Published online Mar 28, 2012. doi: 10.3748/wjg.v18.i12.1379
Revised: September 26, 2011
Accepted: December 31, 2011
Published online: March 28, 2012
AIM: To examine the significance of branched-chain amino acid (BCAA) treatment before transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma (HCC).
METHODS: This study included 99 patients who underwent TACE therapy for HCC at our hospital and were followed up without treatment for at least 6 mo between January 2004 and January 2010. They were divided into 2 groups: those receiving BCAA granules (n = 40) or regular diet (n = 59, control). Data obtained were retrospectively analyzed (prior to TACE, and 1 wk, 1, 3, and 6 mo after TACE) in terms of nutritional condition and clinical laboratory parameters (serum albumin level and Child-Pugh score), both of which are determinants of hepatic functional reserve.
RESULTS: The BCAA group comprised 27 males and 13 females with a mean age of 69.9 ± 8.8 years. The patients of the BCAA group were classified as follows: Child-Pugh A/B/C in 22/15/3 patients, and Stage II/III/IVA HCC in 12/23/5 patients, respectively. The control group comprised 32 males and 27 females with a mean age of 73.2 ± 10.1 years. In the control group, 9 patients had chronic hepatitis, Child-Pugh A/B/C in 39/10/1 patients, and StageI/II/III/IVA HCC in 1/11/35/12 patients, respectively. Overall, both serum albumin level and Child-Pugh score improved significantly in the BCAA group as compared with the control 3 and 6 mo after TACE (P < 0.05). Further analysis was performed by the following categorization: (1) child-Pugh classification; (2) liver cirrhosis subgroup with a serum albumin level > 3.5 g/dL; and (3) epirubicin dose. A similar trend indicating a significant improvement of all variables in the BCAA group was noted (P < 0.05).
CONCLUSION: Treatment with BCAA granules in patients who have undergone TACE for HCC is considered useful to maintain their hepatic functional reserve.
- Citation: Nishikawa H, Osaki Y, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S, Kita R, Kimura T. Branched-chain amino acid treatment before transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2012; 18(12): 1379-1384
- URL: https://www.wjgnet.com/1007-9327/full/v18/i12/1379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i12.1379
Hepatocellular carcinoma (HCC) is the most common carcinoma worldwide[1]. Treatment for HCC varies depending on the disease stage and liver function, and includes radiofrequency ablation, percutaneous ethanol injection therapy, hepatic resection, liver transplantation, transcatheter arterial chemoembolization (TACE), and molecular target therapy[2-4].
TACE is a procedure whereby an embolizing agent is injected into the hepatic artery to deprive the tumor of its major nutrient source via embolization of the nutrient artery, resulting in ischemic necrosis of the tumor. Hepatic arterial embolization, which had been used until early in the 1990s, is divided into two treatment methods: injection of an embolizing agent after intra-arterial injection of an anticancer drug and intra-arterial injection of a mixture of an embolizing agent and an anticancer drug[5,6]. Subsequently, it was revealed that an oil contrast medium or iodized oil (Lipiodol) accumulates within the tumor after injection. This led to introduction of TACE, in which an embolizing agent is injected after injection of a mixture of Lipiodol and an anticancer drug (Lipiodol emulsion)[7,8]. Until the middle of the 1990s TACE had been performed in a large majority of patients with unresectable HCC. With the subsequent introduction of local treatment, however, TACE is now mainly indicated for treatment of an HCC measuring 3 to 5 cm in diameter or treatment of 4 or more HCCs less than 3 cm in diameter that are both unresectable and not indicated for local treatment.
Takayasu et al[9] reported that independent prognostic factors in relation to survival in patients who underwent TACE include (1) degree of hepatic damage; (2) tumor staging; and (3) serum α-fetoprotein level, and recommended TACE, which can sufficiently maintain the volume ratio of a chemoembolized tumorous liver to the entire tumor-free liver as well as of residual hepatic functional reserve, while emphasizing the importance of maintenance of hepatic functional reserve in these patients.
Branched-chain amino acids (BCAAs) are three amino acids possessing branched side chains (i.e., valine, leucine, and isoleucine). Patients with liver cirrhosis are known to have decreased plasma BCAA levels, which can lead to protein-energy malnutrition (PEM). PEM is associated with a high morbidity and mortality due to an increased risk of life-threatening complications, resulting in poor survival and quality of life (QoL)[10].
A considerable proportion of patients with HCC have concurrent liver cirrhosis. In those patients with underlying PEM, interventional therapy such as TACE may further worsen their nutritional condition and even occasionally cause development of ascites and jaundice, resulting in an irreversible outcome[11].
Supplementation with BCAAs in patients with liver disorder has been attracting attention. BCAA treatment can correct malnutrition associated with liver cirrhosis in animals and humans[12-14], and long-term nutritional BCAA supplementation may also be useful for prevention of hepatic failure while it also improves surrogate markers in patients with advanced cirrhosis[15,16]. BCAA supplementation is also effective in down-regulating protein metabolism in liver cirrhosis patients by reducing ammonia (NH3) level, thus improving the nitrogen balance and resulting in better clinical outcomes[17,18]. The mechanism underlying these beneficial effects of BCAAs might be mediated by stimulation of hepatocyte growth factor activity that induces liver regeneration[19]. Therefore, nutritional support may play an important role in management of liver cirrhosis in patients with unresectable HCC. Studies dealing with the effect of treatment with BCAA granules before TACE in patients with HCC, nevertheless, are few as yet to our knowledge. This study was thus performed to investigate the significance of BCAA treatment in HCC patients who had undergone TACE.
This retrospective study included 99 patients who underwent TACE alone for treatment of HCC at our hospital and were followed up thereafter without treatment for at least 6 mo between January 2004 and January 2010. Patients were divided into two groups: those receiving BCAA treatment (n = 40) or regular diet (n = 59, control). BCAA therapy had been started at least one month before the day TACE was performed, and treatment compliance was good in all patients receiving BCAAs.
Dynamic computed tomography (CT) and abdominal echography were performed in all patients. A lesion visualized as a tumor blush in the early phase scan and as a defect area in the late phase scan on dynamic CT was diagnosed as HCC. It has been verified that such lesions appear as blushes on CT hepatic angiography and as defect areas on CT arterial portography during TACE. Two radiologists proficient in diagnostic imaging of the liver made a diagnosis of HCC. No pathological examination was conducted.
BCAA granules, containing 952 mg of L-isoleucine, 1904 mg of L-leucine and 1144 mg of L-valine per sachet, were orally administered to subjects at a dose of one sachet three times daily after meals. The control patients received no such treatment.
Written informed consent was obtained from each patient prior to TACE. The protocol for TACE was approved by the independent ethics committee of the hospital. TACE for HCC was performed in conformity with Japanese guidelines for this therapy[20] and consisted of catheterization via the femoral artery with super-selective cannulation to the hepatic artery feeding the target HCC. Farmorubicin (epirubicin hydrochloride, Pfizer) emulsion was infused at 10 to 60 mg, and Lipiodol (iodine addition products of ethyl esters of fatty acids obtained from poppy seed oil; Mitsui, Japan) was also injected at 2 to 10 mL according to the tumor size and tumor number. This was followed by embolization with gelatin (Spongel; Yamanouchi, Japan), which was injected slowly to prevent reflux into untreated segments. The sites of injection of the embolizing agents were segmental or subsegmental in all patients.
At 1 wk and 1, 3 and 6 mo after TACE, patients underwent hematological and blood biochemical tests and were assessed for their hepatic functional reserve and development of any adverse events. Dynamic CT was carried out to assess for any ascites or recurrence of HCC at 1, 3 and 6 mo after TACE.
Student t test, χ2 test and Fisher’s exact test were used to compare data between BCAA patients and the control. Serum albumin level and Child-Pugh score constituted parameters for assessment of hepatic functional reserve. Absolute changes in serum albumin level observed at 1 wk and 1, 3 and 6 mo after TACE were compared between the two groups and evaluated using Student t test, and the absolute change was defined as the difference found at each assessment time point from the baseline (pre-TACE level). Changes in Child-Pugh score were also evaluated similarly using Student t test at 1, 3 and 6 mo after TACE.
Data were analyzed using SPSS software, version 9.0 (SPSS Inc., Chicago, IL, United States) for Microsoft Windows. Data are expressed as mean ± SD. Values of P < 0.05 were considered to be statistically significant.
Patient demographic characteristics are summarized in Table 1. Significant differences were noted for the following parameters: Child-Pugh score, serum albumin level, prothrombin time, and dose of epirubicin at the time of TACE. A patient in the control group had stage I HCC, for which percutaneous therapy is indicated, but TACE alone was performed because the patient refused percutaneous therapy.
| BCAA group (n = 40) | Control group (n = 59) | P value | |
| Gender | |||
| Male | 27 | 32 | 0.215 |
| Female | 13 | 27 | |
| Age (yr) | 69.9 ± 8.8 | 73.2 ± 10.1 | 0.092 |
| Etiology of liver disease | |||
| Chronic hepatitis C | 28 | 43 | 0.287 |
| Chronic hepatitis B | 2 | 8 | |
| Non B non C | 10 | 10 | |
| Child-Pugh classification | |||
| Chronic hepatitis | 0 | 9 | 0.006 |
| Child-Pugh A | 22 | 39 | |
| Child-Pugh B | 15 | 10 | |
| Child-Pugh C | 3 | 1 | |
| WBC (× 103/μL) | 38.2 ± 10.8 | 44.7 ± 16.0 | 0.082 |
| Hb (g/dL) | 11.9 ± 1.8 | 12.5 ± 1.7 | 0.091 |
| Platelet (×104/mm3) | 10.2 ± 9.4 | 11.4 ± 4.9 | 0.431 |
| Alb (g/dL) | 3.32 ± 0.50 | 3.74 ± 0.51 | < 0.001 |
| T-Bil (mg/dL) | 1.28 ± 0.81 | 1.05 ± 0.63 | 0.123 |
| PT (%) | 77.5 ± 14.1 | 85.9 ± 17.3 | 0.012 |
| AST (IU/L) | 65.8 ± 39.6 | 73.8 ± 56.4 | 0.445 |
| ALT (IU/L) | 48.0 ± 38.8 | 54.2 ± 39.0 | 0.438 |
| AFP (ng/mL) | 626.1 ± 2009.8 | 1109.2 ± 2652.5 | 0.331 |
| PIVKAII (mAU/mL) | 1471.7 ± 5033.5 | 3421.5 ± 8211.2 | 0.183 |
| HCC Stage | |||
| StageI | 0 | 1 | 0.412 |
| Stage II | 12 | 11 | |
| Stage III | 23 | 35 | |
| Stage IVa | 5 | 12 | |
| Max tumor size (cm) | 3.34 ± 1.67 | 3.59 ± 1.47 | 0.422 |
| Epirubicin dose (mg) | 34.8 ± 10.4 | 39.5 ± 9.2 | 0.024 |
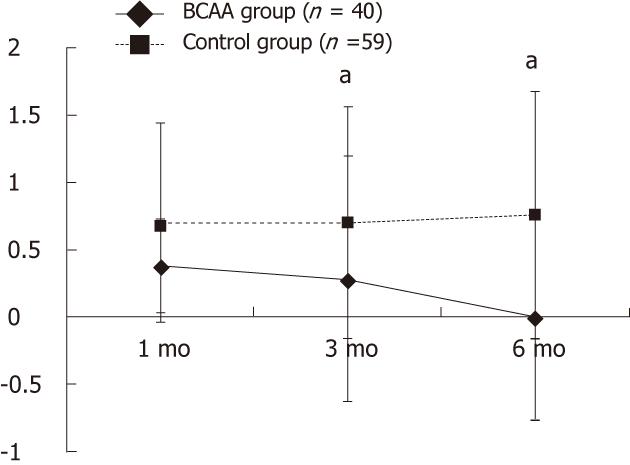
A significant difference in serum albumin level was observed at all assessment time points (P < 0.05). Also, there was a significant difference in Child-Pugh score 3 and 6 mo after TACE (P < 0.05) (Table 1, and Figure 1).
The categorized analysis results are presented below.
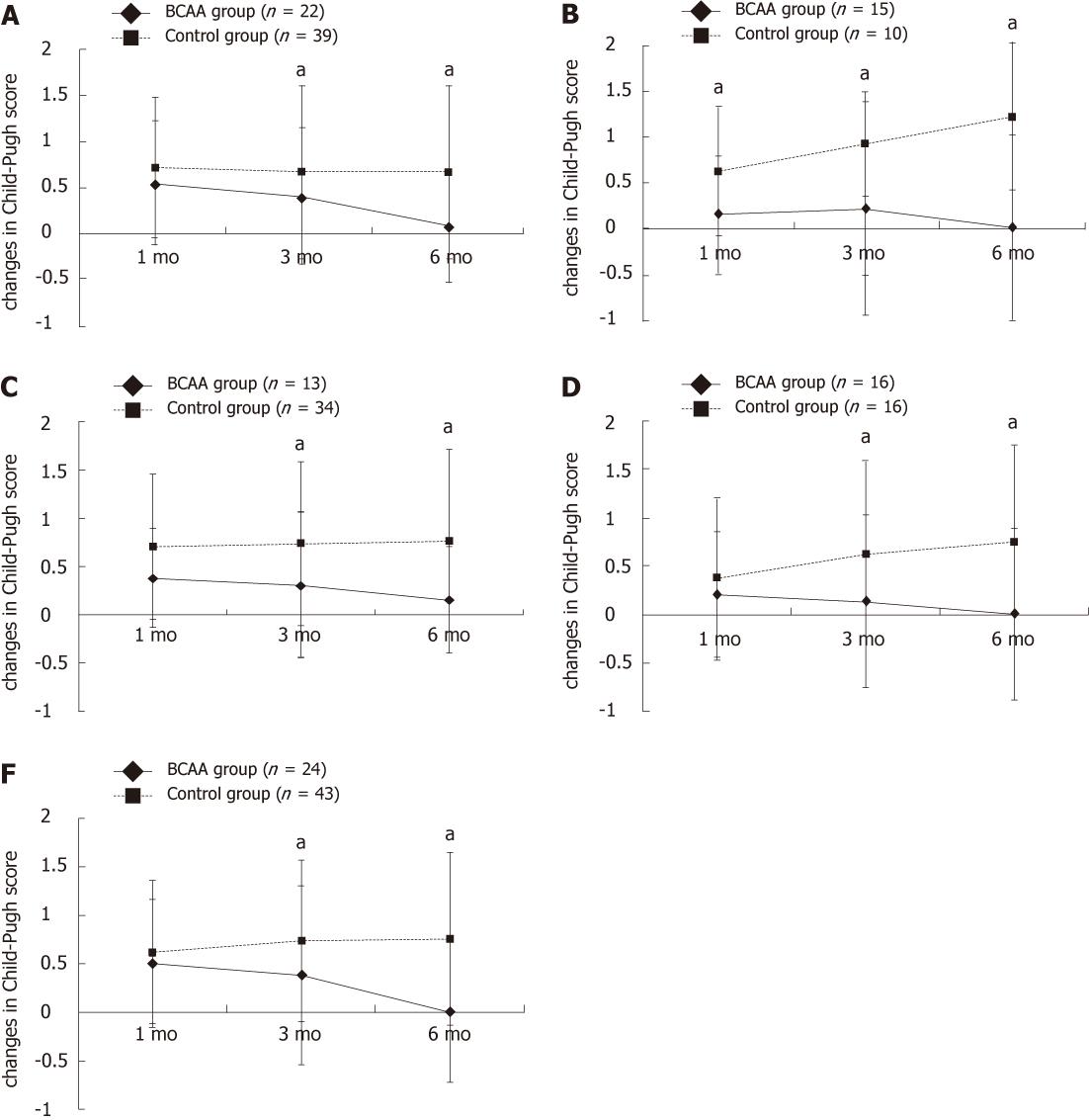
There were 22 Child A patients in the BCAA group and 39 in the control group. A significant difference was noted in serum albumin level 1, 3 and 6 mo after TACE and in Child-Pugh score 3 and 6 mo after TACE (P < 0.05) (Table 1, and Figure 2A).
There were 15 Child B patients in the BCAA group and 10 in the control group. A significant difference was noted in serum albumin level 3 and 6 mo after TACE and in Child-Pugh score 1, 3 and 6 mo after TACE (P < 0.05) (Table 1 and Figure 2B).
There were 13 and 34 patients who fell in this category in the BCAA group and the control group, respectively. A significant difference was observed in both serum albumin level and Child-Pugh score 3 and 6 mo after TACE (P < 0.05) (Table 1, and Figure 2C).
As it is thought that antineoplastic agents used during TACE may cause hepatic impairment in a dose-dependent fashion, the data were further evaluated in patients classified into two subgroups: those treated with low-dose epirubicin (less than 40 mg) or a high-dose epirubicin (40 mg or more).
Sixteen patients each received low-dose epirubicin in the BCAA group and the control group. Serum albumin level was significantly different 1, 3 and 6 mo after TACE and Child-Pugh score 3 and 6 mo after TACE (P < 0.05) (Table 1 and Figure 2D).
Twenty-four and 43 patients received high-dose epirubicin in the BCAA group and the control group, respectively. A significant difference was noted in serum albumin level at all assessment time points and in Child-Pugh score 3 and 6 mo after TACE (P < 0.05) (Table 1 and Figure 2E).
PEM occurs frequently in patients with liver cirrhosis and represents an important predictive factor for the prognosis of liver cirrhosis patients with HCC [18,21]. Supplementation with BCAA formula is reportedly useful for improving PEM and QoL in these patients. However, few studies have assessed the importance of such nutritional intervention in patients with HCC who underwent nonsurgical therapies such as TACE. The purpose of the present study was to investigate to what extent BCAA treatment can contribute to maintaining hepatic functional reserve in HCC patients after TACE.
A significant difference was observed in the overall patient population in terms of change in serum albumin level at all assessment time points. As seen in Table 1, hepatic functional reserve was relatively well maintained in the control group; therefore, anticancer chemotherapy was given at relatively high doses (60% of patients treated with BCAA received epirubicin at 40 mg or more whereas the corresponding percentage for the control group was 72.9%). Patients receiving high-dose anticancer chemotherapy are often unable to sufficiently ingest food over several weeks after TACE. This may account for lower serum albumin levels observed in the control group compared with the BCAA group. Other possible causes of decreased serum albumin levels after TACE include (1) impaired ability of the liver to synthesize serum albumin due to decreased hepatocyte count; (2) inhibition of the synthesis of albumin by inflammatory cytokines; and (3) leakage of albumin due to inflammation of the cauterized areas[22,23].
The assessments in Child-Pugh A patients revealed a significant difference in serum albumin level 1, 3 and 6 mo after TACE and in Child-Pugh score 3 and 6 mo after TACE. TACE is best indicated for Child-Pugh A HCC. In patients undergoing TACE, caution should be exercised to minimize depression of hepatic functional reserve in preparation for the next treatment session. The above results thus suggest the usefulness of BCAA treatment in this regard.
The assessments in the Child-Pugh B subgroup showed a significant difference in Child-Pugh score 1, 3 and 6 mo after TACE. Once hepatic functional reserve has worsened from Child-Pugh B to Child-Pugh C following TACE, the next TACE cannot be performed according to the Barcelona Clinic Liver Cancer guidelines[24]. Therefore, particular caution should be exercised in maintaining hepatic functional reserve at the time of TACE in patients with Child-Pugh B HCC, indicating the indispensability of BCAA therapy.
In Japan, BCAA granules are indicated for the treatment of liver cirrhosis in patients with a serum albumin level of 3.5 g/dL or less. However, conversely, the present study demonstrated similar results between patients with a serum albumin level of more than 3.5 g/dL and those in other categories of serum albumin level. Therefore, treatment with BCAA proved to improve hepatic functional reserve even in cirrhotic patients with HCC whose serum albumin level exceeds 3.5 g/dL. It is thus recommended to actively provide BCAA treatment in such patients.
There was a conspicuous difference between the BCAA and control groups in respect of response to BCAA therapy when assessed in patients receiving high-dose epirubicin compared to those treated with low-dose epirubicin. TACE may cause a marked damage to the liver in HCC patients, eventually leading to a considerable impact on their hepatic functional reserve[9]. BCAA treatment is thus recommended at sufficient doses prior to TACE in patients with advanced HCC in whom high-dose anticancer chemotherapy is anticipated.
TACE is often repeated because a single session of therapy seldom provides complete necrosis of a tumor. The procedure is commonly repeated once every 2 to 3 mo[25-27]. In the present study, however, many patients failed to attain recovery of hepatic functional reserve to a pre-TACE level, particularly in the control group, within 2 to 3 mo of TACE. It is thus estimated that every repeated session of TACE may worsen hepatic functional reserve and thereby shorten the prognosis for survival. Treatment with BCAA would therefore be essential in order to allow for providing TACE periodically while securely maintaining hepatic functional reserve.
One of the findings commonly noted in regard to all the variables assessed in this study is that a significantly greater improvement was noted in both serum albumin level and Child-Pugh score for the BCAA group 6 mo after TACE in comparison to the control group. What is suggested by this fact is simply the usefulness of long-term BCAA treatment prior to TACE. It is also important that patients should be fully instructed on the use of BCAA granules to maintain their treatment compliance.
The present study has several limitations. Firstly, it is a retrospective study. Furthermore, there was a bias in patient demographic characteristics between the BCAA and control groups since BCAA is usually used for patients showing low serum albumin levels. Therefore, pertinent data were evaluated for improvement or exacerbation using absolute serum albumin change as a parameter. The present study did not include assessment for the prognosis for survival, which should be addressed by a prospective study using comparable demographic characteristics among patients.
In conclusion, treatment with BCAAs before TACE in HCC patients is extremely useful in maintaining their hepatic functional reserve.
Patients with hepatocellular carcinoma (HCC) due to liver cirrhosis are known to have decreased plasma branched-chain amino acid (BCAA) levels, which can lead to protein-energy malnutrition (PEM). BCAA treatment can correct malnutrition associated with liver cirrhosis.
Studies dealing with the effect of treatment with BCAA granules before transcatheter arterial chemoembolization (TACE) in patients with HCC are few as yet. In this study, the authors analyzed the effect of BCAA treatment before TACE for HCC patients.
Recent studies imply that by BCAA supplementation, malnutrition associated with liver cirrhosis is corrected and liver function improves. The present study shows that in HCC patients who underwent TACE, liver function was maintained by BCAA supplementation.
This study emphasizes the importance of BCAA treatment before TACE for HCC patients with regard to maintaining liver function.
This is a very good and novel study in which authors analyze the effect of BCAA treatment before TACE for HCC patients. The results are interesting and suggest the usefulness of BCAA treatment before TACE in HCC patients in maintaining their hepatic functional reserve.
Peer reviewer: Sanaa Ahmed Ali, Assistant Professor, Therapeutic Chemistry Department, National Research Centre, El Behooth St., Cairo 12622, Egypt
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 671] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang ZY, Pan LJ. Therapeutic options for intermediate-advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. [PubMed] |
| 5. | Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453-456. [PubMed] |
| 6. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [PubMed] |
| 7. | Bronowicki JP, Boudjema K, Chone L, Nisand G, Bazin C, Pflumio F, Uhl G, Wenger JJ, Jaeck D, Boissel P. Comparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol. 1996;24:293-300. [PubMed] |
| 8. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 9. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 10. | Nutritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J Hepatol. 1994;21:317-325. [PubMed] |
| 11. | Takeshita S, Ichikawa T, Nakao K, Miyaaki H, Shibata H, Matsuzaki T, Muraoka T, Honda T, Otani M, Akiyama M. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Kajiwara K, Okuno M, Kobayashi T, Honma N, Maki T, Kato M, Ohnishi H, Muto Y, Moriwaki H. Oral supplementation with branched-chain amino acids improves survival rate of rats with carbon tetrachloride-induced liver cirrhosis. Dig Dis Sci. 1998;43:1572-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Yoshida T, Muto Y, Moriwaki H, Yamato M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn. 1989;24:692-698. [PubMed] |
| 14. | Hayaishi S, Chung H, Kudo M, Ishikawa E, Takita M, Ueda T, Kitai S, Inoue T, Yada N, Hagiwara S. Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis. Dig Dis. 2011;29:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, Martines D, Abbiati R. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11:92-101. [PubMed] |
| 16. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [PubMed] [DOI] [Full Text] |
| 17. | Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Tomiya T, Omata M, Fujiwara K. Significance of branched chain amino acids as possible stimulators of hepatocyte growth factor. Biochem Biophys Res Commun. 2004;313:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72 Suppl 1:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150-2154. [PubMed] |
| 22. | Ishikawa T, Michitaka I, Kamimura H, Higuchi K, Kubota T, Seki K, Ohta H, Yoshida T, Kamimura T. Oral branched-chain amino acids administration improves impaired liver dysfunction after radiofrequency ablation therapy for hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1491-1495. [PubMed] |
| 23. | Harima Y, Yamasaki T, Hamabe S, Saeki I, Okita K, Terai S, Sakaida I. Effect of a late evening snack using branched-chain amino acid-enriched nutrients in patients undergoing hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2010;40:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 865] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 25. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |