Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1496
Revised: July 7, 2011
Accepted: July 14, 2011
Published online: April 7, 2012
AIM: To define the evolution of ischemic lesions with 7T magnetic resonance imaging (7T-MRI) in an animal model of acute colonic ischemia.
METHODS: Adult Sprague-Dawley rats were divided into two groups. Group I underwent inferior mesenteric artery (IMA) ligation followed by macroscopic observations and histological analysis. In group II, 7T-MRI was performed before and after IMA ligation and followed by histological analysis.
RESULTS: Morphological alterations started to develop 1 h after IMA ligation, when pale areas became evident in the splenic flexure mesentery and progressively worsened up to 8 h thereafter, when the mesentery was less pale, and the splenic flexure loop appeared very dark. The 7T-MRI results reflected these alterations, showing a hyperintense signal in both the intraperitoneal space and the colonic loop wall 1 h after IMA ligation; the latter progressively increased to demonstrate a reduction in the colonic loop lumen at 6 h. Eight hours after IMA ligation, MRI showed a persistent colonic mural hyperintensity associated with a reduction in peritoneal free fluid. The 7T-MRI findings were correlated with histological alterations, varying from an attenuated epithelium with glandular apex lesions at 1 h to coagulative necrosis and loss of the surface epithelium detected 8 h after IMA ligation.
CONCLUSION: MRI may be used as a substitute for invasive procedures in diagnosing and grading acute ischemic colitis, allowing for the early identification of pathological findings.
- Citation: Iacobellis F, Berritto D, Somma F, Cavaliere C, Corona M, Cozzolino S, Fulciniti F, Cappabianca S, Rotondo A, Grassi R. Magnetic resonance imaging: A new tool for diagnosis of acute ischemic colitis? World J Gastroenterol 2012; 18(13): 1496-1501
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1496
Ischemic colitis (IC) was first described by Boley et al[1] and Theodoropoulou et al[2] , and is a relatively common disease, being the most frequent form of intestinal ischemia[3] and the second-most frequent cause of lower gastrointestinal bleeding[4]. IC is the consequence of an acute interruption or chronic decrease in the colonic blood supply[5], which may be either occlusive or non-occlusive in origin[2]. This disease results in ischemic necrosis of variable severity that can range from superficial mucosal involvement to full-thickness transmural necrosis[5,6].
Clinically, IC presents in either a gangrenous (acute fulminant) or non-gangrenous form (acute transient; chronic)[2], with a mortality rate ranging from 10% for the non-occlusive disease to 90% for occlusive mesenteric infarction due to embolus or thrombosis[5]. The left colon is involved in 75% of cases and the right colon in the remaining 25%; the splenic flexure and the sigmoid colon are the areas most frequently affected[3,7].
The presentation of IC is not specific and is highly variable, therefore, its diagnosis largely depends on clinical suspicion[7]. In this context, the role of imaging techniques is controversial. Standard radiology and computed tomography (CT), the latter considered the best diagnostic modality in acute settings[8,9], yield non-specific and late findings, whereas the advantages of magnetic resonance imaging (MRI) are still a matter of debate[5].
The functional and morphological responses to ischemia produced by inferior mesenteric artery (IMA) ligation have been studied in the canine, porcine and rat colon[10-13]. However, to date, nothing is known about the histological evolution of the colonic ischemic injury or the relationship between these lesions and MRI findings. Therefore, the aim of our study was to define the evolution of histological ischemic lesions and to compare anatomopathological features with T2-weighted MRI in an animal model of colonic ischemia.
