Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1545
Revised: April 16, 2011
Accepted: April 23, 2011
Published online: April 7, 2012
Primary esophageal combined carcinoma is very rare. The authors herein report 2 cases. Case 1 was a combined squamous cell carcinoma and small cell carcinoma, and case 2 was a combined squamous cell carcinoma, adenocarcinoma, and small cell carcinoma. Case 1 was a 67-year-old man with complaints of dysphagia. Endoscopic examination revealed an ulcerated tumor in the middle esophagus, and 6 biopsies were obtained. All 6 biopsies revealed a mixture of squamous cell carcinoma and small cell carcinoma. Both elements were positive for cytokeratin, epithelial membrane antigen, and p53 protein, and had high Ki-67 labeling. The small cell carcinoma element was positive for synaptophysin, CD56, KIT, and platelet-derived growth factor-α (PDGFRA), while the squamous cell carcinoma element was not. Genetically, no mutations of KIT and PDGFRA were recognized. The patient died of systemic carcinomatosis 15 mo after presentation. Case 2 was a 74-year-old man presenting with dysplasia. Endoscopy revealed a polypoid tumor in the distal esophagus. Seven biopsies were taken, and 6 showed a mixture of squamous cell carcinoma, small cell carcinoma, and adenocarcinoma. The 3 elements were positive for cytokeratins, epithelial membrane antigen, and p53 protein, and had high Ki-67 labeling. The adenocarcinoma element was positive for mucins. The small cell carcinoma element was positive for CD56, synaptophysin, KIT, and PDGFRA, but the other elements were not. Mutations of KIT and PDGFRA were not recognized. The patient died of systemic carcinomatosis 7 mo after presentation. These combined carcinomas may arise from enterochromaffin cells or totipotential stem cell in the esophagus or transdifferentiation of one element to another. A review of the literature was performed.
- Citation: Terada T, Maruo H. Esophageal combined carcinomas: Immunohoistochemical and molecular genetic studies. World J Gastroenterol 2012; 18(13): 1545-1551
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1545.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1545
Combined esophageal carcinomas are very rare and interesting tumors. A full review of the English literature revealed 24 reporting combined carcinoma of the esophagus[1-24]. Most were small cell carcinomas, and a few were non-small cell carcinomas[1-24]. The author herein reports 2 cases of combined carcinoma of the esophagus. One case is a combined squamous cell carcinoma and small cell carcinoma, and another case is a combined squamous cell carcinoma, adenocarcinoma, and small cell carcinoma
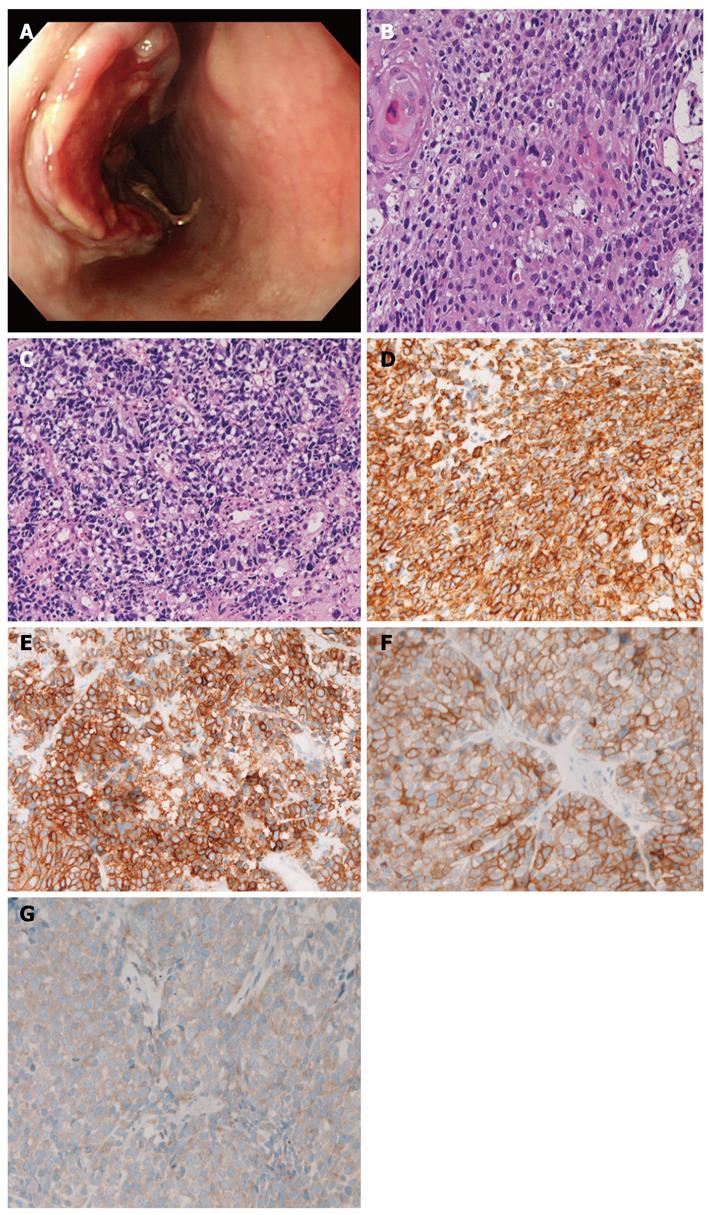
A 67-year-old man was admitted to our hospital with dysphagia. An endoscopic examination revealed an ulcerated tumor (3 cm × 4 cm × 3 cm) in the middle esophagus (Figure 1A), and 6 biopsies were obtained. All 6 biopsies revealed a mixture of squamous cell carcinoma (Figure 1B) and small cell carcinoma (Figure 1C). The squamous element was composed of malignant cells arranged in a layer with focal keratinization (cancer pearls). The small cell carcinoma element consisted of malignant small cells with hyperchromatic nuclei, nuclear molding, absent nucleoli, and very scant cytoplasm. There was a gradual merging of the 2 elements.
The authors performed an immunohistochemical study using Dako Envision method, as previously described[25,26]. The immunohistochemical antibodies used were as follows: cytokeratins (AE1/3, Dako; CAM5.2 Bekton-Dickinson, CA, United States), epithelial membrane antigen (E29, Dako), neuron-specific enolase (BBS/NC/VI-H14, Dako), chromogranin (DAK-A3, Dako), synaptophysin (polyclonal, Dako), CD56 (UJ13A, Dako), p53 protein (DO-7, Dako), Ki-67 (MIB-1, Dako), KIT (polyclonal, Dako), and platelet derived growth factor receptor-α (PDGFRA) (polyclonal, Santa Cruz, CA, United States). The squamous cell carcinoma element was positive for cytokeratin, epithelial membrane antigen, p53 protein, and Ki-67 antigen (57% labeled), but negative for other antigens examined. The small cell carcinoma element was positive for cytokeratin (Figure 1D), p53 protein, Ki-67 (96% labeled), synaptophysin (Figure 1E), CD56, and chromogranin, KIT (Figure 1F), and PDGFRA (Figure 1G).
The authors performed a molecular genetic study for KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes in paraffin sections using microdissection and the polymerase chain reaction-direct sequencing method, as previously described[27-30]. There were no mutations of the KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes.
The patient was diagnosed with combined carcinoma of esophagus (stage II, T2 N0 M0). Surgery was not considered because the tumor contained small cell carcinoma. The patient was treated with cisplatin-based chemotherapy and radiation, but died of systemic carcinomatosis 15 mo after presentation.
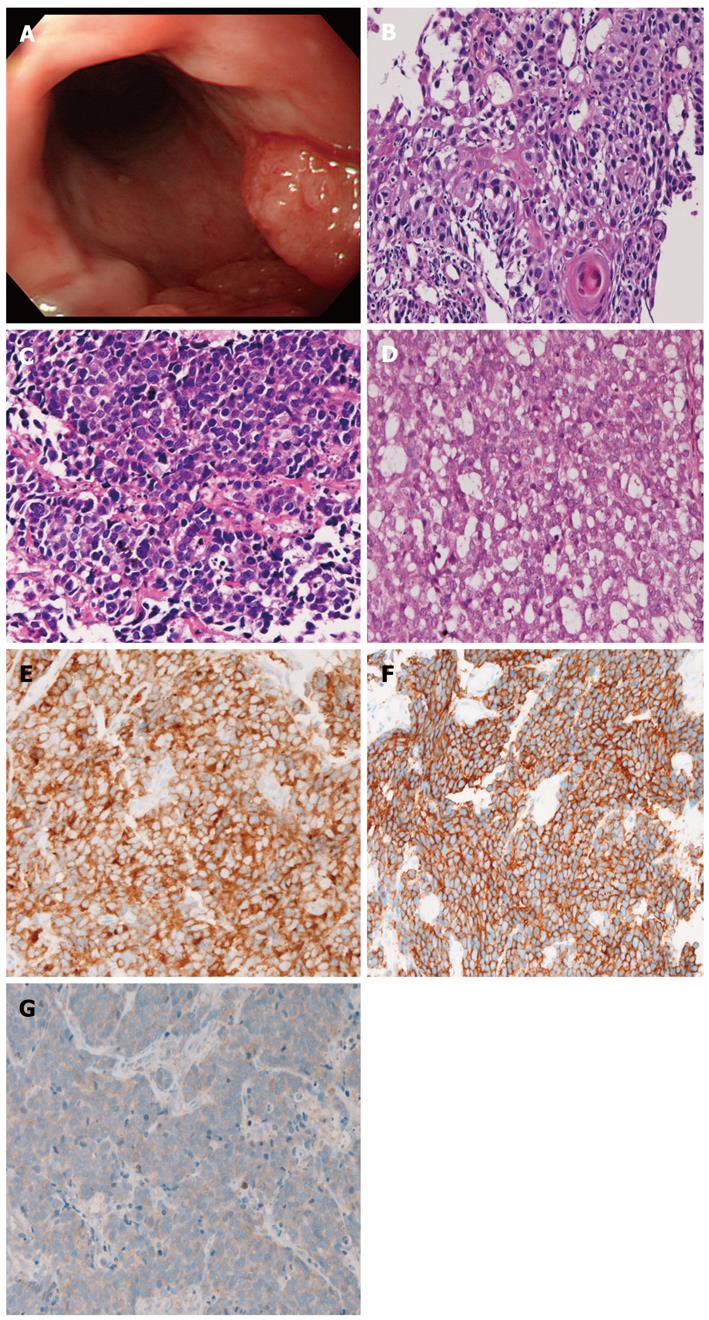
A 74-year-old man presented with dysplasia, and attended our hospital. An endoscopy revealed a polypoid tumor (2 cm × 2 cm × 3 cm) in the middle esophagus (Figure 2A). Seven biopsies were taken, and 6 showed a mixture of squamous cell carcinoma (Figure 2B), small cell carcinoma (Figure 2C), and adenocarcinoma (Figure 2D). The squamous cell carcinoma element showed malignant cells in a layer with focal keratinization. The small cell carcinoma element was composed of small malignant cells with hyperchromatic nuclei, inconspicuous nucleoli, and scant cytoplasm. The adenocarcinoma element showed sheet-like tumor cells with focal acinar formations, in which mucins were identified. The 3 elements were positive for cytokeratins, epithelial membrane antigen, p53 protein, and Ki-67 (labeling: squamous cell carcinoma element, 34%; adenocarcinoma element, 29%; small cell carcinoma element 87%). The squamous cell carcinoma and adenocarcinoma elements were negative for CD56, chromogranin, synaptophysin, neuron-specific elolase, KIT and PDGFRA. In contrast, the small cell carcinoma element was positive for CD56 (Figure 2E), synaptophysin, KIT (Figure 2F), and PDGFRA (Figure 2G). Mutations of KIT and PDGFRA were not found.
The patient was diagnosed with combined carcinoma of the esophagus (stage II, T2 N1 M0). Surgery was not considered because the tumor contained small cell carcinoma. The patient received chemoradiation, but died of systemic carcinomatosis 7 mo after presentation.
The present 2 cases of combined carcinoma of the esophagus were associated with small cell carcinoma. Small cell carcinoma is diagnosed with hematoxylin and eosin (HE) staining and is defined as an undifferentiated carcinoma consisting of small cells with characteristic cellular and nuclear features, such as small-sized cells, scant cytoplasm, hyperchromatic, finely granular, and molded nuclei, and inconspicuous nucleoli, according to the World Health Organization Blue Book[31]. Neuroendocrine features are recognized in more than 90% of small cell carcinoma[31]. Squamous cell carcinoma is characterized by a squamoid cell arrangement and the presence of intercellular bridges and keratinization. Adenocarcinoma is characterized by tubular formations and the presence of mucins. Case 1 in the present study fulfilled these criteria, and was definitely combined small cell carcinoma and squamous cell carcinoma. Likewise, Case 2 was an apparently combined small cell carcinoma, squamous cell carcinoma, and adenocarcinoma. The presence of p53 protein and high Ki-67 labeling supports the above diagnosis.
In the present study, there was gradual merging of the 2 elements in case 1 and of the 3 elements in case 2. These findings may indicate that each element is derived from transdifferentiation of other elements. Traditionally, small cell carcinoma of the esophagus is thought to be derived from enterochromaffin cells or APUD cells present in the normal esophagus. Otherwise, this esophageal tumor arises from totipotent stem cells of the esophagus, as suggested by Ho et al[2]. The present study could not determine the histogenesis of the combined carcinomas associated with small cell carcinomas.
Most of esophageal tumors with multiple differentiation (combined carcinoma) are associated with small cell carcinoma[1-15,17-24], although basaloid cell squamous cell carcinoma also shows multiple differentiation[16]. The cellular origin of small cell carcinoma is unknown. In the full review of the English literature on combined carcinomas of esophageal cancers, Rosen et al[1] reported an epidermoid carcinoma simulating oat cell carcinoma. Ho et al[2] reported that 2 of 4 cases of esophageal small cell carcinoma contained foci of squamous cell carcinoma. Reid et al[3] described a case of esophageal small cell carcinoma with foci of squamous cell carcinoma. Reyes et al[4] reported that foci of squamous cell carcinoma were seen in 4/16 esophageal small cell carcinoma. Sarma[5] mentioned that there were oat cell carcinomas with squamous cell carcinoma foci and adenocarcinoma foci. Doherty et al[6] reported that there were oat cell carcinomas with squamous cell carcinoma in situ, with squamous cell carcinoma, with adenocarcinoma, and with carcinoid. Sato et al[7] reported a case of small cell carcinoma with invasive squamous cell carcinoma. Sasajima et al[8] demonstrated one case of esophageal carcinoma showing multiple differentiations into oat cell carcinoma, adenoid cystic carcinoma, adenocarcinoma, and squamous cell carcinoma. Mori et al[9] reported that 7 squamous cell foci and 2 adenocarcinoma foci were recognized in 10 small cell carcinomas. Attar et al[10] showed concomitant squamous cell carcinoma in small cell carcinoma. Beyer et al[11] mentioned that there was considerable histological heterogeneity in small cell carcinoma. Fujiwara et al[13] reported a case of small cell carcinoma with concomitant squamous cell carcinoma. Takubo et al[14] found a combination of small cell carcinoma and squamous cell carcinoma in 11 of 21 cases, and a combination of small cell carcinoma and mucoepidermid carcinoma in 1 of 21 cases. Medgyesy et al[15] found a combination of small cell carcinoma and adenocarcinoma in 1 of 8 cases, and a combination of small cell carcinoma and squamous cell carcinoma in 1 of 8 cases. Cho et al[16] identified a combination of basaloid squamous cell carcinoma and squamous cell carcinoma in 8 of 18 cases, a combination of basaloid squamous cell carcinoma and adenocarcinoma in 3 of 18 cases, a combination of basaloid squamous cell carcinoma and small cell carcinoma in 2 of 18 cases. Uğraş et al[18] reported a combined carcinoma composed of small cell carcinoma and squamous cell carcinoma. Ishihara et al[19] found an esophageal combined carcinoma consisting of Pagetoid squamous cell carcinoma, choriocarcinoma, and mucoepidermoid carcinoma. Yamamoto et al[20] reported in situ and invasive squamous cell carcinomas were present in 3 of 6 cases of small cell carcinoma. Wu et al[21] reported that small cell carcinoma with squamous cell carcinoma was found in 3 of 9 cases. Yun et al[22] identified squamous differentiation in small cell carcinoma in 2 of 21 cases. Bilbeau et al[23] reported a case of small cell carcinoma with adenocarcinoma in a Barrett’s esophagus. Maru et al[24] reported that a combination of small cell carcinoma and adenocarcinoma was seen in 15 of 40 cases, and a combination of small cell carcinoma and squamous cell carcinoma in 1 of 40 cases. Therefore, this literature review showed that combined carcinoma of the esophagus is not so rare among small cell esophageal carcinomas, and that the majority of combined carcinoma is associated with small cell carcinoma. The review also confirmed that esophageal combined carcinoma composed of small cell carcinoma and squamous cell carcinoma is the most common, followed by a combination of small cell carcinoma and adenocarcinoma. The present 2 cases also are the common type of combined esophageal carcinoma.
As mentioned above, small cell carcinoma is diagnosed by HE staining[31]. About 90% of small cell carcinoma has neuroendocrine features[31]. The neuroendocrine features can be demonstrated by immunohistochemical demonstration of neuroendocrine antigens such as chromogranin, synaptophysin, CD56, and neuron-specific enolase or by ultrastructural demonstration of neuroendocrine secretory vesicles[32]. Yamamoto et al[20] described that CD56, neuron-specific enolase, and chromogranin were positive in a small cell carcinoma component while they were negative in the squamous cell carcinoma component in 3 cases of combined esophageal carcinoma. They also demonstrated that both components were positive for cytokeratins and epithelial membrane antigen. Wu et al[21] described that esophageal small cell carcinomas were positive for neuron-specific enolase, chromogranin A, and synaptophysin in all 9 cases investigated. Yun et al[22] described that the percentage of endocrine markers in 21 esophageal small cell carcinomas was as follows: synaptophysin, 95%; CD56, 76%; chromogranin A, 62%, neuron-specific enolase, 62%, TTF-1, 71%; epithelial membrane antigen, 62%; cytokeratins, 57%; S100 protein, 19%. Maru et al[24] described that chromogranin was positive in 31 of 40 and synaptophysin in all 40 esophageal neoroendocrine carcinomas. In the present case, synaptophysin, CD56 and chromogranin were positive in the small cell carcinoma component in case 1, and CD56 and synaptophysin were positive in the small cell carcinoma component in case 2. In both cases in the present study, all the elements were positive for cytokeratin and epithelial membrane antigen. The non-small cell carcinoma components were negative for the neuroendocrine carcinoma. These findings are compatible with those of previous studies.
The present study has new findings: it showed positive expression of KIT and PDGFRA in the small cell carcinoma element of the 2 combined esophageal carcinomas. The present study also revealed that the squamous cell carcinoma and adenocarcinoma components were negative for KIT and PDGFRA protein and were negative for KIT and PDGFRA mutations in the esophageal combined carcinoma. KIT and PDGFRA are transmembranous receptor tyrosine kinase oncoproteins involved in carcinogenesis[33-35]. The vast majority of small cell carcinoma develops in the lung. In small cell lung carcinoma, KIT is frequently expressed, but no mutations of KIT gene have been recognized[36-46]. In small cell lung carcinoma, protein expression and mutations of PDGFRA are unknown. In extrapulmonary small cell carcinoma, KIT and PDGFRA proteins are frequently expressed, but there have been no mutations of KIT and PDGFRA genes found[46-48]. Many more studies of the KIT and PDGFRA gene status in esophageal combined carcinomas are necessary to elucidate the molecular mechanism of the carcinogenesis.
The biological behavior of these combined carcinomas of the esophagus is not known. However, it is thought that these combined carcinomas behave like small cell carcinoma, because the great majority of these combined carcinomas contain a small cell carcinoma element[1-24]. The option for treatment is not surgery but chemotherapy and radiation as in pulmonary small cell carcinoma[1-24]. The chemotherapy employed was cisplatin and etoposide[1-24]. Adjuvant radiation therapy may be effective. The combined carcinomas of the esophagus have a higher propensity for systemic metastases[1-24]. The survival rate is not clear because of a limited number of cases. However, survival was thought to be similar to that of pulmonary small cell carcinoma[1-24].
In summary, the authors presented 2 rare cases of esophageal combined carcinoma with double (squamous cell carcinoma and small cell carcinoma) and triplicate differentiation (squamous cell carcinoma, small cell carcinoma, and adenocarcinoma). The authors speculates that the combined carcinomas are basically small cell carcinomas with squamous and/or adenocarcinomatous differentiation. The present esophageal combined carcinomas may arise from enterochromaffin or totipotent stem cell of the esophagus. It is also possible that each element of the esophageal combined carcinomas may be derived from transdifferentiation of other elements. There were expressions of KIT and PDGFRA in the small cell carcinoma component of the esophageal combined carcinomas, but were negative for mutations of KIT and PDGFRA.
Peer reviewer: Matthew James Schuchert, MD, FACS, Assistant Professor of Surgery, Heart, Lung and Esophageal Surgery Institute, University of Pittsburgh Medical Center, Shadyside Medical Building, 5200 Centre Avenue, Suite 715, Pittsburgh, PA 15232, United States
S- Editor Tian L L- Editor Cant MR E- Editor Zhang DN
| 1. | Rosen Y, Mon S, Kim B. Small cell epidermoid carcinoma of the esophagus: An oat cell carcinoma. Cancer. 1975;36:1042-1049. |
| 2. | Ho KJ, Herrera GA, Jones JM, Alexander CB. Small cell carcinoma of the esophagus: evidence for a unified histogenesis. Hum Pathol. 1984;15:460-468. [RCA] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Reyes CV, Chejfec G, Jao W, Gould VE. Neuroendocrine carcinomas of the esophagus. Ultrastruct Pathol. 1980;1:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Sarma DP. Oat cell carcinoma of the esophagus. J Surg Oncol. 1982;19:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Doherty MA, McIntyre M, Arnott SJ. Oat cell carcinoma of the esophagus: a report of six British patients with a review of the literature. Int J Radiat Oncol Biol Phys. 1984;10:147-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Sato T, Mukai M, Ando N, Tashiro Y, Iri H, Abe O, Watanabe Y. Small cell carcinoma (non-oar cell type) of the esophagus concomitant with invasive squamous cell carcinoma and carcinoma in situ: A case report. Cancer. 1986;15:328-332. |
| 8. | Sasajima K, Hayashi N, Yamashita K, Onda M, Takubo K. Oat cell carcinoma of the esophagus with multiple differentiation. J Clin Gastroenterol. 1988;10:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mori M, Matsukuma A, Adachi Y, Miyagahara T, Matsuda H, Kuwano H, Sugimachi K, Enjoji M. Small cell carcinoma of the esophagus. Cancer. 1989;63:564-573. |
| 10. | Attar BM, Levendoglu H, Rhee H. Small cell carcinoma of the esophagus. Report of three cases and review of the literature. Dig Dis Sci. 1990;35:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Beyer KL, Marshall JB, Diaz-Arias AA, Loy TS. Primary small-cell carcinoma of the esophagus. Report of 11 cases and review of the literature. J Clin Gastroenterol. 1991;13:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Law SY, Fok M, Lam KY, Loke SL, Ma LT, Wong J. Small cell carcinoma of the esophagus. Cancer. 1994;73:2894-2899. |
| 13. | Fujiwara Y, Nakagawa K, Tanaka T, Utsunomiya J, Nishigami T, Uematsu K. Small cell carcinoma of the esophagus combined with superficial esophageal cancer. Hepatogastroenterology. 1996;43:1360-1369. [PubMed] |
| 14. | Takubo K, Nakamura K, Sawabe M, Arai T, Esaki Y, Miyashita M, Mafune K, Tanaka Y, Sasajima K. Primary undifferentiated small cell carcinoma of the esophagus. Hum Pathol. 1999;30:216-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Medgyesy CD, Wolff RA, Putnam JB Jr, Ajani JA. Small cell carcinoma of the esophagus: the University of Texas M.D. Anderson Cancer Center experience and literature review. Cancer. 2000;88:262-267. |
| 16. | Cho KJ, Jang JJ, Lee SS, Zo JI. Basaloid squamous carcinoma of the oesophagus: a distinct neoplasm with multipotential differentiation. Histopathology. 2000;36:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Bennouna J, Bardet E, Deguiral P, Douillard JY. Small cell carcinoma of the esophagus: analysis of 10 cases and review of the published data. Am J Clin Oncol. 2000;23:455-459. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Uğraş S, Akpolat N, Er M, Yalçýnkaya I, Karaayvaz M. Primary composite tumour with bipartite differentiation of the esophagus. Acta Chir Belg. 2000;100:39-43. [PubMed] |
| 19. | Ishihara A, Mori T, Koono M. Diffuse pagetoid squamous cell carcinoma of the esophagus combined with choriocarcinoma and mucoepidermoid carcinoma: an autopsy case report. Pathol Int. 2002;52:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Yamamoto J, Ohshima K, Ikeda S, Iwashita A, Kikuchi M. Primary esophageal small cell carcinoma with concomitant invasive squamous cell carcinoma or carcinoma in situ. Hum Pathol. 2003;34:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: report of 9 cases and review of literature. World J Gastroenterol. 2004;10:3680-3682. [PubMed] |
| 22. | Yun JP, Zhang MF, Hou JH, Tian QH, Fu J, Liang XM, Wu QL, Rong TH. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Bibeau F, Chateau MC, Guiu M, Assenat E, Azria D, Lavaill R, Ychou M, Boissière-Michot F. Small cell carcinoma with concomitant adenocarcinoma arising in a Barrett's oesophagus: report of a case with a favourable behaviour. Virchows Arch. 2008;452:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Maru DM, Khurana H, Rashid A, Correa AM, Anandasabapathy S, Krishnan S, Komaki R, Ajani JA, Swisher SG, Hofstetter WL. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Oncol. 2009;28:29-34. |
| 28. | Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256-7259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Terada T. Primary extragastrointestinal stromal tumor of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Travis W, Nicholson S, Hirsch FR et al. Small cell carcinoma. WHO classification of tumour. Pathology and genetics of tumours of the lung pleura, thymus and heart. Lyon: IARC press 2004; 31-34. |
| 32. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 33. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 34. | Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumor. Gastroenterol. 2003;125:660-667. [RCA] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 478] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 35. | Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13: 205-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | LaPoint RJ, Bourne PA, Wang HL, Xu H. Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. Appl Immunohistochem Mol Morphol. 2007;15:401-406. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | López-Martin A, Ballestín C, Garcia-Carbonero R, Castaño A, Lopez-Ríos F, López-Encuentra A, Sánchez-Cespedes M, Castellano D, Bartolomé A, Cortés-Funes H. Prognostic value of KIT expression in small cell lung cancer. Lung Cancer. 2007;56:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Micke P, Basrai M, Faldum A, Bittinger F, Rönnstrand L, Blaukat A, Beeh KM, Oesch F, Fischer B, Buhl R. Characterization of c-kit expression in small cell lung cancer: prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188-194. [PubMed] |
| 39. | Camps C, Sirera R, Bremnes RM, Garde J, Safont MJ, Blasco A, Berrocal A, Sánchez JJ, Calabuig C, Martorell M. Analysis of c-kit expression in small cell lung cancer: prevalence and prognostic implications. Lung Cancer. 2006;52:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Rossi G, Cavazza A, Marchioni A, Migaldi M, Bavieri M, Facciolongo N, Petruzzelli S, Longo L, Tamberi S, Crinò L. Kit expression in small cell carcinomas of the lung: effects of chemotherapy. Mod Pathol. 2003;16:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Naeem M, Dahiya M, Clark JI, Creech SD, Alkan S. Analysis of c-kit protein expression in small-cell lung carcinoma and its implication for prognosis. Hum Pathol. 2002;33:1182-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Mojica WD, Saxena R, Starostik P, Cheney RT. CD117+ small cell lung cancer lacks the asp 816---> val point mutation in exon 17. Histopathology. 2005;47:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camacci T, Lucchi M, Mussi A, Basolo F, Pingitore R. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;10:4101-4108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Burger H, Den Bakker MA, Stoter G, Verweij J, Nooter K. Lack of c-kit exon 11 activating mutations in c-kit/CD117-positive SCLC tumor specimens. Eur J Cancer. 2003;39:793-799. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Sihto H, Sarlomo-Rikala M, Tynninen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Terada T. Primary small cell carcinoma of the mediastinum: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2009;26:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Terada T. Primary small cell carcinoma of the ureter: a case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology. 2010;42:101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |