to be established.
This study aimed at investigating risk factors associated with low BMD in patients with CD, focusing on circulating auto-antibodies against OPG.
Seventy consecutive outpatients with CD (13M, 57F; median age 40.5 years, range 20-68 years) who reported no current symptoms, claimed to be adherent to a GFD for at least 2 years and harboured persistent (at least 18 mo) negative CD-related serology (anti-endomysium and anti-transglutaminase IgA antibodies) were recruited. Diagnosis of CD was performed on the basis of clinical presentation, positive CD-related serology and suggestive histological findings on duodenal biopsy[15]. Dietary compliance was assessed by periodic interview during follow-up visits and classified as good according to Leffler et al[16]. Data on height, weight, time since diagnosis, symptoms beginning, age at menarche, cycle regularity, menopausal status, drug use, calcium intake, life style, smoking, and history of fracture were collected. Blood samples were collected in the morning after a 12 h fast in order to measure serum calcium and parathormone levels. Thirty-eight patients (8M, 30F; median age 41 years, range 20-60 years) underwent repeat duodenal biopsy after a period of at least 12 mo since GFD beginning.
A subgroup of 30 patients (8M, 22F; median age 44 years, range 21-60 years), who were typed for HLA-DQ2 and DQ8 haplotype, were selected in order to measure antibodies against OPG.
At least four duodenal biopsies were collected during upper gastrointestinal endoscopy. Intraepithelial lymphocytes have been identified using CD3 immunostaining and a value ≤ 25 lymphocytes⁄100 epithelial cells was considered normal. Histological changes were classified according to Marsh criteria (stage 0: Normal mucosa; stage 1: Increased number of intra-epithelial lymphocytes; stage 2: Crypts proliferation; stage 3a-3b-3c: Respectively mild, moderate and severe villous atrophy)[17].
All patients underwent lumbar spine and femoral neck BMD evaluation by means of DEXA. A T-score 1 to 2.5 and > 2.5 distinguished osteopenia and osteoporosis, respectively.
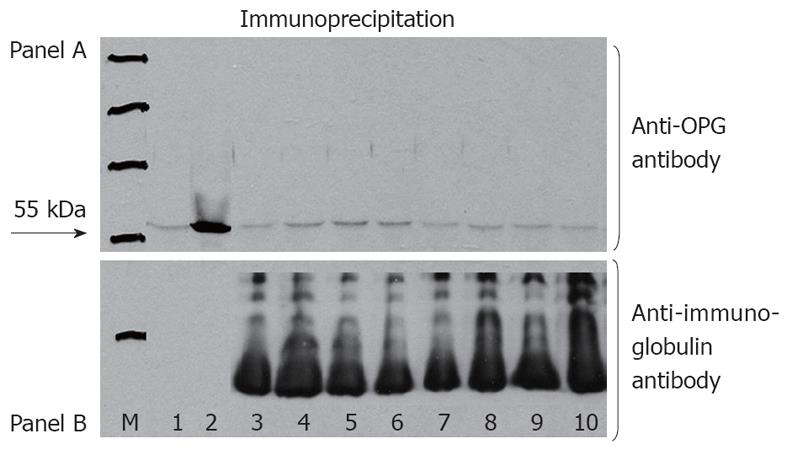
Non-fasting serum samples were obtained from the selected subgroup of 30 CD patients. Measurement was performed according to Riches et al[14]. Briefly, serum samples were incubated at a 1:100, 1:50, and 1:25 dilution with 12.5 ng of homodimeric recombinant human OPG (R and D Systems, Minneapolis, United States) and also with protein G-coated agarose beads (Calbiochem, Darmstadt, Germany) that had been pre-incubated with 5% albumin to reduce non-specific binding. After incubation for 1 h at 37 °C, the beads were washed five times with phosphate-buffered saline, suspended in 30 μL of reducing sample buffer, and incubated at 90 °C for 5 min. After brief centrifugation, the supernatant was loaded onto a 12% polyacrylamide gel, subjected to electrophoresis at 200 V for 60 min, transferred to membrane, and therefore probed with a mouse monoclonal antibody against human OPG (Abcam, Cambridge, United Kingdom). A peroxidase-conjugated donkey anti-mouse antibody (Jackson, Suffolk, United Kingdom) at a 1:5000 dilution was used for detection. Equal loading was assessed by probing the blot with peroxidase-conjugated goat anti-human antibody (Jackson) at a 1:5000 dilution. Immunolabeled bands were detected with the use of a chemiluminescent substrate and a chemiluminescence imager. A homodimeric recombinant human OPG (R and D Systems) was used as positive control. A 55-kDa band indicated the presence of antibodies against OPG.
The study was approved by the local research Ethical Committee, and informed consent was obtained from all participants.
Comparison of proportions was performed using χ2 test. A multivariate analysis was performed using Multivariate Analysis of Variance (MANOVA) to identify variables associated with low BMD. Difference was considered significant if the P value was < 0.05. Data were analyzed using the Statistical Package for Social Services, Version 16.0 (SPSS Inc., IL, United States).
Forty-nine out of the 70 (74%) CD patients displayed low BMD, with 13 (24%) accounting for osteoporosis and 36 (76%) for osteopenia (Table 1). Multiple logistic regression analysis showed that age was the only one variable which positively correlated with low BMD (Table 1). Serum calcium and PTH levels were normal in all patients. A complete healing of duodenal mucosa was found in 31 out of 38 (82%) patients who underwent repeat intestinal biopsies. In this specific subgroup, 20 (64%) patients showed a low BMD compared to 6 out of 7 (86%) patients who were found to carry an incomplete duodenal mucosa healing (n = 1 Marsh 1, n = 2 Marsh 2, n = 4 Marsh 3) (Table 2, P = 0.4).
| Variables | Overall (n = 70) | Normal BMD(n = 21) | Low BMD(n = 49) | P |
| Sex (M/F) | 13/57 | 2/19 | 11/38 | 0.31 |
| Age (yr) | 40.5 ± 10.5 | 31.0 ± 9.7 | 43.0 ± 9.7 | 0.00 |
| Time since diagnosis | 2.8 ± 0.6 | 2.5 ± 0.5 | 2.4 ± 0.4 | 0.37 |
| BMI | 22.2 ± 1.4 | 22.5 ± 1.3 | 22.0 ± 1.4 | 0.40 |
| Smoke | 13 | 3 | 10 | 0.74 |
| Fracture | 0 | 0 | 0 | - |
| Menopausal status | 6 | 0 | 6 | 0.17 |
| Duodenal mucosa healing | Duodenalmucosa lesions | Total | |
| Low BMD | 20 | 6 | 26 |
| Normal BMD | 11 | 1 | 12 |
| Total | 31 | 7 | 38 |
No evidence of the 55-kDa band was found in serum samples of the subgroup of 30 patients who were tested for, indicating no presence of auto-antibodies against OPG (Figure 1). The characteristics of this subgroup of CD patients, including HLA DQ2 and DQ8 status, are shown in Table 3.
| Variables | Overall (n = 30) | Normal BMD (n = 6) | Low BMD (n = 24) |
| Sex (M/F) | 8/22 | 1/5 | 7/17 |
| Age (yr) | 43.5 (21-60) | 31.0 (21-54) | 44.5 (32-60) |
| Time since diagnosis (yr) | 2.8 (1.0-3.5) | 2.7 (1.8-3.2) | 2.9 (1.0-3.5) |
| BMI (kg/m2) | 22.3 (19.2-25.1) | 21.8 (19.2-24.7) | 22.4 (20.0-25.1) |
| Smoke | 4 | 0 | 4 |
| Fracture | 0 | 0 | 0 |
| Menopausal status | 1 | 0 | 1 |
| Duodenal mucosa histology (n = 22) | |||
| Healing | 17 | 5 | 12 |
| Lesions | 5 | 1 | 4 |
| HLA status | |||
| DQ2 | 23 | 4 | 19 |
| DQ8 | 7 | 2 | 5 |
Currently, serology is employed to select individuals needing to underwent intestinal biopsy for diagnosing CD as well as to monitor adherence and response to GFD[18,19]. However, confirming a previous observation[20], this study shows that, despite a persistent negative serology, 18% of CD patients with good adherence to GFD have an incomplete normalization of intestinal mucosa (e.g., 82% negative predictive value in detecting intestinal mucosal recovery).
A GFD normally gains mucosal damage in CD patients restoring calcium absorption, and this can support an improvement in bone mineralization in one year[21]. Nevertheless, a GFD rarely normalizes BMD in adult patients, so nutritional supplementation may be necessary[22,23]. Findings of this study show a higher prevalence (74%) of bone demineralization in adulthood diagnosed CD patients notwithstanding long-term strict adherence to GFD and persistent negative CD-related serology.
No differences in acknowledged risk factors for osteoporosis have been found between patients with low and normal BMD except for age, suggesting that CD is a major one. Even though serum calcium levels may not adequately reflect calcium absorption, no patient showed low levels of serum calcium, and this was in accordance with the finding that calcium absorption returns to normal setting after one year GFD[24]. Furthermore, a significant proportion of patients (64%) on GFD showed low BMD, even if they displayed complete recovery of duodenal lesions as assessed by Marsh classification[25]. Nevertheless, recent observations suggested that normal Marsh grade does not exclude villous atrophy when assessed morphometrically[26].
Despite the high frequency of low BMD, there is still not a consensus about the timing for BMD evaluation in CD patients[27]. A novel finding of this study is that DEXA performed 92% negative predictive value in detecting intestinal mucosa recovery. So, based on this finding, the use of DEXA could be proposed for its additive value in this specific issue (e.g., a non-pathological DEXA has 92% probability to predict intestinal mucosa recovery in CD patients on GFD).
Insight of the molecular mechanism regulating osteoclast formation and activation progressed a lot in the past 10 years, with the identification of the RANKL/RANK signaling system as well as the discovering of OPG, a protein that appeared to protect from excessive bone reabsorption[28,29]. Fiore et al[30] demonstrated that OPG/RANKL ratio was significantly lower in CD patients with normalization of duodenal histology than in healthy controls and it positively correlated with low BMD. It has been hypothesized that in some patients OPG is bound to a plasma protein(s) and this could inactivate it[31].
In this study, circulating antibodies against OPG were not found in 30 CD patients. This contrasts with findings of Riches et al[14] who showed auto-antibodies against OPG in a man with CD on GFD presenting with severe osteoporosis and high bone turnover. As authors demonstrated, these auto-antibodies had the potential to block the inhibitory effect of OPG on RANKL and this lead to the hypothesis that they may play a role in the development of bone derangement. In the same report, authors detected these circulating auto-antibodies in three among 15 additional patients with CD and low BMD, while there was no evidence of them in serum specimens from 10 healthy controls and 14 patients with autoimmune hypothyroidism. If these CD patients were or were not on GFD was not indicated by the authors and data on duodenal mucosa histology were not provided.
It is unlikely that discrepancy between Riches et al[14] and findings of this study relies on the selection of patients neither on the used methodology. Indeed, it seems that the subgroup of 30 CD patients who were tested for antibodies against OPG is representative enough with respect to the variables that may affect the possible appearance of auto-antibodies (e.g., BMD, duodenal histology, HLA). Furthermore, Riches et al[14] anti-OPG antibodies measurement methodology has been strictly followed. A positive control has been checked in order to validate the procedure and several serum sample dilutions have been tested in order to increase sensitivity. Nevertheless, that a long-term GFD, as is the case of this study, may reduce the production of circulating auto-antibodies against OPG towards undetectable levels may be a possibility. At this regard, the occurrence of a limited amount of mucosal antibodies could be taken into account. Furthermore, genetic background affecting the immune system (e.g., auto-antibody development) may be another issue. Indeed, HLA DQ2 heterodimer has been shown to be more involved than HLA DQ8 heterodimer in complicated CD[32]. While for HLA-DQ2 a single deamidation in a gluten peptide is enough to produce a CD4+ T cell response, for HLA-DQ8 it is necessary a deamidation at two positions in the gluten peptide, resulting in a more limited generation of strong antigenic gluten peptides than HLA DQ2 haplotype[33]. Furthermore, the HLA-DQ8 peptidic domain is more easily degraded limiting the availability for antigen presentation[34]. With this in mind, in this study all 30 CD patients who were screened for antibodies against OPG were also tested for HLA DQ2/DQ8 alleles. As expected[35], a proportion of 77% and 23% for DQ2 and DQ8 haplotype, respectively, was found, indicating an unselected sample at this regard. Anyway, although the role of HLA molecules and the association to particular genotypes has been well established in CD pathogenesis, HLA is estimated to contribute only for the 35% of the genetic risk, suggesting that more genetic risk factors had to be involved in CD susceptibility[36].
In conclusion, the negative results of this study indicate that auto-antibodies against OPG, if any, do not play a major role in the pathogenesis of bone demineralization in patients with CD, suggesting that other mechanisms should be investigated.
The authors would like to thank Dr. Heather Mandy Bond for revision of English language.
Evidences indicate that a low bone mineral density (BMD) is found in 20%-50% newly diagnosed patients with celiac disease (CD). Adherence to a strict gluten-free diet (GFD) will reverse the histological changes in the intestine and also the biochemical evidence of calcium malabsorption, resulting in normal BMD in these treated patients. Nevertheless, there may be long-term impairment of bone mineralization in some otherwise healthy CD patients adhering to GFD. Furthermore, osteopenia has been found in treated CD patients who showed improvement or even complete healing of intestinal mucosa.
Other mechanisms of bone injury than calcium malabsorption are probably involved in patients with CD. Mediators of inflammatory immune-mediated responses (e.g., cytokines), parathyroid hormone (PTH), estrogens, androgens, corticosteroids and vitamin D are all acknowledged to affect BMD by modulating the receptor activator of nuclear factor B/receptor activator of nuclear factor B-ligand/osteoprotegerin [RANK/RANK-L/osteoprotegerin (OPG)] system. Recently, circulating neutralizing autoantibodies against OPG have been shown in CD patients with low BMD leading to the hypothesis that blocking the inhibitory effect of OPG on RANKL may have a role in the development of bone derangement in these patients.
This study aimed at investigating risk factors associated with low BMD in patients with CD, focusing on circulating auto-antibodies against OPG. Findings confirm a high prevalence of low BMD in CD patients despite strict adherence to GFD, persistent negative serology, and healing of duodenal lesions. Since auto-antibodies against OPG have not been found in serum samples from a subgroup of patients who were tested for, it can be argued that, if any, the role of antibodies against OPG in the pathogenesis of reduced BMD in patients with CD remains to be established.
The study further supports the importance of early diagnosis in order to avoid bone complications in CD patients. Furthermore, it remarks that autoantibodies against OPG do not play a major role in the pathogenesis of bone demineralization in patients with CD, suggesting that other mechanisms of bone derangement should be investigated.
This is an interesting study showing antibodies to OPG are not present at least systemically to explain persistent low bone density in coeliac subjects. Authors guess there is a small possibility that mucosal antibodies could still be present which should still be alluded to.
Peer reviewer: Adrian Gerard Cummins, Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28-30, 2004. Gastroenterology. 2005;128:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Bianchi ML, Bardella MT. Bone in celiac disease. Osteoporos Int. 2008;19:1705-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Johnston CC, Slemenda CW, Melton LJ. Clinical use of bone densitometry. N Engl J Med. 1991;324:1105-1109. [PubMed] |
| 4. | Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 454] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 938] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 7. | Corazza GR, Di Sario A, Cecchetti L, Tarozzi C, Corrao G, Bernardi M, Gasbarrini G. Bone mass and metabolism in patients with celiac disease. Gastroenterology. 1995;109:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Sategna-Guidetti C, Grosso SB, Grosso S, Mengozzi G, Aimo G, Zaccaria T, Di Stefano M, Isaia GC. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Aliment Pharmacol Ther. 2000;14:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Valdimarsson T, Toss G, Ross I, Löfman O, Ström M. Bone mineral density in coeliac disease. Scand J Gastroenterol. 1994;29:457-461. [PubMed] |
| 10. | Walters JR, Banks LM, Butcher GP, Fowler CR. Detection of low bone mineral density by dual energy x ray absorptiometry in unsuspected suboptimally treated coeliac disease. Gut. 1995;37:220-224. [PubMed] |
| 11. | Pazianas M, Butcher GP, Subhani JM, Finch PJ, Ang L, Collins C, Heaney RP, Zaidi M, Maxwell JD. Calcium absorption and bone mineral density in celiacs after long term treatment with gluten-free diet and adequate calcium intake. Osteoporos Int. 2005;16:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Hofbauer LC. Pathophysiology of RANK ligand (RANKL) and osteoprotegerin (OPG). Ann Endocrinol (Paris). 2006;67:139-141. [PubMed] |
| 14. | Riches PL, McRorie E, Fraser WD, Determann C, van't Hof R, Ralston SH. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med. 2009;361:1459-1465. [PubMed] |
| 15. | Moore JK, West SR, Robins G. Advances in celiac disease. Curr Opin Gastroenterol. 2011;27:112-118. [PubMed] |
| 16. | Leffler DA, Edwards George JB, Dennis M, Cook EF, Schuppan D, Kelly CP. A prospective comparative study of five measures of gluten-free diet adherence in adults with coeliac disease. Aliment Pharmacol Ther. 2007;26:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother. 2000;54:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 18. | Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 479] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 19. | van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010;303:1738-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Lanzini A, Lanzarotto F, Villanacci V, Mora A, Bertolazzi S, Turini D, Carella G, Malagoli A, Ferrante G, Cesana BM. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Bianchi ML, Bardella MT. Bone and celiac disease. Calcif Tissue Int. 2002;71:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Capriles VD, Martini LA, Arêas JA. Metabolic osteopathy in celiac disease: importance of a gluten-free diet. Nutr Rev. 2009;67:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | McFarlane XA, Bhalla AK, Reeves DE, Morgan LM, Robertson DA. Osteoporosis in treated adult coeliac disease. Gut. 1995;36:710-714. [PubMed] |
| 24. | Molteni N, Bardella MT, Vezzoli G, Pozzoli E, Bianchi P. Intestinal calcium absorption as shown by stable strontium test in celiac disease before and after gluten-free diet. Am J Gastroenterol. 1995;90:2025-2028. [PubMed] |
| 25. | Kemppainen T, Kröger H, Janatuinen E, Arnala I, Lamberg-Allardt C, Kärkkäinen M, Kosma VM, Julkunen R, Jurvelin J, Alhava E. Bone recovery after a gluten-free diet: a 5-year follow-up study. Bone. 1999;25:355-360. [PubMed] |
| 26. | Cummins AG, Alexander BG, Chung A, Teo E, Woenig JA, Field JB, Thompson FM, Roberts-Thomson IC. Morphometric evaluation of duodenal biopsies in celiac disease. Am J Gastroenterol. 2011;106:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut. 2000;46 Suppl 1:i1-i8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3723] [Cited by in RCA: 3564] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 29. | Taranta A, Fortunati D, Longo M, Rucci N, Iacomino E, Aliberti F, Facciuto E, Migliaccio S, Bardella MT, Dubini A. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J Bone Miner Res. 2004;19:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Fiore CE, Pennisi P, Ferro G, Ximenes B, Privitelli L, Mangiafico RA, Santoro F, Parisi N, Lombardo T. Altered osteoprotegerin/RANKL ratio and low bone mineral density in celiac patients on long-term treatment with gluten-free diet. Horm Metab Res. 2006;38:417-422. [PubMed] |
| 31. | Rogers A, Eastell R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessment. J Clin Endocrinol Metab. 2005;90:6323-6331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Cassinotti A, Birindelli S, Clerici M, Trabattoni D, Lazzaroni M, Ardizzone S, Colombo R, Rossi E, Porro GB. HLA and autoimmune digestive disease: a clinically oriented review for gastroenterologists. Am J Gastroenterol. 2009;104:195-217; quiz 194, 218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Henderson KN, Tye-Din JA, Reid HH, Chen Z, Borg NA, Beissbarth T, Tatham A, Mannering SI, Purcell AW, Dudek NL. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity. 2007;27:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62:641-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345-350. [PubMed] |
| 36. | van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, Wapenaar MC, Barnardo MC, Bethel G, Holmes GK. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet. 2007;39:827-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (0)] |