All procedures performed on animals were approved by our Institutional Animal Care and Use Committee. Nine adult male Sprague-Dawley rats (250-340 g; Harlan Laboratories, Indianapolis, IN, United States) were used in this study. The rats were maintained on a 12/12 h light/dark cycle and allowed free access to food and water. They were anesthetized with ketamine (100 mg/kg i.m.) and medetomidine (0.25 mg/kg i.m.) injections. Butorphanol (0.1 mg/kg s.c.) was used immediately before the intervention to ensure intraoperative analgesia. Further injections of these drugs were provided throughout the intervention to maintain a sufficient state of anesthesia. Each rat was allowed to breathe spontaneously. Body temperature was monitored with a rectal probe and maintained at 37.0 ± 0.5 °C with a heating blanket regulated by a homeothermic blanket control unit (Harvard Apparatus Ltd., Holliston, MA, United States).
After drug injection, eight rats were prepared for surgery through thoracic and abdominal trichotomy. These areas were then washed with povidone iodine and alcohol. These animals were divided at random into two groups of four rats each. Group I rats underwent IMA ligation followed by macroscopic observation and histological analysis. Before and after IMA ligation, group II rats underwent 7-tesla (7T) MRI followed by histological analysis of a colon specimen after euthanasia. A healthy rat underwent an MRI bowel enema without IMA ligation to enable us to study its bowel anatomy.
After a midline laparotomy, the small and large bowel were exposed from the abdominal cavity and displaced to the left. A photograph of the physiological appearance of the bowel and mesentery was taken with a digital camera (Panasonic Lumix DMC-TZ8, 12.0 megapixels, ISO 2000; Osaka, Japan). The IMA was identified and ligated at the origin with Prolene 7/0 and sectioned.
After IMA ligation, the anesthetized rats of group I were observed for 8 h. The bowel was exposed on gauze moistened with saline to prevent excessive evaporative loss. Photographs of the exposed bowel and mesentery were taken every 10 min for the first hour and every 30 min thereafter. At the end of the observation period, all rats were euthanized with an intrapulmonary injection of Tanax (0.5 mL), and the large bowel was excised for histological analysis.
Abdominal 7T-MRI scans were acquired in group II animals (Bruker BioSpec 70/16US; Bruker Medical Systems, Ettlingen, Germany) before IMA ligation to record the physiological appearance of the abdomen. After IMA ligation, the entire intestine was replaced in the abdomen, and the abdominal wall was closed with Vicryl 2/0 thread.
Each rat underwent 7T-MRI at 1 h, 4 h, 6 h and 8 h after IMA ligation as follows: Tripilot sequence, parameters: TR 100.0 ms; TE 6.0 ms; FOV 8.00 cm; IS 2.00 mm; N slice 3 and RareT2 sequence in axial section, parameters: TR 6060.3 ms; TE 36.0 ms; FOV 7.00 cm; matrix 256 × 256; IS 1.00/1.00 mm; N slice 52; acquisition time: 14 min, 32 s, 688 ms. To obtain bowels for histological analysis, selected rats were sacrified at 1 h, 4 h, 6 h and 8 h after 7T-MRI.
For light microscopy, the large bowels in both groups of rats were excised from the cecum to the rectum, including the mesentery, and stored in 10% buffered formalin acetate for at least 2 d. The samples were divided into three segments: the cecum and proximal colon (first segment), the splenic flexure (second segment), and the distal colon and rectum (third segment). Sections 3 mm in size were obtained at 10-mm intervals from each segment and embedded in paraffin. Transverse sections 3 μm thick were cut and stained with hematoxylin-eosin. The sections were mounted on chrome alum/gelatin-coated slides, dehydrated and coverslipped. Slides were imaged with a Zeiss Axio Skope microscope equipped with a high-resolution digital camera (ORCA-HR C4742-95-12HR, 10 MP; Hamamatsu Photonics, Hamamatsu, Japan)
This procedure was performed on one healthy rat to determine the large bowel anatomy and topography. Under anesthesia, a 16 G angiocatheter was inserted in the rectum and fixed to the anal skin by a purse-string suture. After the injection of 8-10 mL warm water, 7T-MRI was performed using the following protocol: Tripilot sequence, parameters: TR 100.0 ms; TE 6.0 ms; FOV 8.00 cm; IS 2.00 mm; N slice 3 and RareT2 sequence in axial section, parameters: TR 6060.3 ms; TE 36.0 ms; FOV 6.00 cm; matrix 256 × 256; IS 1.00/1.00 mm; N slice 44; acquisition time: 14 min, 32 s, 688 ms. These scans served as anatomic images of reference for comparison purposes to identify ischemic damage in group II rats.
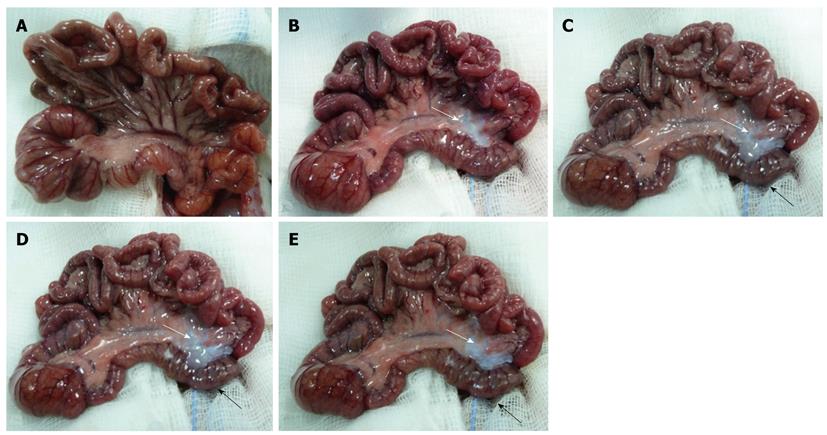
Figure 1A shows the appearance of a rat intestine immediately before IMA ligation; the colon and the ileum were of normal size and presented a uniform serous membrane and rose-colored mesentery. One hour after IMA ligation, pale areas appeared in the splenic flexure mesentery (indicated by a white arrow in Figure 1B); these areas progressively increased (white arrow) and were associated, four hours after IMA ligation, with a change in the color of the splenic flexure loop (black arrow), which appeared dark reddish blue (Figure 1C). Six hours after IMA ligation, the splenic flexure mesentery was very pale (white arrow), and the loop was very dark (black arrow, Figure 1D). Eight hours after IMA ligation, although the loop was even darker (black arrow), the mesentery was less pale (white arrow, Figure 1E). No other macroscopic alterations were noted during the 8 h observation period, and the chronological sequence of the macroscopic morphological changes was the same in all group I animals.
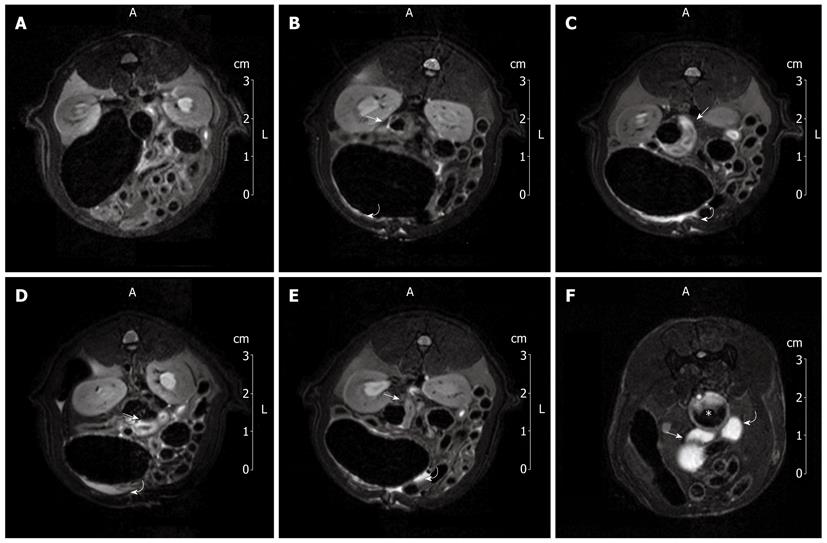
Figure 2A shows a 7T-MRI abdominal scan of a group II rat immediately before IMA ligation. No pathological findings related to ischemic damage were detected. One hour after IMA ligation, the T2 sequences showed minimal findings, namely, hyperintense signals in both the colonic loop wall (arrow) and the intraperitoneal space (curved arrow, Figure 2B). These MRI findings were more pronounced four hours after IMA ligation, suggesting colonic wall edematous thickening and a small amount of peritoneal free fluid (Figure 2C). Six hours after ligation, the amount of peritoneal free fluid increased (curved arrow), as did the colonic wall hyperintensity (arrow, Figure 2D). Eight hours after IMA ligation, MRI showed persistent colonic mural hyperintensity (arrow) associated with a reduction in the peritoneal free fluid (curved arrow), probably related to compensatory intraperitoneal drainage (Figure 2E).
Figure 2F shows the hyperintense signals of the splenic flexure (arrow), descending colon (curved arrow) and rectum (star) distended by instilled water. Comparison of the 7T-MRI and MRI colon enema scans reveals that the splenic flexure was the colonic area most affected by ischemia.
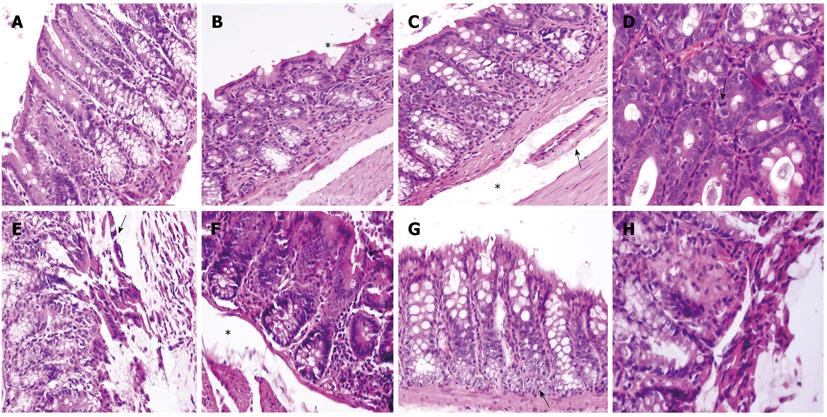
Histology revealed anatomopathological alterations in the second segments (colonic splenic flexure), whereas the first and third segments were unaffected. The histology of the colonic sections obtained one hour after IMA ligation from the proximal colon and the splenic flexure are shown in Figure 3A and B, respectively. In contrast to the normal histological pattern of the proximal colon, the splenic flexure section showed an attenuated epithelium with glandular apex lesions (star). Four hours after IMA ligation, large ischemic lesions appeared at the splenic flexure level: superficial epithelium nuclear pyknosis and epithelium mucin depleted with submucosal edema (star) leading to vessel collapse (arrow, Figure 3C). Six hours after ligation, in the same colonic area, we found a further reduction in crypt goblet cells, clear signs of apoptosis such as nuclear pyknosis and hypereosinophilia, and epithelial regeneration attempts, as shown by the presence of mitotic figures (arrow, Figure 3D). At the end of the observation (8 h), ischemic injury of the splenic flexure was more pronounced; necrosis and a loss of the surface epithelium, crypt drop-out (arrow) with a depletion of goblet cells (Figure 3E) and a markedly edematous submucosa (star, Figure 3F) were observed. Signs of regeneration in response to the injury appeared at the bottom of the crypts (arrow, Figure 3G) near the images of coagulative necrosis (Figure 3H). Ischemic changes were related to vascular anatomy, and a sharp demarcation line often separated the involved from the uninvolved mucosa.
The availability of animal models in which to reproduce human diseases has increased our knowledge of human physiopathology and led to new diagnostic and therapeutic approaches[14]. This study, based on an animal model of colonic ischemic damage, was designed to define the evolution of histological ischemic lesions and to compare anatomopathological features with corresponding 7T-MRI findings. Thus far, the chronological sequence of early colonic ischemic damage has not been described, and the role of MRI is still widely debated. To the best of our knowledge, this is the first study using 7T-MRI for the instrumental evaluation of a human disease reproduced in a rat animal model. We decided to use T2-weighted MRI sequences because, as shown in a previous study[15], they allow us to identify, without using contrast media, the hyperintensity of parietal edema, which is an early sign of intestinal ischemia.
We identified anatomopathological alterations as early as one hour after IMA ligation, namely, a pale mesentery and mild surface epithelium damage at the colonic splenic flexure level. Additionally at one hour after ligation, the MRI study showed a small amount of peritoneal free fluid associated with mild edematous thickening of the colonic splenic flexure wall. Moreover, there was a relevant correlation between the MRI changes observed during the course of bowel ischemia and the pathological damage. Indeed, the morphological and MRI findings showed a progressive and parallel worsening with time up to eight hours when, despite the change in the color of the colonic loop, we observed a mild improvement in mesenteric color associated with a reduction in peritoneal free fluid. Therefore, MRI detected very early signs of colonic ischemia and allowed us to monitor the evolution of the ischemic damage. Consequently, although CT remains a valid diagnostic tool for the visualization of early signs of bowel ischemia and evaluation of colonic ischemic damage[8,9,16], MRI, which does not require the use of a contrast medium or ionizing radiation, can play an important role in the diagnostic and therapeutic management of patients with acute colitis ischemia.
We performed MRI colon enema in a surgically untreated rat to define the large bowel anatomy and topography. We were thus able to identify the specific colonic hyperintensity area detected by MRI after IMA ligation as the colonic splenic flexure. In agreement with a previous study, although the superior mesenteric artery is more important than the IMA in maintaining the cecum and transverse colon perfusion, ligation of the IMA alone produced significant injury in a single, specific colonic area[10]. The major vulnerability of the splenic flexure to ischemic damage may be related to the presence of more limited collateral networks in this region, apparently representing a “watershed” area where the two circulations meet[2]. In this context, the amount of ischemic injury depends on the high variability of the vascular anatomy[7].
The rat model used in this study had several limitations: vascular occlusion was sudden and total, whereas partial occlusion is possible in humans; IMA ligation was performed at the emergence of the vessel, whereas in clinical practice, distal occlusions are also observed; the time window analysis was limited to 8 h, but these patients often reach the hospital a few days after the original occlusive event. However, despite the limitations of the animal model, this study shows that MRI is useful in the diagnosis of acute IC. Moreover, although our MRI scans were performed with a 7T-MRI machine, which is not yet available in clinical practice, both our results and those of previous studies suggest that 7T MRI machines are appropriate for clinical research on humans[17,18].
In conclusion, the assumption of parallels between the experimental colonic ischemic damage in this animal model and humans is reasonable. Our results indicate that MRI allows for the identification of pathological findings of acute IC and their correlation with histopathological features. Therefore, MRI can play a relevant role in the diagnostic management of acute IC and may be substituted for other invasive surgical and endoscopic procedures in diagnosing and grading IC when ischemic injury is suggested. The possibility of assessing the evolution of IC over time and correlating the histological alterations with imaging patterns could result in a more accurate and earlier identification of imaging diagnostic features and thus facilitate more effective treatment.
Ischemic colitis (IC) is a relatively common disease, being the most frequent form of intestinal ischemia and the second-most frequent cause of lower gastrointestinal bleeding. Presentation of IC is not specific and is highly variable, therefore, the diagnosis largely depends on clinical suspicion. In this context, the role of imaging techniques remains controversial.
The role of magnetic resonance imaging (MRI) in the diagnostic management of acute IC is still a matter of debate, and nothing is known about the histological evolution of the acute colonic ischemic injury or the relationship between these lesions and MRI findings.
To be known, this is the first study using 7T-MRI for the instrumental evaluation of a human disease reproduced in a rat animal model. In this study, authors established the chronological evolution of the early functional and morphological responses to ischemia produced by inferior mesenteric artery (IMA) ligation and compared their anatomopathological features with the corresponding 7T-MRI findings.
The possibility of detecting the early signs of colonic ischemic injury with MRI suggests that this technique can play an important role in diagnosing and grading acute IC.
7T-MRI: A non-invasive method of demonstrating internal anatomy based on the principle that atomic nuclei in a strong magnetic field (7 Tesla) absorb pulses of radiofrequency energy and emit them as radio waves that can be reconstructed into computerized images. The concept includes proton-spin tomographic techniques; IC: Inflammation of the colon due to colonic ischemia resulting from alterations in systemic circulation or local vasculature.
This is a good demonstration of the potential use of T2-weighted MRI for detecting edema in IC. In an experimental model, results of MRI are compared with physiological appearance and histology of the bowel at various times after IMA ligation.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
| 1. | Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet. 1963;116:53-60. [PubMed] |
| 2. | Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14:7302-7308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am. 2008;46:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Taourel P, Aufort S, Merigeaud S, Doyon FC, Hoquet MD, Delabrousse E. Imaging of ischemic colitis. Radiol Clin North Am. 2008;46:909-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Stamatakos M, Douzinas E, Stefanaki C, Petropoulou C, Arampatzi H, Safioleas C, Giannopoulos G, Chatziconstantinou C, Xiromeritis C, Safioleas M. Ischemic colitis: surging waves of update. Tohoku J Exp Med. 2009;218:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med. 2003;70:920-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med. 2009;76:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 8. | Angelelli G, Scardapane A, Memeo M, Stabile Ianora AA, Rotondo A. Acute bowel ischemia: CT findings. Eur J Radiol. 2004;50:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Romano S, Romano L, Grassi R. Multidetector row computed tomography findings from ischemia to infarction of the large bowel. Eur J Radiol. 2007;61:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Leung FW, Su KC, Pique JM, Thiefin G, Passaro E, Guth PH. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Dig Dis Sci. 1992;37:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Beuk RJ, Heineman E, Tangelder GJ, Kurvers HA, Bonke HJ, oude Egbrink MG. Effects of different durations of total warm ischemia of the gut on rat mesenteric microcirculation. J Surg Res. 1997;73:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Matthews JG, Parks TG. Ischaemic colitis in the experimental animal. I. Comparison of the effects of acute and subacute vascular occlusion. Gut. 1976;17:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Acosta S, Nilsson TK, Bergqvist D, Björck M. Activation of fibrinolysis and coagulation in non-occlusive intestinal ischaemia in a pig model. Blood Coagul Fibrinolysis. 2004;15:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Grassi R, Cavaliere C, Cozzolino S, Mansi L, Cirillo S, Tedeschi G, Franchi R, Russo P, Cornacchia S, Rotondo A. Small animal imaging facility: new perspectives for the radiologist. Radiol Med. 2009;114:152-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kim MY, Suh CH, Kim ST, Lee JH, Kong K, Lim TH, Suh JS. Magnetic resonance imaging of bowel ischemia induced by ligation of superior mesenteric artery and vein in a cat model. J Comput Assist Tomogr. 2004;28:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology. 1999;211:381-388. |
| 17. | Kraff O, Theysohn JM, Maderwald S, Kokulinsky PC, Dogan Z, Kerem A, Kruszona S, Ladd ME, Gizewski ER, Ladd SC. High-resolution MRI of the human parotid gland and duct at 7 Tesla. Invest Radiol. 2009;44:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kraff O, Bitz AK, Kruszona S, Orzada S, Schaefer LC, Theysohn JM, Maderwald S, Ladd ME, Quick HH. An eight-channel phased array RF coil for spine MR imaging at 7 T. Invest Radiol. 2009;44:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |