Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1517
Revised: August 21, 2011
Accepted: January 22, 2012
Published online: April 7, 2012
AIM: To compare efficacy of proton pump inhibitors (PPIs) with H2-receptor antagonists (H2RAs) plus prokinetics (Proks) for dysmotility-like symptoms in functional dyspepsia (FD).
METHODS: Subjects were randomized to receive open-label treatment with either rabeprazole 10 mg od (n = 57) or famotidine 10 mg bid plus mosapride 5 mg tid (n = 57) for 4 wk. The primary efficacy endpoint was change (%) from baseline in total dysmotility-like dyspepsia symptom score. The secondary efficacy endpoint was patient satisfaction with treatment.
RESULTS: The improvement in dysmotility-like dyspepsia symptom score on day 28 was significantly greater in the rabeprazole group (22.5% ± 29.2% of baseline) than the famotidine + mosapride group (53.2% ± 58.6% of baseline, P < 0.0001). The superior benefit of rabeprazole treatment after 28 d was consistent regardless of Helicobacter pylori status. Significantly more subjects in the rabeprazole group were satisfied or very satisfied with treatment on day 28 than in the famotidine + mosapride group (87.7% vs 59.6%, P = 0.0012). Rabeprazole therapy was the only significant predictor of treatment response (P < 0.0001), defined as a total symptom score improvement ≥ 50%.
CONCLUSION: PPI monotherapy improves dysmotility-like symptoms significantly better than H2RAs plus Proks, and should be the treatment of first choice for Japanese FD.
- Citation: Sakaguchi M, Takao M, Ohyama Y, Oka H, Yamashita H, Fukuchi T, Ashida K, Murotani M, Murotani M, Majima K, Morikawa H, Hashimoto T, Kiyota K, Esaki H, Amemoto K, Isowa G, Takao F. Comparison of PPIs and H2-receptor antagonists plus prokinetics for dysmotility-like dyspepsia. World J Gastroenterol 2012; 18(13): 1517-1524
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1517
Functional dyspepsia (FD) is a condition characterized by persistent upper abdominal symptoms in the absence of any causative organic disease[1]. It is thought to be caused by a combination of different factors, including dysmotility or hypersensitivity of the gastrointestinal (GI) tract, gastric acid secretion, inflammation of the gastric mucosa, altered sympathetic or parasympathetic activity, altered secretion of GI hormones, and psychological factors[2,3]. Treatments vary according to the symptoms, and include gastroprokinetic agents, suppressors of gastric acid secretion, antidepressants, anxiolytics and Chinese herbal medicines. Although it has been shown that gastric acid secretion is normal in patients with FD[4], a subset of these patients will benefit from strong acid suppression by a proton pump inhibitor (PPI)[5]. Inhibitors of acid secretion are therefore widely prescribed to patients with FD worldwide. Although treatment with acid suppression produces symptom relief in a proportion of patients with FD, this effect has not been reported consistently in all studies[6-8]. A Japanese study surveyed the prescribing habits of primary care physicians for functional GI symptoms and evaluated the efficacy and indications of the medications prescribed[9]. It was found that H2-receptor antagonists (H2RAs) are the treatment of first choice for ulcer-like symptoms such as epigastric pain, and prokinetics (Proks) for dysmotility-like symptoms such as epigastric discomfort, heaviness, and bloating. In other words, Japanese primary care physicians prefer H2RA + prokinetic combination therapy for FD symptoms.
For FD patients with at least mild heartburn and/or regurgitation at baseline, omeprazole is associated with higher treatment success rates at 4 wk than ranitidine, cisapride and placebo[10]. In those patients who have either no or minimal heartburn and/or regurgitation at baseline, omeprazole and ranitidine are superior to placebo, although no significant difference is seen between omeprazole and ranitidine[10]. The question of whether more effective acid suppression is efficacious in Japanese patients with FD has yet to be adequately tested.
It is reasonable to think that H2RAs provide adequate relief for FD symptoms in Japanese patients, who have a higher rate of Helicobacter pylori (H. pylori) infection than their western counterparts, as well as lower levels of gastric acid secretion[11].
Complete relief of symptoms is significantly more common with omeprazole than with placebo in subgroups of patients with ulcer-like and reflux-like dyspepsia, whereas, as might be expected, there is no indication of benefit with omeprazole in patients with dysmotility-like dyspepsia[5]. In addition, meta-analyses suggest that H2RA and Proks are superior to placebo in non-ulcer dyspepsia (NUD)[12,13].
In this study, we concentrated on dysmotility-like symptoms in patients with FD, and compared the efficacy of PPI monotherapy and combination therapy with H2RAs and Proks, which is widely prescribed by Japanese primary care physicians for FD symptoms.
This study was a randomized open-label trial conducted in three hospitals (Moriguchi Keijinkai Hospital, Osaka Saiseikai Nakatsu Hospital, and Arisawa General Hospital) and nine general medical clinics (Murotani Clinic, Majima Clinic, Morikawa Clinic, Hashimoto Clinic, Kiyota Clinic, Arisawa General Hospital, Amemoto Clinic, Isowa Clinic, and Mii Clinic) in Japan from January 2009 until April 2010.
The subjects were patients of at least 18 years of age with at least 1 mo of dyspepsia symptoms and no clinically significant findings at endoscopy. The main exclusion criteria were: (1) history of erosive esophagitis, peptic ulcer disease, GI malignancy, primary esophageal motility disorder, previous upper GI surgery; (2) maintenance treatment with a PPI or H2RA within 2 wk of enrollment; and (3) severe concurrent disease. PPI, H2RA and Prok use were not permitted during the 14 d prior to endoscopy, nor during the study. Nonsteroidal anti-inflammatory drugs, acetylsalicylic acid or steroids were not permitted at any time during the study. The study protocol was approved by the relevant Institutional Review Board and/or an Independent Ethics Committee, and informed written consent was obtained from each participating subject.
The investigators referred each enrolled subject for esophagogastroduodenoscopy. After endoscopy, eligible patients underwent a validated 13C urea breath test to determine their H. pylori status. Subjects were randomly allocated to receive one of the following treatments for 4 wk: (1) rabeprazole 10 mg od (PPI); or (2) famotidine 10 mg bid plus mosapride 5 mg tid (H2RA + Prok). Group allocations were assigned in equal numbers using a central computer-generated randomization list stratified for each participating institution. Subject compliance was assessed by counting the returned medication. Subjects were considered to have complied with treatment if they took at least 75% of the dispensed medication. Subjects attended their clinic at randomization and after 4 wk of treatment.
Subjects were asked to assess their dyspepsia symptoms at baseline and after 3 d, 7 d, 14 d and 28 d of treatment using a self-completed questionnaire for dyspepsia symptoms. Dysmotility-like dyspepsia symptoms were assessed using five questions (upper abdominal bloating, postprandial fullness, early satiation, belching, vomiting/nausea), and each response was graded on a five-point frequency scale as follows: 0, never; 1, occasionally; 2, sometimes; 3, often; 4, always. The scores for each question were totaled to give the total symptom score for dysmotility-like dyspepsia symptoms. The total symptom scores at each assessment time point were then expressed as a percentage of the baseline total symptom score.
After 14 d and 28 d of treatment, subject satisfaction was evaluated using a four-grade scale as follows: very satisfied (symptoms disappeared); satisfied (symptoms improved considerably); somewhat satisfied (symptoms improved somewhat); unsatisfied (no improvement or symptoms worse).
The primary efficacy endpoint was the change (%) from baseline in total dysmotility-like dyspepsia symptom score. The secondary efficacy endpoint was subject satisfaction.
The sample size calculation was based on the anticipated difference in symptom improvement rates between the PPI and H2RA + Prok groups. Due to the lack of clinical trials of H2RA + Prok combination therapy, we based our calculations of the sample size on the results of comparative trials of PPIs vs Proks.
The estimated success rate after 4 wk treatment was 23.7% for omeprazole, and 7.5% for cisapride[10]. Assuming a two-tailed α error rate of 0.05 and a power of 80%, with a 30% dropout rate during screening, 77.5 patients were required for each treatment arm.
Data are presented as mean ± SD. The intention-to-treat analysis included all randomized subjects. A subject who withdrew at any time was considered a dropout. We used the Wilcoxon single rank test for paired intra-individual comparisons, the Mann-Whitney U test for comparisons of continuous variables, and the χ2 test for comparisons of categorized variables between the two treatment groups. In addition, we stratified primary endpoint results for differences between treatment groups according to H. pylori status. We performed multiple logistic regression analysis to determine factors (age, sex, H. pylori status, and baseline dysmotility-like dyspepsia symptom score) associated with treatment response (defined as change in total dysmotility-like dyspepsia symptom score of ≥ 50% after 28 d of treatment). P < 0.05 was considered to signify statistical significance for all analyses.
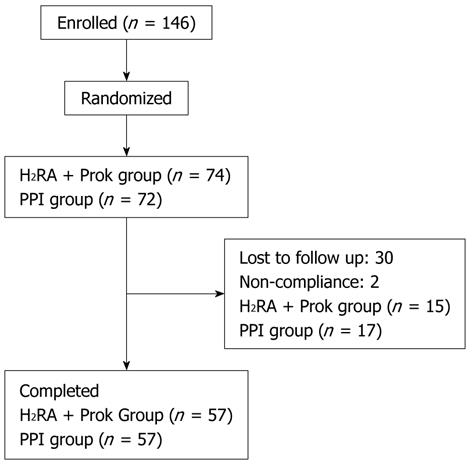
A total of 146 patients were randomized. Thirty-two patients were excluded in the follow-up period (30 lost to follow-up, two for non-compliance), leaving 114 patients for inclusion in the analysis. Fifty-seven patients were randomized to receive PPI treatment, and 57 to receive H2RA + Prok treatment (Figure 1). Baseline demographic characteristics and symptom scores of the patients who completed the treatment period are given in Table 1. There were no significant differences between the characteristics of the two treatment groups at baseline.
| H2RA + Prok(n = 57) | PPI(n = 57) | P value | |
| Age (mean ± SD, yr) | 51.5 ± 14.8 | 52.9 ± 13.8 | 0.6120 |
| Sex (male/female) | 17/40 | 12/45 | 0.3899 |
| Helicobacter pylori infection (%) | 43.8 | 43.8 | > 0.9999 |
| Symptom scores | |||
| Upper abdominal bloating | 1.6 ± 1.2 | 1.8 ± 1.3 | 0.6160 |
| Postprandial fullness | 2.1 ± 1.2 | 2.0 ± 1.2 | 0.7747 |
| Early satiation | 1.7 ± 1.3 | 1.3 ± 1.2 | 0.1934 |
| Belching | 1.7 ± 1.4 | 1.6 ± 1.4 | 0.7211 |
| Vomiting/nausea | 1.5 ± 1.3 | 1.4 ± 1.3 | 0.8361 |
| Total symptom score (dysmotility-like dyspepsia symptoms) | 8.6 ± 3.9 | 8.2 ± 4.7 | 0.5330 |
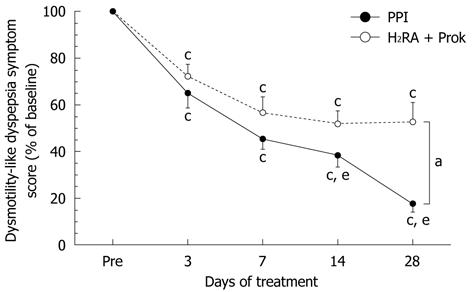
No significant differences were seen between groups in the change in dysmotility-like dyspepsia symptom score from baseline to 3 d or 7 d of treatment. After 28 d of treatment, the change in symptom score was significantly greater in the PPI group (22.5% ± 29.2% of baseline) than in the H2RA + Prok group (53.2% ± 58.6% of baseline) (P < 0.0001), indicating greater improvement in symptoms with PPI treatment (Figure 2). A significant improvement in total symptom score was seen over time in both groups, but in the H2RA + Prok group, the improvements in total symptom score on days 14 and 28 were not significantly greater than at day 7, whereas in the PPI group, the improvements in total symptom score at day 14 (38.4% ± 37.8% of baseline, P = 0.0034) and day 28 (P < 0.0001) were both significantly greater than on day 7 (45.1% ± 33.8% of baseline) (Figure 2).
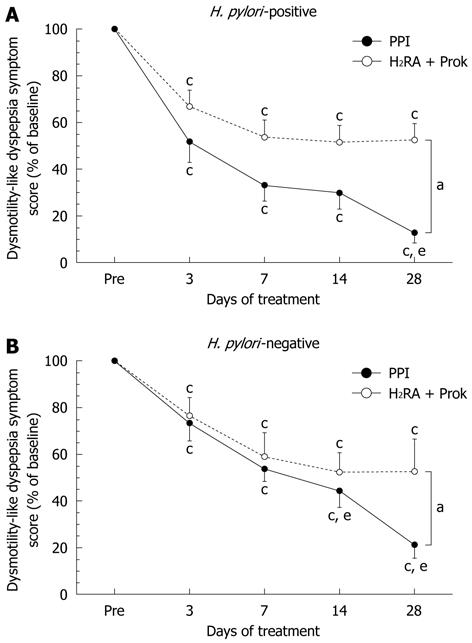
Among H. pylori-positive subjects, a significantly greater improvement in total symptom score was seen in the PPI group (13.4% ± 26.2% of baseline) than in the H2RA + Prok group (53.4% ± 34.7% of baseline) (P < 0.007) by the end of the treatment period. Significant symptomatic improvement was seen over time in both treatment groups, although in the H2RA + Prok group, no further improvement was observed after day 7 (35.7% ± 31.6% of baseline). In the PPI group, there was no significant difference between the changes in total symptom score for days 7 and 14, although there was a statistically significant difference between days 7 and 28 (P = 0.0277) (Figure 3A).
Among H. pylori-negative subjects, a significantly greater improvement in total symptom score was seen in the PPI group (24.1% ± 31.7% of baseline) than in the H2RA + Prok group (53.1% ± 72.2% of baseline, P < 0.0001) on day 28. Significant symptomatic improvement was seen over time in both treatment groups, although the improvements seen in the H2RA + Prok group on days 14 and 28 were not significantly superior to those observed on day 7. In the PPI group, the reductions in total symptom score on days 14 (44.2% ± 42.0% of baseline, P = 0.0177) and 28 (P = 0.0002) were both significantly greater than on day 7 (52.4% ± 34.1% of baseline) (Figure 3B).
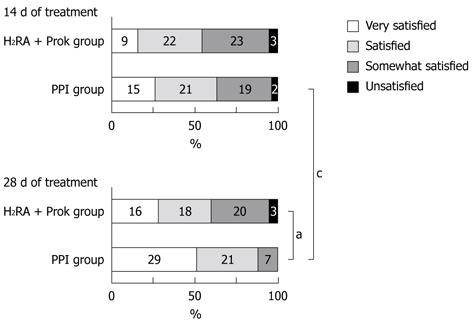
Although no significant difference was seen between the groups on day 14, the proportion of subjects who were satisfied or very satisfied with their treatment was significantly higher in the PPI group than in the H2RA + Prok group on day 28 (87.7% vs 59.6%, P = 0.0012). No significant increase was seen in subject satisfaction in the H2RA + Prok group between days 14 and 28, whereas a significant increase was seen in the proportion of subjects in the PPI group answering satisfied or very satisfied between day 14 (63.2%) and 28 (P = 0.0042) (Figure 4).
In this study, treatment response was defined as an improvement in dysmotility-like dyspepsia symptom score of ≥ 50% after 28 d of treatment. The only factor identified by logistic regression analysis as a positive predictor of treatment response was PPI therapy (Table 2).
| Variable | Estimated OR | 95% CI | P value |
| Age (yr) | 0.999 | 0.962-1.036 | 0.9493 |
| Sex: male | 0.853 | 0.312-2.332 | 0.7563 |
| Helicobacter pylori infection | 0.521 | 0.191-1.419 | 0.2019 |
| Treatment: PPI | 9.055 | 3.231-25.376 | < 0.0001 |
| Dysmotility-like symptom score | 1.062 | 0.940-1.200 | 0.3368 |
The Rome III criteria define FD as “the chronic presence of one or more dyspepsia symptoms (bothersome postprandial fullness, early satiation, epigastric pain, epigastric burning) that are considered to originate from the gastroduodenal region, with no evidence of structural disease (including at upper endoscopy) that is likely to explain the symptoms”[1]. FD is a common condition[14,15], with considerable adverse impact on quality of life[16], and represents a serious problem in everyday clinical practice. Patients with FD present with a variety of symptoms[17,18], so based on the symptom profile, they may be prescribed gastroprokinetic agents, suppressors of gastric acid secretion, antidepressants, anxiolytics or Chinese herbal medicines[19]. A Japanese survey of the prescribing habits of primary care physicians for upper GI symptoms has found that H2RAs are prescribed for epigastric pain and heartburn, and Proks for epigastric discomfort, nausea and loss of appetite[9]. In other words, H2RAs rather than PPIs are prescribed for epigastric pain syndrome[1], characterized by the two symptoms of epigastric pain and epigastric burning, unrelated to meals and considered mainly related to gastric acid. Proks are widely prescribed for postprandial distress syndrome[1], characterized by the two symptoms of postprandial fullness and early satiation, and considered to be strongly related to dysmotility of the GI tract; in particular, gastric accommodation of adaptive relaxation. H2RA + Prok combination therapy is widely prescribed in Japan, where dyspepsia is not a recognized diagnosis for insurance purposes.
It has recently become clear that gastric acid secretion is strongly associated with the onset of dysmotility-like symptoms. Lee et al[20] have examined the influence of acid on gastric hypersensitivity and motility in healthy subjects, and have found that duodenal acidification significantly induces gastric hypersensitivity and impairs gastric motility. Compared with saline, infusion of acid into the duodenum causes not only ulcer reflux symptoms such as heartburn, but also dysmotility-like symptoms such as epigastric discomfort, belching and abdominal bloating[20]. When Miwa et al[21] infused acid into the stomach of Japanese subjects, they induced a variety of dyspeptic symptoms, with no significant change in acid-reflux-related symptoms such as heartburn and epigastric pain, but a significant increase in dysmotility-like symptoms such as epigastric discomfort, stomach fullness, nausea and belching. Samsom et al[22] have reported that decreased acid clearance and increased sensitivity to acid in patients with dyspepsia may lead to dyspeptic symptoms. There have in fact been a number of recent studies reporting the effectiveness of PPIs[7] and H2RAs[23] in the treatment of FD. Empirical omeprazole therapy induces symptom improvement in a higher proportion of patients with uninvestigated dyspepsia (defined as epigastric pain or discomfort with or without heartburn or regurgitation) than ranitidine or cisapride does, but this effect is more marked in patients with gastroesophageal reflux disease[10]. The prevalence of pathological pH monitoring (4%-6% of time at pH < 4) is significantly higher in FD patients with heartburn than those without[17]. In other words, PPIs are more effective than H2RAs in the treatment of FD associated with heartburn and regurgitation.
It is unclear, however, how important suppression of acid secretion is in Japanese patients, who have a high prevalence of H. pylori infection and low levels of gastric acid secretion[11]. A Japanese clinical trial found that H2RA therapy was more effective than mosapride for treatment of FD[24], suggesting that suppression of gastric acid secretion by an H2RA may be sufficient to treat FD in Japanese patients, with no need for a PPI. In this study, we compared PPI monotherapy with H2RA + Prok combination therapy; the therapy most commonly prescribed by Japanese clinicians[9]. In consideration of the superior acid secretion suppression of PPIs over H2RAs, we combined an H2RA with a Prok, and examined only dysmotility-like symptoms, considered less responsive to PPI therapy.
Our results showed no differences between treatment groups for changes in dysmotility-like dyspepsia symptom score on days 3 and 7 of treatment, but significantly greater symptom improvement was seen in the PPI group than the H2RA + Prok group on days 14 and 28 of treatment. The proportion of subjects reporting that they were satisfied or very satisfied was significantly higher in the PPI group than in the H2RA + Prok group on day 28. PPI therapy was the only significant predictor of treatment response, defined as a total symptom score improvement of ≥ 50%.
In a recent meta-analysis of PPI treatment for FD, the dysmotility-like subgroup did not respond to PPI therapy, unlike the reflux and ulcer-like subgroup[25]. The severity of heartburn at baseline in FD patients influences treatment response to PPI or H2RA therapy[10]. We assessed heartburn symptoms at baseline in a separate study, finding no difference between groups in the pretreatment heartburn symptom score (data not shown). Our results suggest that powerful suppression of acid secretion by PPIs is also important in the treatment of dysmotility-like symptoms in Japanese patients with FD. We saw similar symptom score improvements in both groups until day 7 of treatment. This may represent a placebo response, because it is well known that the placebo response can be substantial in trials of GI disorders, including FD[10]. There have been reports of a placebo effect until day 7 of treatment with a PPI[26], and of the need for a 7-d run-in period to minimize the placebo effect in FD clinical trials[27-29], so a similar placebo response lasting until day 7 of treatment was also possible in this study. The absence of any further improvement in dysmotility-like dyspepsia symptoms between day 7 and days 14 or 28 in the H2RA + Prok group, and the lack of significant change in subject satisfaction rates from day 14 to 28, indicates the development of H2RA tolerance[30].
Stratifying the study sample by H. pylori status showed that significantly better symptom improvement was seen in the PPI group than the H2RA + Prok group in both H. pylori-positive and -negative subjects. No influence of H. pylori status was seen in either treatment group in our study. In the studies by Talley et al[5], no significant difference was seen in the rate of complete symptom resolution after 28 d of treatment between H. pylori-positive and -negative subjects in the ulcer-like, reflux-like, or dysmotility-like symptom subgroups in either treatment arm, although these were only exploratory analyses. Although H. pylori status did not significantly influence the response to omeprazole, this does not exclude the possibility of a small true difference between H. pylori-positive and -negative patients in the effect of acid inhibition on FD. Intragastric pH is higher in patients taking a PPI who are infected with H. pylori[31]. PPI therapy may therefore be more effective in H. pylori-positive patients with NUD, as one study has suggested[28]. Pooling all trial data suggests that symptom responses are similar in H. pylori-positive and -negative patients with NUD. H. pylori status is unlikely to have a clinically important impact on the efficacy of treatments in this patient group.
In this study, symptoms improved over time regardless of H. pylori status in the PPI group, whereas no further symptom improvement was seen in the H2RA + Prok group after day 7 of treatment in both H. pylori-positive and -negative subjects, indicating tolerance to treatment. Loss of the suppressive effect on acid secretion by H2RAs has been reported in H. pylori-negative patients as the duration of treatment increases[32], whereas in this study tolerance was seen from day 7 of treatment in the H2RA + Prok group.
Limitations of this study include the small number of subjects and the fact that it was not a placebo-controlled trial. Consideration of the Japanese medical system tells us that clinical trials of treatments for FD, a condition not covered by medical insurance, conducted with the participation of general medical clinics will be limited in scope. Nevertheless, this is the first published report of a randomized comparative trial of PPI monotherapy and H2RA + Prok combination therapy in the treatment of FD in Japanese subjects.
We demonstrated that, even in Japan with its high proportion of H. pylori-positive patients, PPI monotherapy significantly improves not only ulcer and reflux-like symptoms, but also dysmotility-like symptoms, better than H2RA + Prok combination therapy. In particular, tolerance to H2RA + Prok combination therapy was seen regardless of H. pylori status. The prevalence of H. pylori infection is expected to decline in Japan in the future[33], leading to increased gastric acid secretion, so suppression of acid secretion will likely become even more important. The American College of Gastroenterology guidelines for the management of dyspepsia recommend a PPI as the treatment of first choice in regions with a low prevalence of H. pylori infection, with investigations and other therapies for those who fail to respond[29]. In Japan, where the prevalence of H. pylori infection remains high, upper GI endoscopy is considered mandatory for the exclusion of malignancy. Powerful suppression of acid secretion, such as a PPI provides, is the most effective therapy for treatment of all dyspeptic symptoms in Japanese patients, both ulcer- and reflux-like and dysmotility-like symptoms. Of particular interest is our finding that PPI therapy is useful in the treatment of dysmotility-like symptoms, usually considered less responsive to PPIs. In other words, dysmotility-like symptoms of FD are also an acid-related disorder, for which suppression of acid secretion is the most effective therapy. PPIs are extremely effective in the treatment of all symptoms of FD, and should be the treatment of first choice in Japanese patients with FD.
Many people suffer from functional dyspepsia (FD). The disease has a substantial negative effect on quality of life, therefore, daily care is of the utmost importance. However, because there are many causes of FD, including gastrointestinal motility disorders and hypersensitivity of the digestive tract, gastric acid secretion, inflammation of the mucous membrane of the stomach, nervous system and digestive tract hormone disorders, and psychological factors, no method of treatment has been established.
It has recently been clarified that gastric acid secretion plays a major role in the onset of FD symptoms. In Japan, however, where there are many Helicobacter pylori (H. pylori)-positive patients, epigastric pain, epigastric burning and other acid-related symptoms are treated with acid-secretion blockers. In contrast, dysmotility-like symptoms such as painful stomach heaviness after eating and soon feeling full are mainly treated with prokinetic agents and/or antiulcer agents, and the significance of acid secretion suppression is not clear. For this reason, we conducted a prospective randomized treatment study to examine the importance of acid-suppressing drugs for the treatment of various FD symptoms.
Regardless of whether cases were H. pylori-positive or -negative, the improvement rate for dysmotility-like symptoms as well as ulcer and acid-reflux-related symptoms in a proton pump inhibitor (PPI) single therapy group was significantly better compared to a group concomitantly administered histamine H2 receptor antagonists (H2RAs) and prokinetic drugs (Proks). Logistic analysis (multivariable analysis) of factors related to improvements in dysmotility-like symptoms also showed that PPI therapy was the only significant factor. Patient satisfaction was also significantly higher in the PPI single therapy group than in the H2RA and Prok groups. In other words, in Japan, as well as other countries, regardless of whether a patient is H. pylori-positive or -negative, the importance of acid secretion suppression for the treatment of not only ulcer and acid-reflux-related symptoms but also digestive motility disorder symptoms has been established.
It is thought that the H. pylori infection rate in Japan will gradually decrease. Assuming this will lead to more patients with increased acid secretion, acid secretion suppression will become an increasingly important issue. It has been concluded that, regardless of whether a patient is H. pylori-positive or -negative, PPI therapy is extremely effective as a first-choice treatment for not only ulcer and acid-reflux-related symptoms but also dysmotility-like symptoms, in other words, for all FD symptoms.
In the Rome III classification, FD is defined as “one or more of the chronic symptoms of painful postprandial fullness, early feeling of satiety, epigastric pain and epigastric burning for which no causal organic disease is observed during endoscopic and other examinations”. This definition is divided into the subcategories of epigastric pain syndrome, indicated by the two symptoms of epigastric pain related to dietary intake and epigastric burning related to gastric acid, and postprandial distress syndrome (PDS), indicated by the two so-called dysmotility-like symptoms of early satiation and fullness. Many cases of FD are thought to be due to PDS. In Japan, Proks, drugs that improve gastrointestinal motility, are the first-choice treatment for dysmotility-like symptoms. H. pylori, a bacterium that exists in the mucous membrane of the stomach, is thought to cause stomach and duodenal ulcers, and stomach cancer. In H. pylori-positive patients, the bacterium causes chronic atrophic gastritis, progressing to gastric cancer, and low gastric acid output. The H. pylori-positive rate in Japan is tending to decrease, which could result in more Japanese patients with excessive gastric acid output.
A good paper and can be accepted for publication. Only problem is the number of patients recruited for the study was too low.
Peer reviewer: Andrew Seng Boon Chua, MD, Department of Gastroenterology, Gastro Centre Ipoh, 1, lorong Rani, 31, lebuhraya Tmn Ipoh, Ipoh Garden South, IPOH 30350, Malaysia
S- Editor Shi ZF L- Editor Kerr C E- Editor Zheng XM
| 1. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 2. | Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239-1255. [PubMed] |
| 3. | Choung RS, Talley NJ. Novel mechanisms in functional dyspepsia. World J Gastroenterol. 2006;12:673-677. [PubMed] |
| 4. | Nyrén O, Adami HO, Gustavsson S, Lindgren PG, Lööf L, Nyberg A. The "epigastric distress syndrome". A possible disease entity identified by history and endoscopy in patients with nonulcer dyspepsia. J Clin Gastroenterol. 1987;9:303-309. [PubMed] |
| 5. | Talley NJ, Meineche-Schmidt V, Paré P, Duckworth M, Räisänen P, Pap A, Kordecki H, Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther. 1998;12:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Wong WM, Wong BC, Hung WK, Yee YK, Yip AW, Szeto ML, Fung FM, Tong TS, Lai KC, Hu WH. Double blind, randomised, placebo controlled study of four weeks of lansoprazole for the treatment of functional dyspepsia in Chinese patients. Gut. 2002;51:502-506. [PubMed] |
| 7. | Peura DA, Kovacs TO, Metz DC, Siepman N, Pilmer BL, Talley NJ. Lansoprazole in the treatment of functional dyspepsia: two double-blind, randomized, placebo-controlled trials. Am J Med. 2004;116:740-748. [PubMed] |
| 8. | Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2004;CD001960. [PubMed] |
| 9. | Hongo M, Kanatsuka H, Sugawara A, Nagasaki Y, Endo Y, Karahashi K, Shoji T, Sagami Y, Aoki I. Primary care in the treatment of functional gastrointestinal symptoms in Japan: prescription preferences and impression of results. Aliment Pharmacol Ther. 2005;21 Suppl 2:47-54. [PubMed] |
| 10. | Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, Barkun A, Thomson A, Smyth S, Escobedo S, Lee J, Sinclair P. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488. [PubMed] |
| 11. | Haruma K, Kamada T, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Inoue M, Kishimoto S, Kajiyama G, Miyoshi A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277-283. [PubMed] |
| 12. | Allescher HD, Böckenhoff A, Knapp G, Wienbeck M, Hartung J. Treatment of non-ulcer dyspepsia: a meta-analysis of placebo-controlled prospective studies. Scand J Gastroenterol. 2001;36:934-941. [PubMed] |
| 13. | Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Nishi N, Chayama K, Haruma K. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Thjodleifsson B. Natural history of functional dyspepsia: a 10-year population-based study. Digestion. 2010;81:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Okumura T, Tanno S, Ohhira M, Tanno S. Prevalence of functional dyspepsia in an outpatient clinic with primary care physicians in Japan. J Gastroenterol. 2010;45:187-194. [PubMed] |
| 17. | Tack J, Caenepeel P, Arts J, Lee KJ, Sifrim D, Janssens J. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut. 2005;54:1370-1376. [PubMed] |
| 18. | Laheij RJ, De Koning RW, Horrevorts AM, Rongen RJ, Rossum LG, Witteman EM, Hermsen JT, Jansen JB. Predominant symptom behavior in patients with persistent dyspepsia during treatment. J Clin Gastroenterol. 2004;38:490-495. [PubMed] |
| 19. | Halder SL, Talley NJ. Functional Dyspepsia: A New Rome III Paradigm. Curr Treat Options Gastroenterol. 2007;10:259-272. [PubMed] |
| 20. | Lee KJ, Vos R, Janssens J, Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am J Physiol Gastrointest Liver Physiol. 2004;286:G278-G284. [PubMed] |
| 21. | Miwa H, Nakajima K, Yamaguchi K, Fujimoto K, Veldhuyzen VAN Zanten SJ, Kinoshita Y, Adachi K, Kusunoki H, Haruma K. Generation of dyspeptic symptoms by direct acid infusion into the stomach of healthy Japanese subjects. Aliment Pharmacol Ther. 2007;26:257-264. [PubMed] |
| 22. | Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515-520. [PubMed] |
| 23. | Bytzer P. H(2) receptor antagonists and prokinetics in dyspepsia: a critical review. Gut. 2002;50 Suppl 4:iv58-iv62. [PubMed] |
| 24. | Seno H, Nakase H, Chiba T. Usefulness of famotidine in functional dyspepsia patient treatment: comparison among prokinetic, acid suppression and antianxiety therapies. Aliment Pharmacol Ther. 2005;21 Suppl 2:32-36. [PubMed] |
| 25. | Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337. [PubMed] |
| 26. | van Rensburg C, Berghöfer P, Enns R, Dattani ID, Maritz JF, Gonzalez Carro P, Fischer R, Schwan T. Efficacy and safety of pantoprazole 20 mg once daily treatment in patients with ulcer-like functional dyspepsia. Curr Med Res Opin. 2008;24:2009-2018. [PubMed] |
| 27. | Bolling-Sternevald E, Lauritsen K, Aalykke C, Havelund T, Knudsen T, Unge P, Ekström P, Jaup B, Norrby A, Stubberöd A. Effect of profound acid suppression in functional dyspepsia: a double-blind, randomized, placebo-controlled trial. Scand J Gastroenterol. 2002;37:1395-1402. [PubMed] |
| 28. | Blum AL, Arnold R, Stolte M, Fischer M, Koelz HR. Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group. Gut. 2000;47:473-480. [PubMed] |
| 29. | Talley NJ, Vakil N, Lauritsen K, van Zanten SV, Flook N, Bolling-Sternevald E, Persson T, Björck E, Lind T. Randomized-controlled trial of esomeprazole in functional dyspepsia patients with epigastric pain or burning: does a 1-week trial of acid suppression predict symptom response? Aliment Pharmacol Ther. 2007;26:673-682. [PubMed] |
| 30. | Komazawa Y, Adachi K, Mihara T, Ono M, Kawamura A, Fujishiro H, Kinoshita Y. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:678-682. [PubMed] |
| 31. | Gillen D, Wirz AA, Neithercut WD, Ardill JE, McColl KE. Helicobacter pylori infection potentiates the inhibition of gastric acid secretion by omeprazole. Gut. 1999;44:468-475. [PubMed] |
| 32. | Fujisawa T, Adachi K, Komazawa Y, Mihara T, Azumi T, Katsube T, Furuta K, Kazumori H, Kinoshita Y. Helicobacter pylori infection prevents the occurrence of the tolerance phenomenon of histamine H2 receptor antagonists. Aliment Pharmacol Ther. 2004;20:559-565. [PubMed] |
| 33. | Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094-2099. [PubMed] |