Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1485
Revised: October 2, 2011
Accepted: January 18, 2012
Published online: April 7, 2012
AIM: To investigate the possible mechanism by which hepatitis B virus X protein (HBx) mediates apoptosis of HepG2 cells.
METHODS: HBx expression vector pcDNA3.1-X was transfected into HepG2 cells to establish an HBx high-expression cellular model as pcDNA3.1-X transfected group. The pcDNA3.1-X and pSilencer3.1-shHBX (HBx antagonist) were cotransfected into HepG2 cells to establish an HBx low-expression model as RNAi group. Untransfected HepG2 cells and HepG2 cells transfected with negative control plasmid were used as controls. Apoptosis rate, the expression of Fas/FasL signaling pathway-related proteins and the phosphorylation levels of MLK3, MKK7 and JNKs, which are upstream molecules of death receptor pathways and belong to the family of mitogen-activated protein kinases (MAPKs), were measured in each group.
RESULTS: Compared with HepG2 cell group and RNAi group, apoptosis rate, the expression of Fas and FasL proteins, and the activation of MLK3, MKK7 and JNKs were increased in the pcDNA3.1-X transfected group. The activation of JNKs and expression of FasL protein were inhibited in the pcDNA3.1-X transfected group when treated with a known JNK inhibitor, SP600125. When authors treated pcDNA3.1-X transfected group with K252a, a known MLK3 inhibitor, the activation of MLK3, MKK7 and JNKs as well as expression of FasL protein was inhibited. Furthermore, cell apoptosis rate was also significantly declined in the presence of K252a in the pcDNA3.1-X transfected group.
CONCLUSION: HBx can induce HepG2 cell apoptosis via a novel active MLK3-MKK7-JNKs signaling module to upregulate FasL protein expression.
- Citation: Tang RX, Kong FY, Fan BF, Liu XM, You HJ, Zhang P, Zheng KY. HBx activates FasL and mediates HepG2 cell apoptosis through MLK3-MKK7-JNKs signal module. World J Gastroenterol 2012; 18(13): 1485-1495
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1485
Hepatitis B virus (HBV) is one of the major pathogenic causes of primary hepatocellular carcinoma (HCC), among which, HBV X gene is considered as a key gene that plays a critical role in the occurrence and progression of HBV-related HCC[1]. It has been proposed that HBx is a cellular transactivator that may indirectly stimulate a variety of viral and host gene promoters by interacting with transcription factors, including AP-1, ATF/CREB, ERCC, and is involved in several signal transduction pathways, including mitogen-activated protein kinase, Ras-Raf-mitogen-activated protein kinase, and JAK/STAT signaling pathways, therefore HBx affects several cellular processes, such as proliferation and differentiation[2-4]. In contrast to its proliferative effects, HBx also participates in the apoptotic destruction of liver cells during the virus infection. Several mechanisms might be involved in this process: (1) HBx induces cell apoptosis on its own or sensitizes cells to apoptotic stimuli such as tumor necrosis factor α (TNF-α) or UV irradiation[5-8]; (2) HBx increases expression of IL-18 and enhances transcription activity of Egr-2 and Egr-3, which up-regulates FasL expression and induces the apoptosis of hepatic cells by the death receptor pathway[9,10]; (3) HBx may directly not only target to mitochondria to enhance translocation of Bax to mitochondria but also interact with the mitochondrial protein voltage-dependent anion channel (HVDAC3) to induce cell death by causing loss of mitochondrial membrane potential; and (4) HBx also interacts with heat shock protein 60 which is also localized in mitochondria to enhance HBx-mediated apoptosis[11-13].
Cell death signals from the extracellular environment or internal sensors for the cellular response are major constituents of apoptotic machinery. Cell surface death receptors that transmit cell death signals are activated by specific death ligands. It is demonstrated that Fas is one of the best-characterized death receptors. Upon binding of FasL onto Fas, apoptotic signals are subsequently transmitted via death adaptor molecule FADD which can mediate the activation of caspase 8, and active caspase 8 can proteolytically activate downstream effector caspases, such as caspase 3, to trigger apoptosis[14,15]. It is reported that liver cell apoptosis is mediated by Fas[16-18]. Understanding the molecular mechanism responsible for the regulation of Fas and FasL may aid in developing novel therapeutic strategies for HCC.
The mixed lineage kinases (MLKs) are a family of serine/threonine protein kinases that function in a phosphorelay module to control the activity of specific mitogen-activated protein kinases (MAPKs). The family includes three subgroups: MLKs (mixed lineage kinases, including MLK1-4), dual leucine zipper-bearing kinases (DLKs), and Zipper Sterile-a-Motif Kinases (ZAKs). MLKs as mitogen activated protein kinase kinase kinases (MAPKKKs) could activate MKKs, such as MKK4 and/or MKK7, which in turn, activate c-Jun N-terminal kinases (JNKs)[19-21]. Ischemic brain injury studies provide the evidence that the ischemia-stimulating factor can activate MLK3-MKK7-JNKs signaling module to activate death receptor pathway, leading to the neural cell apoptosis[22,23]. It is noteworthy that hepatic cells also express MLK3, MKK7, JNK proteins[24,25]. Therefore, it is of significance to clarify whether the HBx can also activate MLK3-MKK7-JNKs signaling module and induce apoptosis of hepatic cells.
In this study, we demonstrated that HBx induces the apoptosis of hepatic cells depending on activating MLK3-MKK7-JNKs signaling module to upregulate FasL protein expression. On this basis, this study gives a new insight into a better understanding of how HBx mediates apoptosis in hepatocytes, and lays the foundation for further revealing the role of HBx in HBV-related liver oncogenesis and development.
The RPMI 1640 medium, liposome Lipofectamine 2000, and Trizol reagent were obtained from Invitrogen (Carlsbad, CA). Mouse monoclonal anti-HBx antibody was from Chemicon (Temecula, CA). Rabbit polyclonal anti-phospho-MLK3 and rabbit polyclonal anti-phospho-MKK7 were from Cell Signaling Technology (Beverly, MA). Mouse monoclonal anti-phospho-JNKs, rabbit polyclonal anti-Fas, and rabbit polyclonal anti-FasL were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-GAPDH antibody, goat anti-mouse IgG-AP, goat anti-rabbit IgG-AP, BCA Protein Assay Kit, BCIP/NBT Alkaline Phosphatase Color Development Kit, the BeyoECL Plus Western blotting detection System, Caspase3 activity assay kit, Caspase8 activity assay kit, and Hoechst33258 staining solution were from Beyotime Institute of Biotechnology (Jiangsu, China). In situ cell death detection kit was from Roche (Mannheim, Germany). Annexin V/PI apoptosis kit was from Biovision (Mountain View, CA). Primers were synthesized by Shanghai Sangon Biological Engineering Technology and Services (Shanghai, China). TIANScript RT Kit was from TIANGEN Biotech (Beijing, China). SP600125 and K252a were obtained from Sigma (St. Louis, MO). Twenty mmol/L stock solution of SP600125 and 20 μmol/L stock solution of K252a were prepared in dimethyl sulfoxide (DMSO) and stored at -20 °C in the dark. SP600125 and K252a were prepared freshly for each experiment by serial dilution into 0.01% DMSO in RPMI 1640 medium. All other chemicals and reagents were of analytical grade.
Plasmid pcDNA3.1-X containing the full length HBx sequence, was constructed in mammalian expression vector pcDNA3.1 (Invitrogen) as described previously[26].
To construct the expression vector for shRNA targeting HBx, pSilencer3.1-shHBX, two chemically synthesized oligonucleotides encoding HBx specific shRNA with the following sense sequences: 5’-GATCCGGTCTTACATAAGAGGACTTTCAAGAG AAGTCCTCTTATGTAAGACCTTTTTTGGAAA-3’ and antisense sequences: 5’-AGCTTTTCCAAAAAAGGTCTTACATAAGAGGACTTCTCTTGAAAGTCCTCTTAT GTAAGACCG-3’ were annealed and cloned into BamH I-HindIII sites of the linearized pSilencer3.1-H1-nero vector (Ambion, Austin, TX).
Human hepatocarcinoma cell line, HepG2 cell, obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) was cultured in RPMI 1640 medium supplemented with 100 mL/L fetal bovine serum, 2 mmol/mL L-glutamine, 100 μg/mL streptomycin and 100 units/mL penicillin at 37 °C in 5% CO2. When the cell fusion rate reached 80%, HepG2 cells were transfected with negative control plasmid pcDNA3.1, pcDNA3.1-X, cotransfected pcDNA3.1-X with either pSilencer3.1-shHBX or pSilencer3.1-H1 in a ratio of 1:3, in the presence of the liposome Lipofectamine 2000 according to the manufacturer’s instructions.
The total RNA of HepG2 cells transfected with various plasmids was prepared with Trizol reagent according to the manufacturer’s instructions. The reverse transcription was performed with TIANScript RT Kit. The specific primers used are shown in Table 1, the amplification condition was 94 °C for 45 s, 58 °C (55 °C-60 °C) for 35s, 72 °C for 1 min for 35 cycles and a final extension at 72 °C for 5 min each. The PCR products were subjected to electrophoresis in 1% agarose gel and visualized by ethidium bromide staining.
| Gene | Sense | Antisense | Product length (bp) |
| HBX | 5’-TGTGAAGCTTATGGCTGCTAGGC-3’ | 5’-TGTGGAATTCTTAGGCAGAGGTG-3’ | 465 |
| Fas | 5’-GTGAACACTGTGACCCTT-3’ | 5’-TCATTGACACCATTCTTTCG-3’ | 349 |
| FasL | 5'-CTGGGGATGTTTCAGCTCTTC-3’ | 5'-CTTCACTCCAGAAAGCAGGAC-3' | 304 |
| Bax | 5’-TTTGCTTCAGGGTTTCATCC-3’ | 5’-CAGTTGAAGTTGCCGTCAGA-3’ | 246 |
| BcL-2 | 5’-GTGGAGGAGCTCTTCAGGGA-3’ | 5’-AGGCACCCAGGGTGATGCAA-3 | 304 |
| β-actin | 5’-GGCATCGTGATGGACTCCG-3’ | 5’-GCTGGAAGGTGGACAGCGA-3 | 607 |
For protein extracts, cells were lysed using cell lysis buffer [20 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 1% TritonX-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 1% Na3VO4, 0.5 μg/mL leupeptin and other phosphatase inhibitors, 1 mmol/L phenylmethanesulfonyl fluoride (PMSF)]. The lysates were collected, and centrifuged at 10 000 ×g at 4 °C for 5 min. The bicinchoninic acid (BCA) Protein Assay Kit was used to measure the protein concentrations. Total protein of 100 μg of each above lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes, which were blocked with 3% bovine serum albumin (BSA) in tris buffered saline (TBS) containing 0.01% Tween-20 for 3 h at room temperature, and then incubated with specific primary antibodies: mouse monoclonal anti-HBx antibody (1:250), rabbit polyclonal anti-phospho-MLK3 (1:500), rabbit polyclonal anti-phospho-MKK7(1:500), mouse monoclonal anti-phospho-JNKs (1:400), rabbit polyclonal anti-Fas (1:500), rabbit polyclonal anti-FasL (1:500), and mouse monoclonal anti-GAPDH antibody (1:500), respectively, overnight at 4 °C. The membranes were then incubated with goat anti-mouse IgG-AP (1:500), goat anti-rabbit IgG-AP (1:500), goat anti-mouse IgG-HRP (1:2000) and goat anti-rabbit IgG-HRP (1:2000) for 2 h at room temperature separately. The labeled bands were detected with NBT/BCIP Alkaline Phosphatase Color Development Kit or the BeyoECL Plus Western blotting detection system.
The enzyme activities of caspase3, caspase8 and caspase9 were quantified using caspase3, caspase8 and caspase9 activity assay kit, respectively. Adherent and floating cells were collected and lysed in caspase lysis buffer. Caspase3 enzyme activity in 30 μg cell lysate was measured by cleavage of Ac-DEVD-pNA colorimetric substrate. Caspase8 enzyme activity in 30 μg cell lysate was measured by cleavage of Ac-IETD-pNA colorimetric substrate. Caspase9 enzyme activity in 30 μg cell lysate was measured by cleavage of Ac-LEHD-pNA colorimetric substrate. The absorbance at A405 nm was quantified in a microtiter plate reader after incubated at 37 °C for 2 h.
Cells were adjusted to a density of 2 × 105 cells/mL, added to 24-well plates in 0.5 mL each well. After transfection and incubation for 72 h, cell apoptosis was analyzed by three methods: (1) terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining: cell apoptosis was analyzed using the in situ cell death detection kit, according to the manufacturer’s protocol. The number of TUNEL-positive cells was divided by the total number of cells to determine the ratio of TUNEL-positive cells. Five optical fields, about 200 cells were selected randomly and analyzed; (2) Hoechst 33258 staining: cells were fixed with 4% paraformaldehyde, washed twice with phosphate-buffered saline (PBS) and stained with Hoechst 33258 staining solution according to the manufacturer’s instructions. The morphologic changes of apoptotic cells, including reduction in volume and nuclear chromatin condensation, were observed under a fluorescence microscope. Five optical fields containing about 200 cells were selected randomly and analyzed; and (3) flow cytometry: cell apoptosis was evaluated by double staining with fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI) using annexin V/PI apoptosis kit. Cells were washed with PBS twice and stained with Annexin V and PI for 5 min at room temperature in the dark. The level of apoptosis was determined by measuring the fluorescence of the cells with a flow cytometer (Becton-Dickinson, San Diego, CA).
All experiments were performed three times. Semiquantitative analysis of the bands was performed with the Image J analysis software (Version 1.30v, Wayne Rasband, NIH, United States). The data were presented in mean ± SD and analyzed by one-way ANOVA (SPSS version 13.0). P < 0.05 was considered statistically significant.
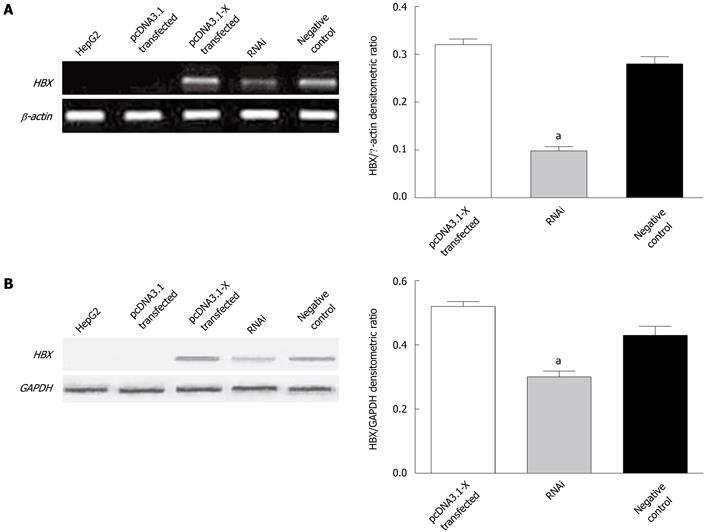
To investigate the potential apoptotic ability of HBx, HBx expressing plasmid pcDNA3.1-X was transiently transfected into a human HCC cell line, HepG2. RT-PCR and Western blotting analysis demonstrated that HepG2 cells transfected with pcDNA3.1-X could steadily express HBx. To further identify the function of HBx upon apoptosis of HepG2, the expression vector for shRNA targeting HBx named pSilencer3.1-shHBX was constructed. When the shRNA expression plasmid was transfected to HepG2 cells in combination with pcDNA3.1-X, the expression of HBx was specifically inhibited by shRNA against HBx, while universal negative control plasmid pSilencer3.1-H1 did not display any effect on HBx expression (Figure 1).
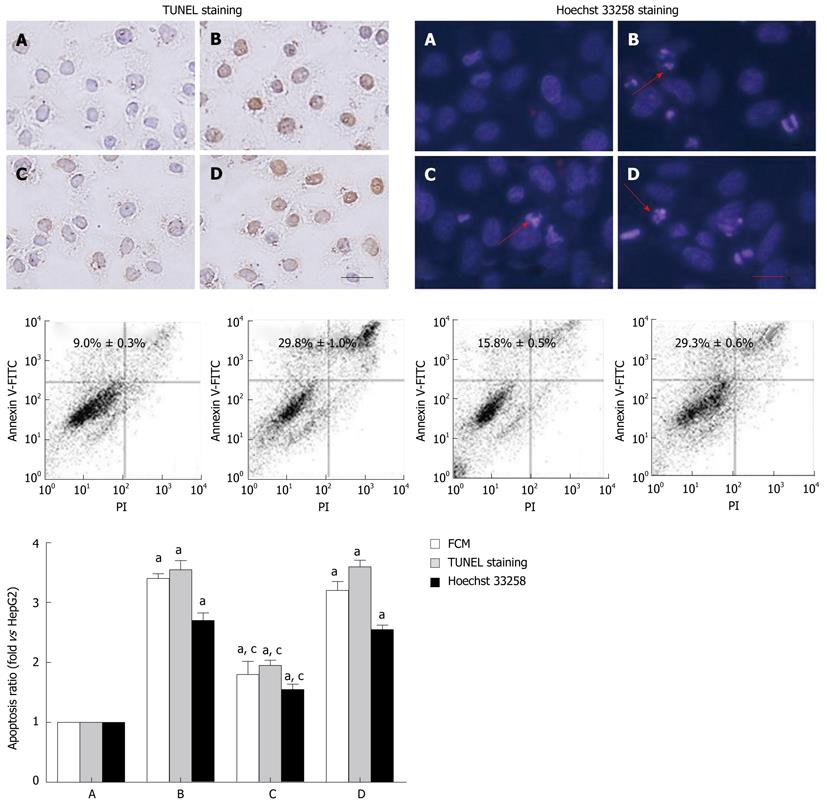
To investigate the roles of HBx in cell apoptosis, TUNEL assay was used to detect the presence of DNA strand breaks. The in situ TUNEL staining showed that the TUNEL-negative cells appeared with blue nucleus and TUNEL-positive cells with yellow nucleus. To further confirm the occurrence of apoptosis, the apoptosis of HepG2 cells and HepG2 transfected with HBx were detected by Hoechst 33258 staining and flow cytometry (FCM). In Hoechst 33258 staining, reduced cell sizes and increased nuclear chromatin condensation were detected in the apoptotic cells. As it is shown in Figure 2, when compared with HepG2 cells group, the apoptosis rate of pcDNA3.1-X transfected group and negative control group was increased, while, in contrast, that of RNAi group was decreased. It indicated that the high expression of HBx could promote the apoptosis of HepG2 cells, and inhibiting the expression of HBx protein could reduce the apoptosis rate of HepG2 cells.
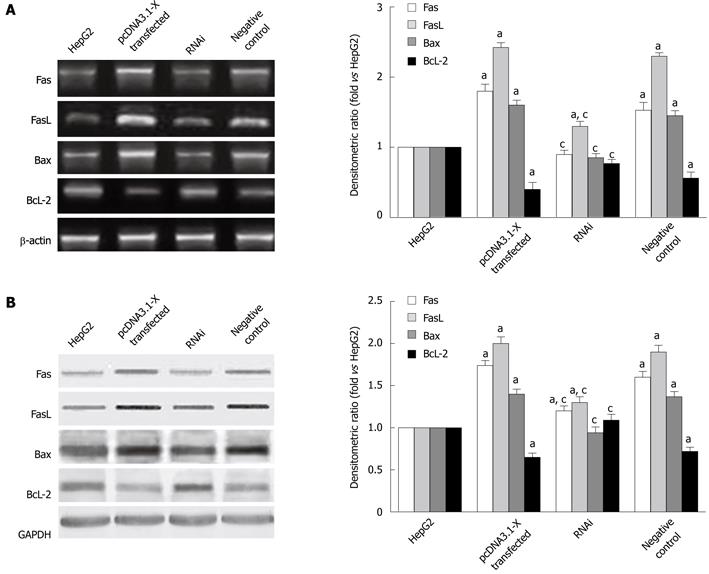
To determine which signaling pathway was involved in cell apoptosis by HBx, expression of Fas, FasL and apoptotic regulators Bax, Bcl-2 in HBx-transfected HepG2 cells were examined by RT-PCR and Western blotting analysis. The results showed that the Fas, FasL, Bax mRNA and protein expression were increased induced by HBx, while the Bcl-2 mRNA and protein were decreased after transfection with HBx in HepG2 cells for 48 h (Figure 3). When HepG2 cells were cotransfected with pcDNA3.1-X and pSilencer3.1-shHBX for 48 h, the levels of Fas, FasL, Bax mRNA and protein were decreased. However, Bcl-2 mRNA and protein were increased after cotransfection with pcDNA3.1-X and pSilencer3.1-shHBX in HepG2 cells.
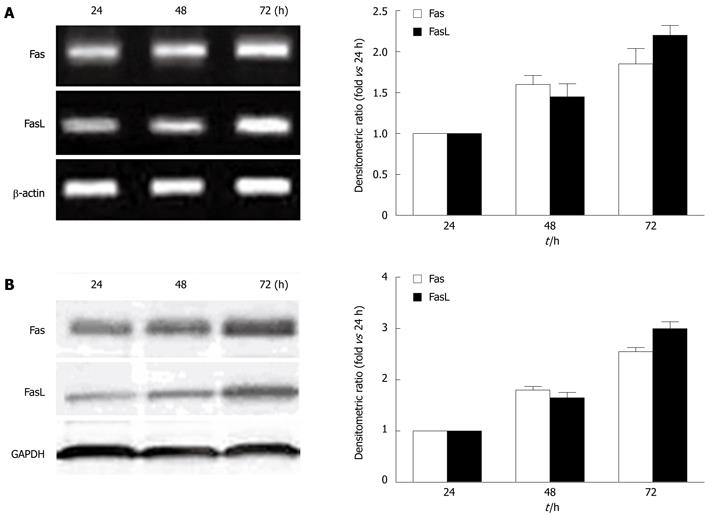
In order to determine the dynamic effect of HBx on the Fas/FasL signaling pathway, we detected the gene and protein expression of Fas and FasL at 24 h, 48 h, and 72 h after transfection with HBx, respectively, and found that the Fas and FasL mRNA expression was increased by HBx in a time-dependent manner (Figure 4A). In parallel, the levels of Fas and FasL proteins in pcDNA3.1-X transfected cells were increased by HBx in a similar manner (Figure 4B).
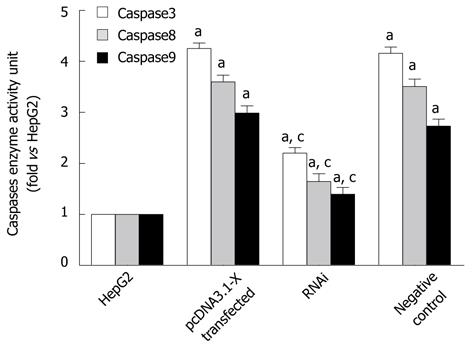
To further determine the effect of HBx on the Fas/FasL signaling pathway, enzyme activity of caspase8, caspase9 and caspase3 were also detected by spectrophotometric test. The results showed that the activation of caspase8, caspase9 and caspase3 was increased in HBx-transfected HepG2 cells (Figure 5).
It could be concluded that HBx could mediate cell apoptosis by upregulating Fas/FasL signaling pathway-related protein expression to activate the Fas/FasL signaling pathway.
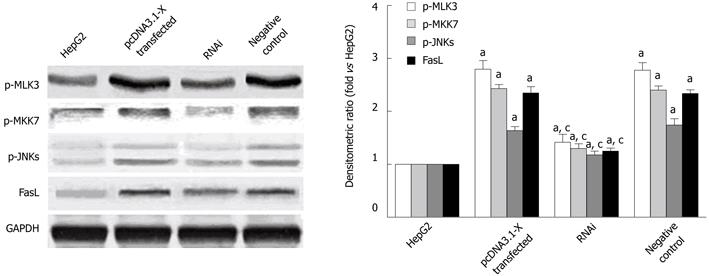
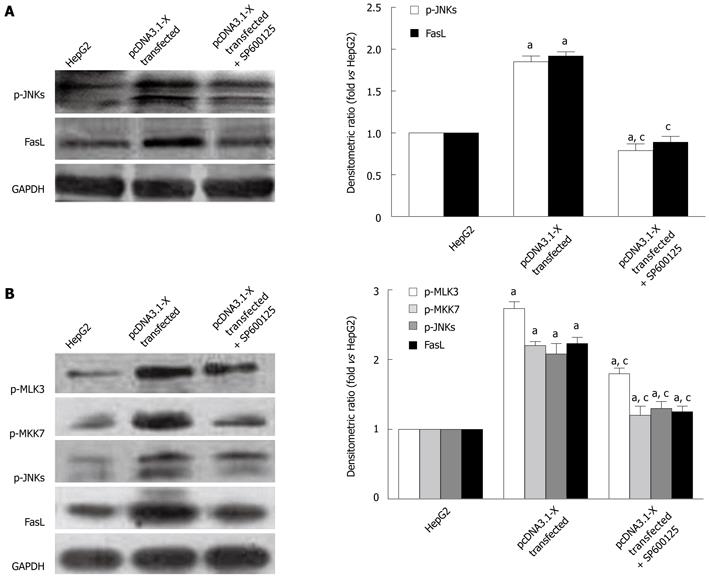
The upstream mechanisms of HBx on expression of Fas/FasL signaling pathway-related proteins were further investigated. Previous studies showed that MLK3, MKK7 and JNKs which belong to MAPK signaling pathways, could form MLK3-MKK7-JNKs signaling module and the activation of the signaling module could upregulate the expression of Fas/FasL signaling pathway-related protein, leading to cell apoptosis[22,23]. To find out whether the upregulation of Fas/FasL signaling pathway-related proteins induced by HBx was dependent on activation of MLK3-MKK7-JNKs signaling module, the phosphorylation levels of MLK3, MKK7 and JNKs were detected. The phosphorylation levels of MLK3, MKK7 and JNKs proteins in pcDNA3.1-X transfected group were increased remarkably compared with the HepG2 cells. When silencing HBx protein expressed by pSilencer3.1-shHBX, phosphorylation of MLK3, MKK7 and JNKs was inhibited (Figure 6). The changing tendency of expression level of FasL protein was consistent with phosphorylation levels of MLK3, MKK7 and JNKs proteins. When we treated HepG2 cells transfected with HBx with a known JNK inhibitor, SP600125, the activation of JNKs and expression of FasL protein were inhibited (Figure 7A). Furthermore, the phosphorylation levels of MLK3, MKK7 and JNKs and the protein expression of FasL also concomitantly decreased in pcDNA3.1-X transfected group when treated with K252a, a known MLK3 inhibitor[27,28] (Figure 7B). The results demonstrated that HBx could activate MLK3-MKK7-JNKs signal module and upregulate Fas/FasL death receptor pathway-related protein expression.
To determine the relationship between activation of MLK3-MKK7-JNKs signal module and HBx-stimulated cell apoptosis, the apoptosis rate of the pcDNA3.1-X transfected group treated with K252a or not was determined by flow cytometry. As expected, apoptosis rate of the HBx-transfected HepG2 cells was suppressed in the presence of the MLK3 inhibitor (Figure 8), indicating that HBx could activate the MLK3-MKK7-JNKs signaling modular and its downstream death receptor pathway, and induce the apoptosis of the hepatocarcinoma cell line.
HBx protein has been reported to be either a promoter or an inhibitor of cell apoptosis[11,29-31]. The dual activity of HBx protein on cell apoptosis suggests that the expression of HBx gene and its physiological role depend on cellular environments and infection stage[30,32-33]. Naturally, HBx shows extremely low levels of expression during the early stage of HBV infection, which may contribute to the activation of transcription and virus replication[33,34]. With the development of chronic HBV infection, expression of HBx protein increased and activated apoptosis to contribute to virus spread and the progression of chronic hepatitis and HCC[32,35,36]. It was reported that HBx could induce the apoptosis of hepatic cells by the death receptor pathway and the mitochondrial pathway. However, the question how HBx activates the death receptor pathway to induce the apoptosis of hepatic cells remains unclear. The further elucidation of signaling pathways that regulate apoptosis by HBx would help us understand the effectiveness of HBX in the development of HCC.
In this study, the regulation mechanism of apoptosis by HBx in HepG2 cells was investigated. First, the ability of HBx to induce apoptosis using transient transfection of HBx was tested. As a result, after transfected with HBx, HepG2 cells exhibited more conspicuous apoptotic nuclear condensation and a higher level of cell death, which is in agreement with the previous reports that HBx could induce cell apoptosis on its own[5-6,37,38]. Second, the expression level of the Fas/FasL signaling pathway-related proteins was examined in HepG2 cells. The expression of Fas/FasL signaling pathway-related proteins were remarkably upregulated in pcDNA3.1-X transfected group. RNA interference targeting HBx in HepG2 cells could reduce the cell apoptosis and down-upregulate the Fas/FasL signaling pathway-related proteins at the same time. Based on the above results, it was reasonable to conclude that HBx is able to induce apoptosis by upregulating Fas/FasL signaling pathway-related protein expression in HepG2 cells, which was similar to the previous report that HBx played a role in inducing apoptosis of hepatocyte via Fas/FasL system[39,40].
Recent studies have found that the MAPKs were involved in the signal transduction for apoptosis. The animal model experiments for cerebral ischemia demonstrated that MLK3-MKK7-JNKs signaling modules, a member of the MAPK family, could activate its downstream Fas/FasL signaling pathway and induce the apoptosis of nerve cells[22-23]. In addition, it was reported that hepatic cells could also express MLK3, MKK7 and JNKs[24-25]. We supposed HBx protein could act as the stressor to activate the signal module of MLK3-MKK7-JNKs, and to induce the apoptosis of the hepatic cells.
On the basis of this hypothesis, the phosphorylation levels of MLK3, MKK7 and JNKs were detected in all groups. It was found that the phosphorylation levels of MLK3, MKK7 and JNKs were increased obviously in the pcDNA3.1-X group, indicating that the HBx might increase the phosphorylation levels of these proteins. When we treated pcDNA3.1-X group with SP600125, the activation of JNKs and expression of FasL were significantly inhibited. Moreover, when pcDNA3.1-X group was treated with K252a, the expression levels of p-MLK3, p-MKK7, p-JNKs and FasL were also significantly decreased, and then cell apoptosis rate obviously declined, suggesting that the HBx protein could act as a stressor and activate the MLK3-MKK7-JNKs signaling module and its downstream Fas/FasL death receptor pathway to induce the apoptosis of HepG2 cells. This would provide us a new research target and a novel concept for blocking the HBx-induced apoptosis process.
The ratio of Bax and Bcl-2 could change the mitochondrial membrane potential and result in the release of cytochrome C, the activation of caspase9, and then further activate caspase3 to induce apoptosis[41,42]. As shown in Figures 3 and 5, HBx up-regulated the expression of Bax, down-regulated the expression of BcL-2, and increased the activation of caspase9 at the same time. This data indicated that the apoptosis induced by HBx also referred to the mitochondrial pathway. Nevertheless, effects of activating the mitochondrial pathway on apoptosis needs to be further investigated.
In conclusion, this study demonstrated that the high expression level of HBx protein could activate MLK3-MKK7-JNKs module and upregulate FasL protein. It has provided a novel insight into the mechanism of HBx-induced heptocarcinoma cell apoptosis.
We thank Dr. Wei-Dong Du from University Clinic Ulm, Ulm, Germany for proof- reading the article.
Hepatitis B virus (HBV) infection is one of the most widely spread viral diseases and strongly associated with the development of hepatocellular carcinoma (HCC). However, the mechanism of HBV-mediated HCC development is not clearly elucidated. Although it has been shown that HBx, the protein encoded by the X gene of the HBV genome, could induce cell apoptosis on its own or sensitize cells to apoptotic stimuli, such as tumor necrosis factor α (TNF-α) or UV irradiation, the mechanism of HBx-mediated apoptosis remains controversial.
HBx, the protein encoded by the X gene of the HBV genome, is a multifunctional regulatory protein and has been implicated in HBV-mediated hepatocarcinogenesis. In this study, the authors further investigated the regulation mechanism of apoptosis mediated by HBx in HepG2 cells.
Previous researches showed that HBx participated in the apoptotic destruction of liver cells during the virus infection. Although some studies found that HBx could mediate apoptosis by the death receptor pathway or affecting mitochondrial physiology, the mechanism of HBx-mediated apoptosis is still not clear. In the present study, the authors showed that HBx could induce HepG2 cells apoptosis via a novel active MLK3-MKK7-JNKs signaling module and upregulate Fas/FasL signaling pathway-related protein expression.
This study provided a new insight into a better understanding of how HBx mediated apoptosis, and lay the foundation for further clarifying the role of HBx in HBV-related liver oncogenesis and development.
Apoptosis is a common form of cell death and often referred to as programmed death. The characteristics of apoptosis include activation of cysteine proteases (caspases) and endonucleases, condensation of the nuclear chromatin and cytoplasm, cleavage of the DNA into oligonucleosomal fragments, and segmentation of the dying cell into membrane-bound apoptotic bodies. Fas is one of the best-characterized death receptor. Upon binding of FasL (Fas ligand) onto Fas, apoptotic signals are subsequently transmitted in cytoplasm to trigger apoptosis. The mixed lineage kinase 3 (MLK3) is a member of the mixed lineage kinases (MLKs), a family of serine/threonine protein kinases functioning in a phosphorelay module to control the activity of specific mitogen-activated protein kinases (MAPKs).
In this manuscript, the authors demonstrate that hepatitis B virus X protein induced apoptosis through Fas/FasL signaling via MLK3-MKK7-JNKs signaling module in HepG2 cells. A series of experiments are well-planned and well-performed and this manuscript is well written. Although interesting, this manuscript could be strengthened if several points were addressed.
Peer reviewer: Kotaro Miyake, MD, PhD, Department of Surgery, Institute of Health Biosciences, The University of Tokushima Graduate School, 3-18-15 Kuramoto, Tokushima 770-8503, Japan
S- Editor Shi ZF L- Editor Ma JY E- Editor Zhang DN
| 1. | Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 2. | Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725-12734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Miao J, Chen GG, Chun SY, Lai PP. Hepatitis B virus X protein induces apoptosis in hepatoma cells through inhibiting Bcl-xL expression. Cancer Lett. 2006;236:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Pollicino T, Terradillos O, Lecoeur H, Gougeon ML, Buendia MA. Pro-apoptotic effect of the hepatitis B virus X gene. Biomed Pharmacother. 1998;52:363-368. [PubMed] |
| 7. | Kim WH, Hong F, Jaruga B, Zhang ZS, Fan SJ, Liang TJ, Gao B. Hepatitis B virus X protein sensitizes primary mouse hepatocytes to ethanol- and TNF-alpha-induced apoptosis by a caspase-3-dependent mechanism. Cell Mol Immunol. 2005;2:40-48. [PubMed] |
| 8. | Kim SY, Kim JK, Kim HJ, Ahn JK. Hepatitis B virus X protein sensitizes UV-induced apoptosis by transcriptional transactivation of Fas ligand gene expression. IUBMB Life. 2005;57:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lee MO, Choi YH, Shin EC, Kang HJ, Kim YM, Jeong SY, Seong JK, Yu DY, Cho H, Park JH. Hepatitis B virus X protein induced expression of interleukin 18 (IL-18): a potential mechanism for liver injury caused by hepatitis B virus (HBV) infection. J Hepatol. 2002;37:380-386. [PubMed] |
| 10. | Yoo YG, Lee MO. Hepatitis B virus X protein induces expression of Fas ligand gene through enhancing transcriptional activity of early growth response factor. J Biol Chem. 2004;279:36242-36249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kim HJ, Kim SY, Kim J, Lee H, Choi M, Kim JK, Ahn JK. Hepatitis B virus X protein induces apoptosis by enhancing translocation of Bax to mitochondria. IUBMB Life. 2008;60:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74:2840-2846. [PubMed] |
| 13. | Tanaka Y, Kanai F, Kawakami T, Tateishi K, Ijichi H, Kawabe T, Arakawa Y, Kawakami T, Nishimura T, Shirakata Y. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem Biophys Res Commun. 2004;318:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 15. | Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 712] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 16. | Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin Liver Dis. 2007;27:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, Kassahn D, Torgler R, Mueller C, Schneider P. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 19. | Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 463] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 20. | Handley ME, Rasaiyaah J, Chain BM, Katz DR. Mixed lineage kinases (MLKs): a role in dendritic cells, inflammation and immunity? Int J Exp Pathol. 2007;88:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Liu XM, Pei DS, Guan QH, Sun YF, Wang XT, Zhang QX, Zhang GY. Neuroprotection of Tat-GluR6-9c against neuronal death induced by kainate in rat hippocampus via nuclear and non-nuclear pathways. J Biol Chem. 2006;281:17432-17445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Pei DS, Wang XT, Liu Y, Sun YF, Guan QH, Wang W, Yan JZ, Zong YY, Xu TL, Zhang GY. Neuroprotection against ischaemic brain injury by a GluR6-9c peptide containing the TAT protein transduction sequence. Brain. 2006;129:465-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Kim KY, Kim BC, Xu Z, Kim SJ. Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-beta-induced apoptosis in hepatoma cells. J Biol Chem. 2004;279:29478-29484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Wada T, Joza N, Cheng HY, Sasaki T, Kozieradzki I, Bachmaier K, Katada T, Schreiber M, Wagner EF, Nishina H. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat Cell Biol. 2004;6:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | You HJ, Pei DS, Sun W. Cloning of human HBx-cDNA and construction of the eukaryotic expression vector. Acta Academiae Medicinae Xuzhou. 2006;3:191-193. |
| 27. | Pan J, Wang G, Yang HQ, Hong Z, Xiao Q, Ren RJ, Zhou HY, Bai L, Chen SD. K252a prevents nigral dopaminergic cell death induced by 6-hydroxydopamine through inhibition of both mixed-lineage kinase 3/c-Jun NH2-terminal kinase 3 (JNK3) and apoptosis-inducing kinase 1/JNK3 signaling pathways. Mol Pharmacol. 2007;72:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Roux PP, Dorval G, Boudreau M, Angers-Loustau A, Morris SJ, Makkerh J, Barker PA. K252a and CEP1347 are neuroprotective compounds that inhibit mixed-lineage kinase-3 and induce activation of Akt and ERK. J Biol Chem. 2002;277:49473-49480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Diao J, Khine AA, Sarangi F, Hsu E, Iorio C, Tibbles LA, Woodgett JR, Penninger J, Richardson CD. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276:8328-8340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Kim H, Lee H, Yun Y. X-gene product of hepatitis B virus induces apoptosis in liver cells. J Biol Chem. 1998;273:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Stürzbecher HW, Hoeijmakers JH, Harris CC. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55:6012-6016. [PubMed] |
| 32. | Jin YM, Yun C, Park C, Wang HJ, Cho H. Expression of hepatitis B virus X protein is closely correlated with the high periportal inflammatory activity of liver diseases. J Viral Hepat. 2001;8:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Moriarty AM, Alexander H, Lerner RA, Thornton GB. Antibodies to peptides detect new hepatitis B antigen: serological correlation with hepatocellular carcinoma. Science. 1985;227:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Antonucci TK, Rutter WJ. Hepatitis B virus (HBV) promoters are regulated by the HBV enhancer in a tissue-specific manner. J Virol. 1989;63:579-583. [PubMed] |
| 35. | Hwang GY, Lin CY, Huang LM, Wang YH, Wang JC, Hsu CT, Yang SS, Wu CC. Detection of the hepatitis B virus X protein (HBx) antigen and anti-HBx antibodies in cases of human hepatocellular carcinoma. J Clin Microbiol. 2003;41:5598-5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Wang WL, London WT, Lega L, Feitelson MA. HBxAg in the liver from carrier patients with chronic hepatitis and cirrhosis. Hepatology. 1991;14:29-37. [PubMed] |
| 37. | Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071-22078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965-6973. [PubMed] |
| 39. | Zhang SJ, Chen HY, Chen ZX, Wang XZ. Possible mechanism for hepatitis B virus X gene to induce apoptosis of hepatocytes. World J Gastroenterol. 2005;11:4351-4356. [PubMed] |
| 40. | Lin N, Chen HY, Li D, Zhang SJ, Cheng ZX, Wang XZ. Apoptosis and its pathway in X gene-transfected HepG2 cells. World J Gastroenterol. 2005;11:4326-4331. [PubMed] |
| 41. | Lok J, Martin LJ. Rapid subcellular redistribution of Bax precedes caspase-3 and endonuclease activation during excitotoxic neuronal apoptosis in rat brain. J Neurotrauma. 2002;19:815-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Perfettini JL, Reed JC, Israël N, Martinou JC, Dautry-Varsat A, Ojcius DM. Role of Bcl-2 family members in caspase-independent apoptosis during Chlamydia infection. Infect Immun. 2002;70:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |